Abstract
Purpose: The current work describes the synergistic enhancement of hyperthermic cancer therapy by selective thermal sensitization and induction of vascular injury at the tumor site. The specificity of this response was mediated by CYT-6091: a pegylated colloidal gold-based nanotherapeutic designed to selectively deliver an inflammatory cytokine, tumor necrosis factor alpha (TNF), to solid tumors.
Materials and methods: FSaII murine fibrosarcoma-bearing C3H mice received an intravenous injection of either soluble TNF or CYT-6091 (50–250 µg/kg TNF). Four hours later the tumors were exposed to localized heating (42.5 or 43.5°C, 60 min). Tumor responses were assessed by growth delay and/or perfusion.
Results: Both soluble TNF and CYT-6091 reduced tumor perfusion by 80% of control (no treatment), 4 hours post administration. However, soluble TNF was toxic to the tumor burdened mice and resulted in 40% mortality alone and 100% mortality when combined with hyperthermia. Conversely, no toxicities were noted with CYT-6091 alone or when combined with hyperthermia. Additionally, CYT-6091 combined with heat yielded significant tumor regression in vivo as compared to heat or CYT-6091 alone as demonstrated by tumor growth delay. Pretreatment with soluble TNF or CYT-6091 followed by heating reduced in vitro tumor and endothelial cell survival by 40–50% (TNF) and 70–75% (CYT-6091) of the control cell (i.e. tumor and endothelial) values, respectively.
Conclusions: CYT-6091, by selectively delivering TNF to solid tumors, improves the safety of TNF treatment. In addition, the targeted delivery of TNF augments cancer thermal therapy efficacy possibly by inducing a tumor-localized inflammatory response.
Introduction
The major physiological factors influencing the response of solid tumors to hyperthermia are: (1) blood flow, (2) intra-tumor pressure, (3) per-meability, (4) pH, and (5) pO2 Citation[1]. Among these, the blood flow is a major barrier to hyperthermia treatment as it dissipates heat allowing tumor and associated stromal cells to survive Citation[1]. Therefore, to improve efficacy of hyperthermia, alone or in combination with other therapies, more attention has been paid to a variety of vascular targeting agents. Promising results have been achieved by agents such as arsenic trioxide Citation[2], prostaglandins Citation[3], indomethacin Citation[4], interleukins Citation[5] and tumor necrosis factors Citation[6], which accentuate the thermal injury to the tumor vasculature when combined with heating. In recent years, a number of small molecule vascular targeting agents (CA4P Citation[7], DMXAA Citation[8], FAA Citation[9]) have also been shown to enhance hyperthermia, validating the general approach of targeting tumor vascular structures and characteristics to improve thermal therapy [10,11].
Among these adjuvants, tumor necrosis factor alpha (TNF) is of particular interest because of its multifunctional anti-cancer effects. The major effects are direct tumor cell lysis, anti-tumor immunotherapeutic response, apoptotic and pro-inflammatory pathways Citation[12–14]. TNF has also been shown to reduce interstitial fluid pressure and cause acute hyperpermeability leading to accumulation of more drug in the tumo Citation[15], Citation[16]. This in turn reduces blood flow causing tumor-associated vascular destruction and hemorrhagic necrosis of tumor. Furthermore, we and others have shown enhancement of thermal therapy with soluble TNF in vitro Citation[6], Citation[17], in vivo Citation[6], Citation[18] and in limited clinical use Citation[19]. These studies have shown that, at the doses employed (in vitro: 10,000 units/ml, in vivo: 50 µg/kg or 1000 units/mouse, clinical: 1 × 10(6) U), TNF alone has a weak tumor growth inhibitory effect, but, when given before thermal therapy, exhibits significant thermal sensitizing activity, particularly at the level of the tumor vasculature. Unfortunately, while the cytokine TNF can lead to excellent anti-tumor response (alone and with hyperthermia), it is associated with systemic toxicity (hypotension, congestion, septic shock)Citation[12], Citation[13], Citation[20], and has therefore been discontinued, with the exception of isolated limb perfusion, in clinical practice Citation[21]. Thus, improvements in the safety and efficacy of combination treatment may be expected from approaches which can alter the biodistribution of TNF by targeting its delivery specifically to solid tumors.
