Abstract
Purpose: Patients with recurrent cervical carcinoma within a previously irradiated area respond poorly to chemotherapy. We have treated these patients with simultaneous cisplatin and hyperthermia (CDDP + HT) and investigated response, toxicity, palliative effect and survival.
Materials and methods: Between 1992 and 2005 47 patients received CDDP + HT. Response was evaluated by gynaecologic examination and CT-scan. The Common Toxicity Criteria (CTC) were used for evaluation of toxicity and palliative effect. The Kaplan-Meier method was used to estimate survival, and Cox regression analysis to evaluate the influence of prognostic factors.
Results: The objective response rate was 55%, palliation was achieved in 74% and operability in 19% of patients. Two patients are currently disease free at 9 years and 18 + months following treatment and 2 remained disease free until death by other causes. The median survival was 8 months and was influenced by duration of disease free interval and tumour diameter. Grade 3–4 haematological toxicity was observed in 36% of patients and renal toxicity was maximum grade 2.
Conclusion: CDDP + HT results in a high response rate and acceptable toxicity in patients with recurrent cervical cancer.
Introduction
Patients with cervical cancer stage IB2 or higher are usually treated with radiotherapy, which can be combined with chemotherapy or hyperthermia. The probability to achieve permanent local tumour control decreases with higher stages. Overall, 22% of patients have persistent or recurrent disease after radiotherapy Citation[1]. When such a failure to achieve a complete response or a recurrence is unresectable, therapeutic options are limited. The role of chemotherapy in metastatic or locally recurrent cervical cancer is modest. The currently most active and widely used agent is cisplatin, resulting in response rates range around 23% with a median survival of 6–8 months Citation[2], Citation[3].
If the disease recurs within a previously irradiated pelvic area, prognosis is particularly poor. A probable explanation for this is the decreased blood flow to the pelvis caused by vascular changes following radiotherapy. Published results in this subgroup of patients are scarce, but generally response rates are lower than in not previously irradiated tumours.
For years now, attempts have been made to improve the effect of chemotherapy and overall survival by combining cisplatin with other agents in various phase II and III trials. Thus far these new strategies were not very successful in previously irradiated patients Citation[4–7]. Recently a phase III randomized trial comparing cisplatin alone to cisplatin and topotecan showed a modest but significant advantage in survival of the combination treatment. In this study 56–58% of patients had received prior radiotherapy, but no attempt has been made to collect response and recurrence data relative to prior radiotherapy, because the endpoint was survival Citation[3].
Preclinical studies have shown that the addition of hyperthermia to cisplatin can greatly enhance its effectiveness. Hyperthermia has a cell killing effect preferentially in tumour tissue. There is no intrinsic difference in thermotolerance between normal cells and tumour cells, but there is a difference in physiology. Tumour tissue has chaotic vasculature resulting in hypoxia and low pH areas Citation[8]. Hypoxia and low pH are usually not found in normal tissue and these conditions make tumour cells more sensitive to hyperthermia Citation[9]. In addition, hyperthermia is known to enhance the effect of several chemotherapeutic agents. Hyperthermia enhances the effect of platinum analogues by increasing the cellular uptake and thereby increasing platinum-DNA adduct formation. The effect of cisplatin is enhanced with a factor 4 to 8, depending on temperature level and duration of treatm Citation[10], Citation[11].
We tested the feasibility and effectiveness of the combination of cisplatin and hyperthermia in patients with recurrent cervical cancer after previous irradiation in a phase I/II study. In this study, 6 weekly treatments of cisplatin and hyperthermia (CDDP + HT) applied simultaneously appeared feasible and resulted in 53% response Citation[12]. Similar results were found in another phase I/II study on cisplatin and hyperthermia, using a similar treatment scheme Citation[13]. Following the completion of the phase I/II study, patients with unresectable local tumour recurrences in a previously irradiated area have been considered for this combined treatment as a standard treatment approach in our hospital.
Herewith we report long-term follow-up on the initial study group Citation[12] plus 28 additional patients that have been treated between 2000 and 2005. We analysed treatment, toxicity and follow-up data of all patients with recurrent cervical carcinoma treated in our hospital with CDDP + HT. Further, we evaluated the palliative effect of this treatment.