One such approach is a recently introduced nanotherapeutic agent, CYT-6091 (formerly PT-cAu-TNF) (CytImmune Sciences, Inc., Rockville, MD), which is currently in a phase I clinical trial as a stand-alone treatment against advanced solid organ malignancies Citation[22]. CYT-6091 is manufactured by covalently linking molecules of TNF and a Thiol derivatized polyethylene glycol (PEG) onto the surface of 30-nm colloidal gold particles Citation[23]. CYT-6091 avoids uptake by the RES and selectively delivers TNF to solid tumors by passive (i.e. enhanced permeability and retention effect) and active (TNF receptor targeting) mechanisms Citation[23]. By altering the biodistribution of TNF, CYT-6091 has been shown to improve the dose-to-dose safety and/or efficacy of TNF treatment Citation[23]. This means that TNF when administered as CYT-6091 is as or more effective but less toxic. For example, Paciotti et al. Citation[23] observed that 7.5 µg of CYT-6091 was as effective as 15 µg of TNF. With 15 µg of TNF 40% of the animals died due to systemic toxicity, whereas none of the animals receiving CYT-6091 died.
We have previously shown enhancement of thermal therapy (heat or cold) by the use of TNF or CYT-6091 in murine mammary carcinoma Citation[24] and human prostate cancer modelsCitation[17], Citation[25], Citation[26]. The results have shown that, at the doses employed, TNF or CYT-6091 has a weak tumor growth inhibitory effect on its own, but when given before thermal therapy exhibited significant thermal sensitization. Further work in other solid tumor models is still necessary to define the precise conditions under which this non-toxic thermal therapy enhancement can occur. The goal of this study was to extend our knowledge of this augmentation by further study of the toxicity, dosage, timing and heating regimes of CYT-6091 enhancement of thermal therapy in another well studied solid tumor, a murine fibro-sarcoma (FSaII). Our previous study showing heat enhancement using CYT-6091 was in a murine SCK model of mammary carcinoma Citation[24]. FSaII differs from SCK in physiology, vasculature and growth kinetics Citation[27], Citation[28]. Our hypothesis for the current study was that non-toxic, synergistic enhancement of FSaII tumor thermal therapy can be obtained using properly sequenced CYT-6091 nanoparticle-delivery of TNF to the vasculature. We aimed to thereby demonstrate that tumors with characteristically different composition and treatment sensitivity can be universally impacted with this powerful new treatment approach.
Materials and methods
Cell lines and culture conditions
The FSaII murine fibrosarcoma cell line was a kind gift of Dr Herman Suit (MGH, Boston). The cells were cultured in RPMI 1640 medium (GIBCO BRL, Grand Island, NY) supplemented with 10% bovine serum (Hyclone Laboratories, Logan, UT) and antibiotics under a humidified 5% CO2 atmosphere. FSaII cells in exponential growth phase in culture were dispersed into a single cell suspension by treatment with 0.25% trypsin (Difco Laboratories, Detroit, MI) in RPMI 1640 medium at 37°C for 10 min. The resulting single cells were washed, resuspended in fresh RPMI 1640 medium with 10% calf serum, and then counted using a hemacytometer before use in experiments.
Microvascular endothelial cells (MVECs) were released from newborn human foreskin within 24 hr of collection as described by Davison et al. Citation[29]. Cells were grown in 25 cm2 T-flasks coated with 1% gelatin in a 37°C, 5% CO2 incubator for 3 or 4 passages before they were used for experiments. The culture medium consisted of 800 ml/L of MCDB 131 medium, 2.6 × 10−1 g/L of Dibutryl cAMP, 200 ml/L of heat inactivated male human serum, 400 mg/L of heparin, antibiotics, 1.5 g/L of L-glutamine, and 1 ml/L of hydrocortisone.
Drugs
Recombinant human TNF and CYT-6091 (formerly PT-cAu-TNF) were provided by CytImmune Sciences, Inc. (Rockville, MD). A solution of pegylated colloidal gold particles lacking TNF (PT-cAu, CytImmune Sciences, Inc.) was used as a control. For in vivo experiments, a fresh preparation of drug was made prior to each experiment by dissolving the necessary amount in PBS to get a concentration corresponding to 50, 125 and 250 µg/kg TNF in 0.1 ml of solution. In this way the response due to exact amount of free vs. bound TNF could be studied. Injections were made intravenously through the tail vein.
FSaII cell survival study in vitro
An in vitro clonogenic assay Citation[24] was used to assess whether TNF enhanced the thermal killing of FSaII and MVEC cells in vitro. For these studies, 200 FSaII or 1000 MVEC cells were plated in 25 cm2 tissue culture flasks and were incubated overnight. Subsequently, the cells were treated with increasing doses (10–1000 ng/ml) of either soluble TNF or CYT-6091. The cells were incubated for 4 h at 37°C, 5% CO2 prior to exposing them to the thermal treatment. For thermal treatment, the flasks were immersed into a 42.5°C water bath for 1 h. The cells were then gently rinsed with drug-free medium and cultured in fresh medium in an incubator for 7–10 days to allow colony formation. At the conclusion of the study the colonies were fixed, stained and counted Citation[24].