Materials and methods
All patients with recurrent cervical carcinomas treated from 1992 to 2005 with CDDP + HT were included in this analysis. Before the start of treatment all patients were informed about the rationale, procedure and side-effects of combined treatment and they were eligible for CDDP + HT after informed consent. They were required to have cytologically or histologically proven locoregional recurrent or residual cervical carcinoma and radiotherapy in the past. At diagnosis, patients underwent a gynaecologic examination under anaesthesia and a CT-scan was made of thorax and abdomen. The results were discussed in a multidisciplinary team of a radiologist, pathologist, gynaecologic, medical and radiation oncologists. If patients were not amenable to surgery or radiotherapy, CDDP + HT was considered the treatment of choice. Exclusion criteria for CDDP were inadequate bone marrow or renal function, for HT a pacemaker or a metal implant in the pelvic region larger than 10 cm.
Chemotherapy
In the phase I/II study patients received 50–80 mg/m2 cisplatin weekly with a maximum of 6 courses. After completion of the study, 70 mg/m2 was the recommended dose, because it was well tolerated without dose-limiting toxicity.
The administration of chemotherapy started with 4 hours of prehydration with 1 litre of normal saline. Before starting infusion of cisplatin, anti-emetics were administered both orally, one hour in advance, and IV, 30 minutes before starting cisplatin. Cisplatin was infused in 250 ml saline over 3 hours. Cisplatin infusion and heating were started simultaneously.
After cisplatin infusion all patients received 3 litres of normal saline in 12 hours as posthydration. Each patient was given oral anti-emetics at discharge.
Deep locoregional hyperthermia
Patients were mildly sedated using either 1 mg lorazepam or xanax 0.5 mg. For all treatments, the BSD-2000 3D system (BSD Medical Systems, Utah, USA) was used. For thermometry, Bowman probes were placed intraluminally in bladder, vagina and rectum within closed tip catheters. Thermal mapping along the catheters was performed every 5 min with a step size of 1 cm with a maximum map length of 14 cm. Pulse rate and blood pressure were automatically measured before and every 5 minutes during treatment and oral temperature was measured at 0, 15, 30, 60 and 90 minutes.
Heating was started with a power output of 400 W at 77 MHz. Patients were carefully instructed to report any discomfort due to too high temperatures in normal tissue during treatment. Treatment settings for power, phase and frequency were adjusted accordingly if complaints occurred. If no complaints occurred, 100 W was added to the power output every 5 minutes. Treatment objective was to achieve intraluminal temperatures of 40–43°C as homogeneously as possible. In all patients 90 minutes were scheduled for each hyperthermia treatment; 30 minutes of heating up and 60 minutes actual treatment time.
Response evaluation and toxicity
CT-scan and gynaecologic examination were done 2–4 weeks after the last course of therapy, or earlier during the course of treatment if the patient's condition warranted such. Toxicity and pain were evaluated during treatment using the CTC (Common Toxicity Criteria) scale, version 2.0.
The response noted in this study is the maximum response achieved during follow-up for the duration of at least one month. A response was defined as a partial or complete response. A complete response was defined as the disappearance of all visible and palpable lesions, a partial response as a decrease of at least 50% of all visible and palpable lesions. Palliative effect was defined as a decrease in pain after the last course of therapy of at least one point on the CTC-scale lasting for at least one month. The duration of response was calculated from the date of maximum response to the date of local progression. The duration of palliation was calculated from the date of maximum tumour response to the date progression of pain was diagnosed. After treatment, progression of pain was assessed retrospectively from the patient's medical files. The date of progression was defined as the date the patient mentioned an increase in pain of at least one point on the CTC scale, or sought medical attention because of pain.
The influence of age, performance status, lymph node status, presence or absence of pain and distant metastasis, previous platinum-containing chemotherapy or hyperthermia, time between primary treatment and recurrence and maximum tumour diameter on overall and progression free survival was evaluated using Cox regression analysis. For survival analysis, the Kaplan-Meier method was used. P-values less than 0.05 were considered significant.
Results
Patient characteristics
From 1992 to 2005 47 patients with progressive recurrent or residual intrapelvic tumours were treated with CDDP + HT. Nineteen were treated within the framework of the phase I/II study. Median time of follow-up was 7.8 months (range: 1–106). All patients had received full dose radiotherapy to the pelvis (mean dose of 50.2 Gy) combined with brachytherapy (n = 43), a central boost with external radiotherapy (n = 4) or a boost to the iliac nodes (n = 1).
Patient characteristics are summarised in . There were no differences in patient characteristics when patients treated within the phase I/II trial are compared to those treated after the study closed. One patient received two series of CDDP + HT for two consecutive recurrences. She was included once in response and toxicity evaluation and survival analysis. All patients were in good general condition, WHO performance status (WHO-PS) 0–1, except for one patient who was confined to bed due to severe pain caused by the tumour recurrence (WHO-PS 3).
Table I. Patient characteristics.
Of 47 residual or recurrent tumours, 37 were first recurrences or residual tumours, nine were second recurrences and one was a third recurrence. Eight patients had been treated with cisplatin-containing chemotherapy prior to CDDP + HT. Three patients had received five weekly courses of 40 mg/m2 cisplatin concurrently with radiotherapy. One patient who presented with distant metastasis at primary diagnosis received six three-weekly courses of cisplatin (75 mg/m2) and taxol, and two received cisplatin (75 mg/m2) and taxol as neoadjuvant treatment in three three-weekly courses. A seventh patient received seven courses of cisplatin 70 mg/m2 concurrent with four applications of brachytherapy perioperatively. The last patient received six three-weekly courses of BEMP chemotherapy (Bleomycin, Vindesine, Cisplatin 50 mg/m2 and Mitomycine C) as induction therapy because of lymphogenic metastasis. None of these eight patients received 80 mg/m2 cisplatin during CDDP + HT.
Treatment characteristics
Six weekly treatments were scheduled for every patient.
A total of 226 (80%) courses of chemotherapy were administered to forty-seven patients, of these 216 were combined with hyperthermia.
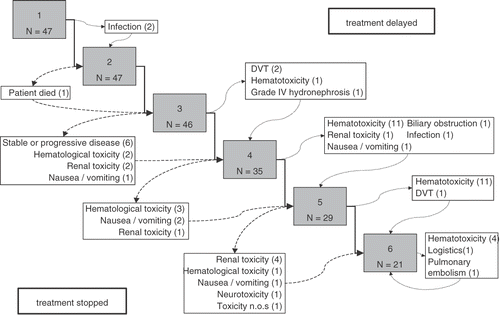
Reasons for administering cisplatin without hyperthermia were patient refusal (5 courses, same patient), technical problems (2 courses), logistical problems (2 courses) and a deep venous thrombosis (1 course) established the day of scheduled hyperthermia.The treatment had to be postponed 39 times in 21 patients.
Reasons to stop or delay chemotherapy are given in . Most common cause of delay in our patient group was haematological toxicity (27 times), occurring after the 4th or 5th course of cisplatin. Vascular events (deep venous thrombosis, pulmonary embolism) were the reason to delay four treatments.
The dose of cisplatin was 70 mg/m2 in 33 patients, given once weekly. Ten patients participating in the phase I/II study received 60 mg/m2 (n = 5) or 80 mg/m2 (n = 5).
A lower dose was given in four patients. In two patients this was because of a poor renal function. Another patient received 40 mg/m2 CDDP the first three treatments and then continued with 70 mg/m2. The last patient was treated with CDDP + HT after she did not respond to radiotherapy which was first combined with hyperthermia (four treatments), thereafter with CDDP (40 mg/m2, three courses) and she then continued with CDDP + HT at 50 mg/m2 (three courses). CDDP was started at 40 mg/m2 for fear of excess toxicity and when no complaints or deterioration of renal function occurred, she continued at 50 mg/m2.
The mean temperatures achieved during hyperthermia were 40.6 ± 0.6°C in the lumen of the bladder, 40.4 ± 0.7°C in the vagina and 40.6 ± 0.7°C in the rectum.
The mean increase in systemic temperature was 1.0 ± 0.5°C.
Toxicity
For toxicity evaluation, all patients were eligible. Forty-five per cent of patients completed all six scheduled courses of chemotherapy. Most severe toxicity observed in each treatment series is listed in . CTC grade 3–4 leucopenia occurred in 36% of treatment series, grade 3–4 neutropenia in 35% and grade 3–4 trombopenia in 15%. The incidence of other grade 3–4 adverse events did not exceed 10%. Neutropenic fever was not observed. No difference in clinically relevant toxicity was observed when the patients treated in the phase I/II study were compared to the patients treated after the study closed.