Tumor implantation
About 2 × 105 FSaII cells suspended in 0.05 ml serum-free medium were injected subcutaneously into the shaved right hind limbs of 20–23 g female C3H mice. Palpable tumors measuring an average of 7–8 mm in diameter were present 7–9 days later. Animals meeting this criterion for tumor size were randomized into the treatment groups shown in . All animal procedures and care were performed using protocols approved by the University of Minnesota Institutional Animal Care and Use Committee in accordance with federally approved guidelines.
Table I. Experimental groups for in vivo studies.
Anesthesia
The mice were anesthetized with an intraperitoneal injection of a mixture of 100 mg/kg ketamine and 10 mg/kg xylazine during all hyperthermia treatment and ultrasound imaging. When necessary a second dose of 20 mg/kg ketamine and 2 mg/kg xylazine was administered.
Hyperthermia
For hyperthermia treatment, anesthetized mice were immobilized on a specially designed Plexiglas jig and the tumor-bearing leg was vertically extended and anchored to an independent support on the jig Citation[24]. The jig was then placed on a Plexiglas shelf positioned over a water bath. Hyperthermic treatment was administered by immersing the anchored leg into a bath containing water preheated to 42.5°C or 43.5°C. The tumors were incubated in the water bath for 60 min. These animals were subsequently used to determine tumor blood flow or to gauge the anti-tumor response as described below.
Animal survival
Following treatments, animals were closely observed for redness, swelling, or inflammation on tumor-bearing leg, limpness, piloerection, lethargy, or death.
Blood flow studies by 86Rb uptake method
At specific time points after intravenous injections of drug, the blood perfusion in tumors was measured by the 86Rb uptake method Citation[24]. Mice were anesthetized and 5 µCi of 86RbCl diluted in 0.1 ml PBS, was injected through the lateral tail vein. The mice were sacrificed 60 sec later by cervical dislocation. The tumor and tail from each mouse was removed and weighed, and the 86Rb activity in a reference aliquot of 5 µCi and the tissues was counted in a well-type gamma counter (1282 Compugamma, Pharmacia LKB Wallac, Turku, Finland). By comparing the activity in the tumor to that in the total amount injected, the percentage uptake per gram of tumor tissue was calculated. Animals in which the tail activity (corrected for any volume lost due to missed injection) exceeded 5% of the total injected dose were excluded from the data analysis Citation[18].
Perfusion studies by contrast enhanced ultrasonography (CEUS)
To determine the longer term (i.e. days) anti-vascular effects of treatment, if any, tumor blood perfusion was imaged using CEUS. CEUS was performed on days 3 and 7 after treatment using a modified Technos MPX system from ESAOTE S.p.A. (Genoa, Italy). Before acquiring the in vivo power Doppler images, the contrasting agent, BR14 (2.5 µl/kg of body weight, Braco Research S.A., Geneva, Switzerland) was injected intravenously Citation[30]. Images were taken from a plane that bisected the center of the tumor. Data acquisition was conducted using a transmission pulse set to 1 cycle with a transducer operating at 8 MHz with an electrical focus of 25 mm. Each data set was collected at 1-sec intervals and a frame data rate of 9 frames/sec.
The quantification of 2-D CEUS image was performed using ‘color range’ and ‘histogram’ tools in Adobe Photoshop (Adobe Systems Inc. San Jose, CA). The ‘color range’ selects color subset within an entire image and the total number of pixels in a selection was obtained using ‘histogram’. Thus the perfused area was calculated as: Perfused area (%) = 100 × colored pixels/total pixels. The colored pixels in the CEUS are assumed to represent the perfused area. The total pixels include perfused area, tumor, skin and other surrounding tissue in the image.
Growth delay assay
Following the experimental treatments, the tumors were measured using metric-scale calipers (Scienceware, Pequannock, NJ), and the tumor volume was estimated with the formula a2b/2, where a and b are the shortest and the longest diameter of the tumor, respectively. The tumor size was measured until the mean volume for each group reached at least 6 times the mean volume on the day of treatment and/or more than 2 animals in a group were sacrificed due to tumor burden.
Statistics
Data sets were analyzed using a commercially available software package (InStat 2.03, Graphpad Software, San Diego, CA). A mean of measured values ± SE was calculated for all groups. One way ANOVA (analysis of variance) was used to determine the differences between control and treatment data sets. A p value ≤ 0.05 was considered significant.