Table II. Most severe toxicity observed per treatment series.
Hyperthermia-related toxicity occurred in five (11%) of 47 treatment series. All incidents were subcutaneous burns that healed without any further medical attention. These incidents did not cause any treatment delay. Neurotoxicity was not observed as a direct effect of hyperthermia.
Response rate and survival
Overall pelvic response rate (complete or partial responses) is 58% (26 of 45, 95% confidence interval 0.42–0.72) including three complete responses. Fifty-three per cent of patients responded in the phase I/II study. The response rate of patients treated since completion of the study is 57% (16 of 28).
Of eight patients with distant metastasis, the response of lesions outside the hyperthermia target area is listed in .
Table III. Responses inside and outside the heated area.
Response evaluation could not be performed in two patients. One patient refused response evaluation because of lack of consequence and another died suddenly after three combined treatments, probably due to myocardial infarction. They were both considered not evaluable for response.
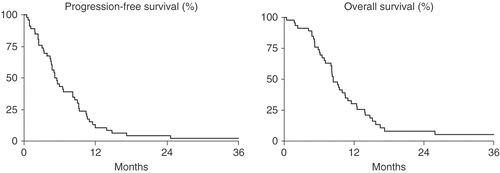
All patients were evaluable for survival. Median duration of survival is eight months with a trend towards better survival for responders (median of ten months for responders vs. six for non-responders, p = 0.05). Overall and progression-free survival is depicted in ; two years after treatment, 16% of responders are still alive, compared to none of the non-responders. Forty-six patients were evaluable for progression free survival. This was four months; 1.6 months for non-responders and 5.6 months for responders.
Additionally, Cox regression analysis showed that overall survival was slightly but significantly influenced by time interval between primary treatment for cervical carcinoma and date of first CDDP + HT (hazard ratio 0.97, p = 0.03) and the maximum diameter of the recurrence (hazard ratio = 1.10, p = 0.04). The mean intraluminal temperatures achieved in the responders were not statistically different from those achieved in non-responders, assessed by an independent samples t-test.
Of eight patients who had received platinum-containing chemotherapy in the past, four responded, three had stable disease and for one patient response could not be evaluated.
The median survival of patients with distant metastasis (7 months) was not significantly different from patients without distant metastasis (8 months, p = 0.8).
Salvage surgery
Of 26 responders, nine obtained a response that enabled salvage surgery. Three patients underwent a total pelvic exenteration, another three an anterior exenteration and one patient had a vaginal tip resection. A suboptimal debulking was performed twice because during surgery the iliac vessels proved to be invaded by the tumour in one patient and the sacral plexus in the other patient. Two of the total pelvic exenterations and two of the anterior exenterations were microscopically radical, the others microscopically irradical. The vaginal tip resection was a radical procedure. Overall survival in this subgroup was twelve months (range: 5–97) (excluding one patient who underwent a total pelvic exenteration in the presence of pulmonary metastasis). There are two long-term disease-free survivors in this subgroup. One patient died of lung cancer, eight years after CDDP + HT followed by a microscopically radical total pelvic exenteration. A second patient is now disease free 18 months after combined treatment followed by a vaginal tip resection.
Palliation
Thirty-eight patients had tumour related pain at the start of treatment. CDDP + HT resulted in a significant reduction of pain in 28 cases (74%). The median duration of palliation is five months (range: 2–18). Two complete responders with pain had significant palliation. Of 18 partial responders with pain, only one did not experience a significant palliation. Of seven patients with pain and progressive disease after treatment, three (43%) had significant palliation and of 11 patients with pain and stable disease following treatment, six (55%) experienced significant palliation. Information on the duration of palliation is unknown for seven patients, due to return of the patient to the referring clinic (n = 4), patient refusing medical interference (n = 1) or surgery before progression of pain (n = 1). One patient died after the third treatment.
Discussion
A locoregional recurrent cervical carcinoma in a previously irradiated area is a major clinical problem. In the majority cases this recurrence is unresectable due to invasion of adjacent organs or bony structures such as the pelvic sidewall. The effectiveness of chemotherapy, including cisplatin-based regimens, in previously irradiated areas is poor. These recurrences frequently cause pain, fistulae, hydronephrosis and lymphedema. Hence, the development of effective treatment strategies is warranted.
In this study, CDDP + HT resulted in a 55% response rate. These results confirm those of the previous phase I/II studies in which response rates of approximately 50% were achieved, with acceptable toxicity Citation[12], Citation[13]. The toxicity profile in the current study is similar to that found in our phase I/II study, without clinically relevant toxicity. None of our patients has developed neutropenic fever and neurotoxicity did not exceed CTC grade 2 in any of the patients.
The additive effects of hyperthermia when combined with chemotherapy can be explained by spatial cooperation. Drug concentration will be less in insufficiently perfused tumour regions, where cells are specifically sensitive to hyperthermia. In addition, heat potentiates many drugs. Generally, interaction is only seen when the two treatments are given in close sequence. The most important mechanisms for an interactive effect are an increased intracellular drug uptake, enhanced DNA damage and higher intratumour drug concentrations, resulting from an increase in blood flow. An interactive effect was observed for virtually all cell lines treated at temperatures above 40°C for platinum analogues, with enhancement ratios of between 4 and 8, depending on temperature and exposure time. Increased renal toxicity has only been observed with core temperatures of 41°C and high Citation[10], Citation[11].
Four randomized trials have compared chemotherapy alone to chemotherapy and hyperthermia. In two, intravesical mitomycine C was combined with local hyperthermia to the bladder and the combined approach lead to a significantly higher response rate and less recurrences in patients with superficial bladder canc Citation[14], Citation[15]. In another trial, patients with metastatic liver cancer were treated with intra-arterial chemotherapy or the same chemotherapy plus hyperthermia Citation[16]. In this trial, response rate in the chemotherapy alone group was 7% and in the combination therapy group 40%. In the fourth, patients with advanced carcinoma of the oesophagus also responded better to the combined approach Citation[17].
It is difficult to compare the results of our study to those published by others on chemotherapy without hyperthermia in recurrent cervical cancer. First, a schedule of 70 mg/m2 cisplatin once weekly for 6 courses is not commonly prescribed. Second, the results achieved in this particular group of patients with a tumour in a previously irradiated volume are usually not reported separately. Studies reporting results of chemotherapy in patients with recurrent cervical cancer in previously irradiated areas are summarised in . Only those studies that specify results in this particular subgroup are mentioned.
Table IV. Reports in literature on response rate for irradiated recurrent cervical carcinomas (reponses/no. evaluable)*.
Following cisplatin alone, response rates have ranged from 0 to 50%. Taking all available data together, the overall response rate is 21%. When cisplatin is combined with various other chemotherapeutic agents, the response rate in previously irradiated areas has remained relatively low: 4 to 24%, for an overall response rate of 15%. When the results of our current study and the study by Rietbroek et al. Citation[13] are combined, the response rate is 54%, which appears to be superior to that obtained by chemotherapy alone.
We found one other study reporting the palliative effect of treatment in patients with recurrent cervical carcinoma. Chambers et al. achieved pain relief in 66% of the patients treated with a combination of bleomycin, vincristin, mitomycine C and cisplatin Citation[18]. The 74% palliative effect in this study is similar to that. No quality of life analysis was performed in either study, but it seems reasonable to assume that relief of pain has a positive effect on the quality of life.
Several patients, three of forty-five (7%) obtained long-term disease free survival with this treatment. One patient is currently free of disease, nine years after her second series of CDDP + HT, another died of a non-related cause eight years after CDDP + HT followed by a pelvic exenteration and yet another died of late complications of radiotherapy, becoming disease free after CDDP + HT followed by a pelvic exenteration. A fourth patient is currently disease free 18 months after CDDP + HT and additional surgical treatment. It would be interesting to know what factors are responsible for these remarkable responses. However, we have not been able to identify any distinguishing parameters in this patient group, which could be due to the heterogeneity of the group.
As a phase III trial comparing cisplatin alone to CDDP + HT for recurrent, previously irradiated cervical carcinoma was not feasible due to the limited number of patients, and in view of the good results that we have seen, we consider this combined treatment a standard treatment approach for patients with locally recurrent or residual cervical carcinoma within a previously irradiated area. The high response rate and acceptable toxicity found in these patients further cleared the way for combining radiotherapy with cisplatin and hyperthermia for primary cervical carcinoma, a combination that proved to be quite promising Citation[19].