Results
Tumor and endothelial cell survival in vitro
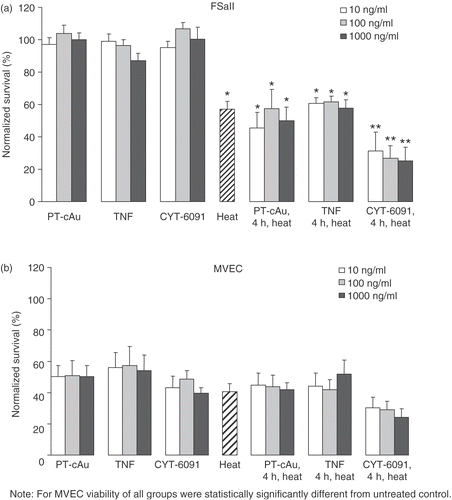
The effects of 10, 100 and 1000 ng/ml soluble TNF, CYT-6091 and the pegylated gold control, PT-cAu, on the thermosensitivity of tumor (FSaII) and endothelial (MVEC) cells are shown in . The number of colonies measured for each cell type following the designated treatments was normalized to the number of colonies of non-treated control cells. Exposing the tumor cells to PT-cAu, soluble TNF or CYT-6091 at the concentrations tested did not change the tumor cell clonogenicity. These same treatments reduced endothelial cell clonogenicity to 40–60% of control values. Hyperthermia reduced tumor and endothelial cell clonogenicity to 57% and 40% of control, respectively. Nevertheless, neither PT-cAu nor soluble TNF, regardless of dose, improved the hyperthermic killing of the cells in vitro since the number of colonies measured in these combinations treatments were not significantly different from those measured with heating alone. In contrast, CYT-6091 augmented the hyperthermic killing of both cell types in vitro since it reduced the number of viable colonies detected from heat alone by an additional 10–30%. This synergy was observed at all doses of CYT-6091 tested. However, in tumor cells the cell viability of the combined treatment group was statistically significantly different from the single treatment group, whereas in endothelial cells there was no statistically significant difference between the combined and single treatment groups.
Figure 1. (a) murine fibrosarcoma cells (FSaII) and microvascular endothelial cells (MVEC) assessed by clonogenic cell survival, assay. *Indicates statistically significant difference from untreated control. **Indicates statistically significant difference from untreated control and heat alone. Cells were treated for 4 hours with 10–1000 ng/ml of soluble TNF or CYT-6091, or PT-cAu, before heating for 60 minutes at 42.5°C. Viability is expressed as mean ± SEM obtained from 5–6 different experiments and normalized to untreated control.
Animal survival
The toxicity of systemically administered TNF in both animals and man is well documented Citation[15], Citation[31]. Our data show that a single intravenous injection of soluble TNF, at a dose of 250 µg/kg caused 40% of the mice to die. In addition, when combined with hyperthermia, this treatment resulted in a 100% mortality rate. In contrast, mice receiving CYT-6091 treatment, at the same dose of TNF, either alone or in combination with hyperthermia, showed no signs of toxicity and survived to scheduled sacrifice.
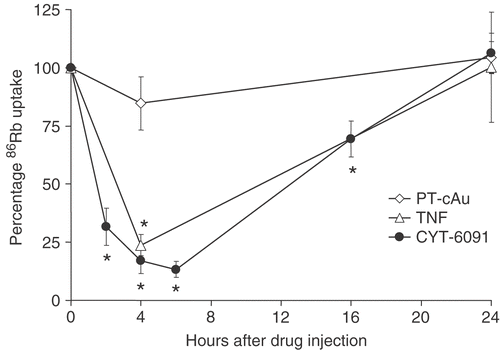
Tumor blood flow
The effects of soluble TNF, CYT-6091 and PT-cAu alone without hyperthermia on the blood flow as measured by 86Rb uptake in tumors are shown in . 86Rb uptake decreased over time and within 4 h of injection both soluble TNF and CYT-6091 reduced tumor blood flow by an average of 80%, at nadir, when compared to untreated controls. The blood flow then recovered considerably but was still reduced by 30% compared to untreated controls at 16 h. Within 24 h, blood flow within the tumor returned to control levels. A slight drop in 86Rb uptake observed 4 h post PT-cAu administration was not statistically significant from untreated control.
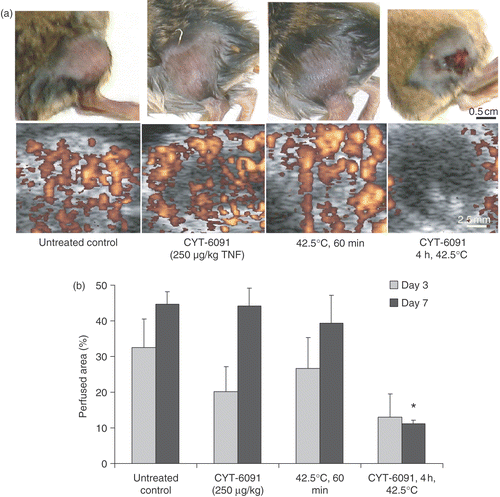
Tumor perfusion imaging
The long-term effects of CYT-6091 with or without hyperthermia on tumor perfusion as measured by the CEUS are shown in . The tumor perfusion 3 days post either treatment was reduced though not significantly different from control (). However, in CYT-6091 and hyperthermia groups, the perfusion recovered by day 7, whereas the perfusion defects persisted in the combination group. The CEUS data show that the initial ischemic response led to permanent perfusion defects in tumors treated with CYT-6091 + hyperthermia. These data also support a strong anti-vascular synergy between CYT-6091 and hyperthermic treatment, since, as shown in , the effect of CYT-6091 with hyperthermia on tumor blood flow was greater than the sum of the two individual treatments.
Figure 3. (a) Representative images of murine fibrosarcoma bearing hindlimb of C3H mice and the corresponding tumor perfusion defects imaged using contrast enhanced ultrasonography (bottom), 7 days post treatments. (b) Perfused murine fibrosarcoma tumor area obtained by quantification of contrast enhanced ultrasonographs, imaged 3 and 7 days post treatments. Results are expressed as mean ± SEM of 3 animals. *Indicates statistically significant difference from untreated controls.
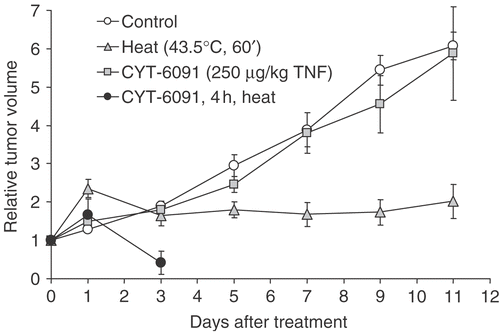
Tumor growth delay
The synergy between CYT-6091 and 43.5°C hyperthermic treatments was readily evident when gauging tumor regression in this model. As shown in , CYT-6091 had little to no effect on the growth of tumors in vivo. Although direct hyperthermic treatment of these tumors resulted in significantly blunted tumor growth, tumor regression was not observed and all tumors began to regrow by day 14. Combining CYT-6091 with hyperthermia markedly damaged the tumor since in all animals no palpable tumors were present after day three. However, there was also severe skin damage and sloughing of the tumor tissue in all animals on the third day post CYT-6091 + heat (43.5°C) treatment, and these animals were therefore sacrificed. In further studies, we used a milder heating (at 42.5°C for 60 min) combined with lower CYT-6091 doses (50, 125 and 250 µg/kg TNF).
Figure 4. Murine fibrosarcoma tumor volume following hyperthermia and/or CYT-6091 treatments. Results are expressed as mean ± SEM of 5–6 animals and normalized to pretreatment volumes.
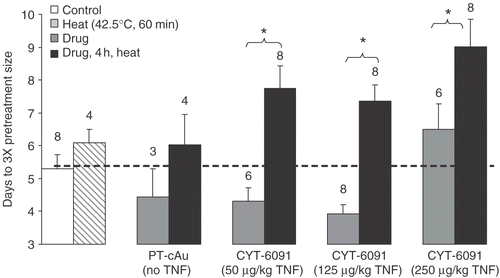
The delay in days for tumors treated at milder temperature with lower CYT-6091 doses to grow 3-fold their pretreatment volume is shown in . Hyperthermia or 250 µg/kg CYT-6091 produced a mild effect delaying the tumor growth by a day. PT-cAu and CYT-6091 (50 or 125 µg/kg) alone appeared to stimulate the tumor growth though it was not found to be statistically significantly different than control. In contrast, the combination of CYT-6091 and hyperthermia produced synergistic growth delay at all doses of CYT-6091 with milder hyperthermia.
Figure 5. Days (mean ± SEM) for murine fibrosarcoma tumors to grow 3-fold their pretreatment volumes following various treatments shown. The numbers on the plot represent number of animals (N) in each group. In addition to the statistics shown, the results of the combination groups were found to be statistically significantly different from untreated control.
Discussion
The routine use of hyperthermia in cancer treatment has been hindered by the inability to achieve complete destruction of the solid tumor mass. In effect, the vascular (and other) mediated dissipation of heat throughout the tumor establishes temperature gradients which allow residual tumor and supporting cells to survive and in turn minimize treatment efficacy. Current efforts to address this limitation have either focused on using higher temperatures or combining hyperthermia with other conventional therapies such as chemotherapy and/or radiation therapy Citation[32], Citation[33]. Many of the chemotherapeutic combinations seek to mechanistically attack the tumor at the level of the vasculature thereby lowering the thermal thresholds of injury and at times also achieving synergistic destructio Citation[6], Citation[34]. Previous work from our group and others has shown a potent synergistic effect from the use of the inflammatory cytokine TNF in vitro, in vivo and in clinical use Citation[6], Citation[17–19]. Unfortunately, systemic administration of TNF at doses required for notable anti-tumor activity has dose-limiting toxicity Citation[12], Citation[13], Citation[20], Citation[24].