References
- Randall ME, Michael H, Vermorken J, Stehman F. Uterine cervix. Principles and practice of gynaecologic oncology, WJ Hoskins, CA Perez, RC Young, RR Barakat, M Markham, ME Randall, et al. Williams and Wilkins, Philadelphia, Lippincott 2005; 743–822
- Moore DH, Blessing JA, McQuellon RP, Thaler HT, Cella D, Benda J, Miller DS, Olt G, King S, Boggess JF, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: A Gynaecologic Oncology Group study. J Clin Oncol 2004; 22: 3113–3119
- Long HJ, Bundy BN, Grendys EC, Jr, Benda JA, McMeekin DS, Sorosky J, Miller DS, Eaton LA, Fiorica JV. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: A Gynaecologic Onology Group Study. J Clin Oncol 2005; 23: 4626–4633
- Papadimitriou CA, Sarris K, Moulopoulos LA, Fountzilas G, Anagnostopoulos A, Voulgaris Z, Gika D, Giannakoulis N, Diakomanolis E, Dimopoulos MA. Phase II trial of paclitaxel and cisplatin in metastatic and recurrent carcinoma of the uterine cervix. J Clin Oncol 1999; 17: 761–766
- Piver MS, Ghamande SA, Eltabbakh GH, O’Neill-Coppola C. First-line chemotherapy with paclitaxel and platinum for advanced and recurrent cancer of the cervix ã A phase II study. Gynecol Oncol 1999; 75: 334–337
- Cadron I, Jakobsen A, Vergrote I. Report of an early stopped randomized trial comparing cisplatin vs. cisplatin/ifosfamide/5-fluorouracil in recurrent cervical cancer. Gynaecol Obstet Invest 2005; 59: 126–129
- Matulonis UA, Campos S, Duska L, Krasner CN, Atkinson T, Penson RT, Seiden MV, Verrill C, Fuller AF, Goodman A. Phase I/II dose-finding study of combination cisplatin and gemcitabine in patients with recurrent cervix cancer. Gynecol Oncol 2006; 103: 160–164
- Reinhold HS, Endrich B. Tumour microcirculation as a target for hyperthermia. Int J Hyperthermia 1986; 2: 111–137
- Raaphorst GP. Fundamental aspects of hyperthermic biology. An introduction to the practical aspects of clinical hyperthermia, SB Field, JW Hand. Taylor and Francis, London 1990; 10–54
- Dahl O. Interaction of heat and drugs in vitro and in vivo. Thermoradiotherapy and thermochemotherapy, MH Seegenschmiedt, P Fessenden, CC Vernon. Springer, Berlin 1995; 103–121
- Rietbroek RC, van der Vaart PJM, Haveman J, Blommaert FA, Geerdink A, Bakker PJM, Veenhof CHN. Hyperthermia enhances the cytotoxicity and platinum-DNA adduct formation of lobaplatin and oxaliplatin in cultured SW 1573 cells. J Cancer Res Clin Oncol 1997; 123: 6–12
- de Wit R, van der Zee J, van der Burg ME, Kruit WH, Logmans A, van Rhoon GC, Verweij J. A phase I/II study of combined weekly systemic cisplatin and locoregional hyperthermia in patients with previously irradiated recurrent carcinoma of the uterine cervix. Br J Cancer 1999; 80: 1387–1391
- Rietbroek RC, Schilthuis MS, Bakker PJ, van Dijk JD, Postma AJ, Gonzalez GD, Bakker AJ, van der Velden J, Helmerhorst TJ, Veenhof CH. Phase II trial of weekly locoregional hyperthermia and cisplatin in patients with a previously irradiated recurrent carcinoma of the uterine cervix. Cancer 1997; 79: 935–943
- Colombo R, Da Pozzo LF, Lev A, Freschi M, Gallus G, Rigatti P. Neoadjuvant combined microwave induced local hyperthermia and topical chemotherapy versus chemotherapy alone for superficial bladder cancer. J Urol 1996; 155: 1227–1232
- Colombo R, Da Pozzo LF, Salonia A, Rigatti P, Zvi L, Baniel J, Caldarera E, Pavone-Macaluso M. Multicentric study comparing intravesical chemotherapy alone with local microwave hyperthermia for profylaxis of recurrence of superficial transitional cell carcinoma. J Clin Oncol 2003; 21: 4270–4276