To avoid toxicity, TNF is currently being administered by a variety of techniques including isolated limb perfusion technique (ILP), liposomal delivery, and gene therapy. In ILP, the vasculature of a tumor-bearing limb is temporarily isolated and a high dose of TNF (3–4 mg) is administered into the perfusion circuit of the limb Citation[21]. This limits the biodistribution of TNF to the limb without exposing the entire body to the drug. However, ILP treatments are limited due to relatively few tumor occurrences in the limb, surgical complexities, cost and potential morbidity Citation[35]. Delivery of TNF using a liposomal delivery system has shown some promise in animal cancer models but requires very high doses of the drug Citation[36]. Gene therapy with TNF has also been explored but the limitations are lack of selective transduction of tumor cells to minimize toxicity and inefficient gene transfer to every cell Citation[37], Citation[38].
Our approach to limit the toxicity of TNF was to target its delivery to solid tumors using the nanotherapeutic, CYT-6091. CYT-6091 selectively delivers TNF to solid tumors by avoiding uptake in the reticuloendothelial system and using passive (i.e. the enhanced permeation and retention phenomenon) and active (TNF receptor) tumor targeting to sequester TNF in solid tumors Citation[23]. The typically high permeability and abnormal morphology (incomplete lining) of tumor blood vessels likely facilitate uptake and retention of CYT-6091 in the tumor interstitium Citation[16], Citation[39]. In addition, CYT-6091 is suggested to cause further TNF-induced vascular leak and reduction of interstitial pressure (i.e. local hypotension). In effect, by delivering TNF to solid tumors, CYT-6091 improves the therapeutic index of TNF against solid tumors Citation[23]. In the present study, we confirmed that CYT-6091 improves the safety of TNF administration and synergizes with hyperthermia to induce complete responses in FSaII-tumor bearing C3H mice. In contrast, in mice treated with soluble TNF, alone or in combination with hyperthermia, there was a 40% or 100% mortality rate, respectively.
We have previously observed the role of CYT-6091 in enhancement of hyperthermic injury in a murine mammary carcinoma model (SCK) Citation[24] and cryothermic injury in a human prostate cancer model (LNCaP) Citation[26]. In the present study, the goal was to extend our knowledge of this enhancement by further study of the toxicity, timing, dosage and heating regimes of CYT-6091 enhancement of thermal therapy in FSaII murine fibrosarcoma model Citation[40]. Based on our previous results, we began testing a TNF dose of 250 µg/kg. Intravenous injection of TNF in the free or bound form reduced the tumor blood flow drastically at 2–6 h after injection (). The rationale for choosing a time point when blood flow is low is that the heat dissipation by tumor vasculature would be expected to be low, increasing heat-induced damage. The blood flow at 6 h was lower than at 4 h, but there was no statistically significant difference between the two. Therefore we chose 4 h as the time interval between TNF treatment and hyperthermia to keep the time interval as small as possible; an advantage for clinical applications. Also, Paciotti et al. Citation[23] observed that intravenous injection of CYT-6091 in MC-38 tumor-burdened C57/BL6 mice elevated intratumor TNF concentration from a baseline value of 0.8 ng TNF/mg protein at 5 min to 6–7 ng TNF/mg protein at 4 h. Therefore to remain consistent with the previous results we chose 4 h.
With a 4-h time interval between CYT-6091 and hyperthermia at 43.5°C for 1 h the tumors were dramatically reduced in 3 days after treatment (). The hyperthermia temperature of 43.5°C was chosen because the conventional treatment goal in adjunctive synergistic hyperthermia is to achieve a temperature of 43°C for 1 h everywhere in the tumo Citation[41], Citation[42]. Unfortunately, the combined treatment with 43.5°C commonly led to the tumor sloughing off the rear leg, which warranted early sacrifice of these animals. We subsequently lowered the heating temperature to 42.5°C and investigated three different TNF doses–50, 125 and 250 µg/kg. With these thermal and TNF doses growth delay was noted, but no qualitative normal tissue damage was apparent and the tumor did not slough. In general, if the combined treatment had caused any form of irreversible skin damage in the area adjacent to the tumor it would have led to ulceration, cessation of blood flow to the foot or limping within few days. Paciotti et al. Citation[23] and Farma et al. Citation[43] have also demonstrated that the vascular effects of CYT-6091 were restricted to the tumor neovasculature and not normal tissue. The TNF dose of 250 µg/kg delayed tumor growth whereas the other two doses may have caused a transient stimulation in growth as can be seen in (day 1) and . This phenomenon has been noted in other TNF related studies Citation[44]. TNF is known to be both a necrosis factor and a growth promoting factor for tumors. Particularly at low doses, TNF can contribute to the tissue remodeling and stromal development necessary for growth and spread of the tumor Citation[45].
It should be pointed out that CEUS yields local data dependent on the plane through which the tumor is bisected by the US wave, whereas 86Rb uptake method gives a quantitative assessment of blood flow in the entire tumor. With the CEUS technique, blood perfusion only in vessels with diameter ≥200 µm is likely to be clearly visible in tumors. This conclusion is based on our ability to measure 200 µm cellulose channels with a LA 522 high-frequency probe in vitro (data not shown). Thus, CEUS is considered a local measure of tumor vascular physiology, whereas 86Rb uptake method gives bulk assessment of tumor blood flow. However, in situations where there is significant edema or tissue ablation, the radioisotope method may be inaccurate because it is based on tissue weight. Therefore we did not use the radioisotope uptake method at extended time points and instead developed the CEUS method to assess the permanent anti-vascular effects of treatment.
The combination of CYT-6091 and hyperthermia attacks the tumor vasculature () thereby inducing a synergistic tumor growth delay in appropriate dosage and heating regimes as shown (). This synergistic effect observed in vivo was also apparent in primary cultures of murine fibrosarcoma tumor corroborating the role of CYT-6091 in enhancing direct cellular hyperthermic injury. All single agent treatments (i.e., those without heat) reduced the viability of MVEC cells equally, suggesting that the true in vivo effects of endothelium exposed to TNF are mediated by multiple other factors such as the inflammatory cascade, subsequent microcirculatory stasis and other possible events in vascular damage and breakdown.
In summary, a synergistic thermal enhancement with CYT-6091 was observed in FSaII tumor model similar to that previously observed in SCK tumor model Citation[24] even though the two murine models differ in vascularity and growth kinetics Citation[27], Citation[28]. The SCK tumor is a hyper-vascular, aggressive and metastatic line while FSaII grows more slowly (5–7 days to 1 cm2 for SCK compared to 10–12 days for FSaII), and does not metastasize to a lethal extent for months after inoculation. Historically, we have observed that SCK is more sensitive to heat and more responsive in general to vessel-targeting agents as compared to FSaII tumo Citation[27], Citation[28]. This can also be seen in our blood flow results with Rb uptake assays. In FSaII tumor, the blood flow recovered completely within 24 hours of CYT-6091 administration (), whereas in SCK the blood flow was still 60% of controls at 24 hours Citation[24]. A similar trend was observed in the growth delay; for example, the days for FSaII vs. SCK tumors to enlarge by 3-fold of the pretreatment volume were: heat (42.5°C, 60 min) = 6 vs. 7.5 days, 125 µg/kg CYT-6091 + heat = 7 vs. 8 days and 250 µg/kg CYT-6091 + heat = 9 vs. 11 days respectively Citation[24]. Thus, growth of SCK tumors was delayed more than FSaII tumors with same treatments, even though untreated SCK tumors grow faster than FSaII. Interestingly, these trends do not occur in vitro, suggesting that the tumor microenvironment and differences in vascular and stromal composition in vivo are the reasons that the tumors respond differently to various treatments. For example, the in vitro clonogenic cell survival post CYT-6091 + heat was 25–30% in both FSaII and SCK cells Citation[24].
We are currently evaluating the effect of the combination treatment on normal tissues in murine tumor models. Also, the biodistribution of gold nanoparticles and TNF in various tissues is presently being studied in our lab using atomic emission spectrometry and ELISA methods, respectively. In the future, the tumor blood flow (vs. normal tissue) at different time points following the combined treatment will be quantified using the Rb uptake method. Investigating the treatment efficacy at higher doses of CYT-6091 and lower hyperthermia temperatures is suggested by our results to date.
Conclusion
As hypothesized, non-toxic synergistic enhancement of FSaII tumor thermal therapy was achieved. The optimal conditions were: 250 µg/kg CYT-6091 followed with hyperthermia (42.5°C, 60 min) 4 h later. In view of these results, nanoparticle-attached TNF appears to have potential as a clinical agent owing to the possibility that much of the previous clinical problems with systemic TNF cytotoxicity might be avoided. Achieving hyperthermic injury at normally sublethal temperatures could improve the effectiveness of a variety of minimally-invasive ablative thermal therapy techniques already in clinical use or in development as well as traditional hyperthermia in combination with radiotherapy. Our results support continued evaluation of the combination of CYT-6091 with hyperthermia in a translational cancer model.