- Kondo M, Itani K, Yoshikawa T, Tanaka Y, Watanabe N, Hiraoka M, Noguchi M, Miura K. A prospective randomized clinical trial comparing intra-arterial chemotherapy alone and when combined with hyperthermia for metastatic liver cancer. Gan To Kagaku Ryoho 1995; 22: 1807–1811
- Sugimachi K, Kuwano H, Ide H, Toge T, Saku M, Oshiumi Y. Chemotherapy combined with or without hyperthermia for patients with oesophageal carcinoma: A prospective randomized trial. Int J Hyperthermia 1994; 10: 485–493
- Chambers SK, Flynn SD, Del Prete SA, Chambers JT, Schwartz PE. Bleomycin, vincristine, mitomycin C, and cis-platinum in gynecologic squamous cell carcinomas: A high incidence of pulmonary toxicity. Gynecol Oncol 1989; 32: 303–309
- Westermann AM, Jones EL, Schem BC, van der Steen-Banasik EM, Koper P, Mella O, Uitterhoeve ALJ, de Wit R, van der Velden J, Burger C, et al. First results of triple-modality treatment combining radiotherapy, chemotherapy and hyperthermia for treatment of patients with stage IIB, II and IVA cervical carcinoma. Cancer 2005; 104: 763–770
- Thigpen T, Shingleton H, Homesley H, Lagasse L, Blessing JA. Cisplatinum in treatment of advanced or recurrent squamous cell carcinoma of the cervix. Cancer 1981; 48: 899–903
- Potter ME, Hatch KD, Potter MY, Shingleton H, Baker VV. Factors affecting the response of recurrent squamous cell carcinoma of the cervix to cisplatin. Cancer 1989; 63: 1283–1286
- Daly M, Cowie VJ, Davis JA, Habeshaw T, Junor EJ, Paul J, Pyper E, Reed M, Soukop M, Yosef H, et al. A short and intensive single-agent cisplatin regimen for recurrent carcinoma of the uterine cervix. Int J Gynaecol Cancer 1996; 6: 61–67
- de Murua EO, George M, Pejovic MH, Dewailly J, Wolff JP. Combination cyclophosphamide, adriamycin, and cis-platinum in recurrent and metastatic cervical carcinoma. Gynecologic Oncol 1987; 26: 225–227
- Lele SB, Piver MS. Weekly cisplatin induction chemotherapy in the treatment of recurrent cervical carcinoma. Gynecol Oncol 1989; 33: 6–8
- Ramm K, et al. Bleomycin-ifosfamide-cis-platinum (BIP) in pelvic recurrence of previously irradiated cervical carcinoma: A second look. Gynecol Oncol 1992; 46: 203–207
- Brader KR, Vergrote IB, Kaern J, Tropé CG. Chemotherapy for cervical carcinoma: Factors determining response and implications for clinical trial design. J Clin Oncol 1998; 16: 1879–1884
- Rose PG, Blessing JA, Gershenson DM, McGhee R. Paclitaxel and cisplatin as first-line therapy in recurrent or advanced squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. J Clin Oncol 1999; 17: 2676–2680
- Burnett AF, Roman LD, Garcia AA, Muderspach LI, Brader KR, Morrow CP. A phase II study of gemcitabine and cisplatin in patients with advanced, persistent or recurrent squamous cell carcinoma of the cervix. Gynecol Oncol 2000; 76: 63–66
- Fiorica J, Holloway R, Ndubisi B, Orr J, Grendys E, Boothby R, DeCesare S, LaPolla J, Hoffman M, Patel J. Phase II trial of topotecan and cisplatin in persistent or recurrent squamous and non-squamous carcinomas of the cervix. Gynecol Oncol 2002; 85: 89–94
- Gebbia V, Caruso M, Testa A, Mauceri G, Borsellino N, Chiarenza M, Pizzardi N, Palmeri S. Vinorelbine and cisplatin for the treatment of recurrent and/or metastatic carcinoma of the uterine cervix. Oncology 2002; 63: 31–37