Acknowledgements
The authors thank Hui Yao for CEUS images, and Esaote, S.p.A., Italy for Ultrasound system and contrast agent. The work was funded by: U.S. Army Department of Defense; Grant Numbers: DAMD 17-03-1-0432 and DAMD 17-01-1-33, National Cancer Institute; Grant Number: CA44114 and Biomedical Engineering Institute, University of Minnesota.
References
- Song CW, Park HJ, Lee CK, Griffin R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia 2005; 21(8)761–767
- Huilgol NG. A phase I study to study arsenic trioxide with radiation and hyperthermia in advanced head and neck cancer. Int J Hyperthermia 2006; 22(5)391–397
- Morita M, Kuwano H, Matsuda H, Mori M, Sugimachi K. Increased hyperthermic response with prostaglandin E1 in VX2 liver carcinoma in rabbits. J Natl Cancer Inst 1991; 83(19)1395–1400
- Shenoy MA, Singh BB. Studies on indomethacin as a potentiator for hyperthermic and radiation responses in a mouse fibrosarcoma. Cancer Lett 1989; 45(3)227–232
- Song CW, Lin JC, Lyons JC. Antitumor effect of interleukin 1 alpha in combination with hyperthermia. Cancer Res 1993; 53(2)324–328
- Watanabe N, Niitsu Y, Umeno H, Sone H, Neda H, Yamauchi N, Maeda M, Urushizaki I. Synergistic cytotoxic and antitumor effects of recombinant human tumor necrosis factor and hyperthermia. Cancer Res 1988; 48(3)650–653
- Murata R, Overgaard J, Horsman MR. Combretastatin A-4 disodium phosphate: A vascular targeting agent that improves that improves the anti-tumor effects of hyperthermia, radiation, and mild thermoradiotherapy. Int J Radiat Oncol Biol Phys 2001; 51(4)1018–1024
- Murata R, Horsman MR. Tumour-specific enhancement of thermoradiotherapy at mild temperatures by the vascular targeting agent 5,6-dimethylxanthenone-4-acetic acid. Int J Hyperthermia 2004; 20(4)393–404
- Horsman MR, Murata R, Overgaard J. Improving local tumor control by combining vascular targeting drugs, mild hyperthermia and radiation. Acta Oncol 2001; 40(4)497–503
- Thorpe PE. Vascular targeting agents as cancer therapeutics. Clin Cancer Res 2004; 10(2)415–427
- Dong D, Ko B, Baumeister P, Swenson S, Costa F, Markland F, Stiles C, Patterson JB, Bates SE, Lee AS. Vascular targeting and antiangiogenesis agents induce drug resistance effector GRP78 within the tumor microenvironment. Cancer Res 2005; 65(13)5785–5791
- Watanabe N, Niitsu Y, Umeno H, Kuriyama H, Neda H, Yamauchi N, Maeda M, Urushizaki I. Toxic effect of tumor necrosis factor on tumor vasculature in mice. Cancer Res 1988; 48(8)2179–2183
- Waterston A, Bower M. TNF and cancer: Good or bad?. Cancer Therapy 2004; 2: 131–148
- Alikhani M, Alikhani Z, Raptis M, Graves DT. TNF-alpha in vivo stimulates apoptosis in fibroblasts through caspase-8 activation and modulates the expression of pro-apoptotic genes. J Cell Physiol 2004; 201(3)341–348
- Havell EA, Fiers W, North RJ. The antitumor function of tumor necrosis factor (TNF), I. Therapeutic action of TNF against an established murine sarcoma is indirect, immunologically dependent, and limited by severe toxicity. J Exp Med 1988; 167(3)1067–1085
- van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: Molecular insights, antitumor effects, and clinical utility. Oncologist 2006; 11(4)397–408
- Han B, Iftekhar A, Bischof JC. Improved cryosurgery by use of thermophysical and inflammatory adjuvants. Technol Cancer Res Treat 2004; 3(2)103–111
- Lin JC, Park HJ, Song CW. Combined treatment of IL-1 alpha and TNF-alpha potentiates the antitumour effect of hyperthermia. Int J Hyperthermia 1996; 12(3)335–344
- Maeda M, Watanabe N, Yamauchi N, Tsuji Y, Niitsu Y. Successful treatment of a case of hepatocellular carcinoma with tumor necrosis factor and local hyperthermia. Gastroenterol Jpn 1991; 26(6)774–778
- Mocellin S, Rossi CR, Pilati P, Nitti D. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev 2005; 16(1)35–53
- Eggermont AM, de Wilt JH, ten Hagen TL. Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol 2003; 4(7)429–437
- http://clinicalstudies.info.nih.gov/cgi/detail.cgi, ?A_2006-C-0167.html
- Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. Colloidal gold: A novel nanoparticle vector for tumor directed drug delivery. Drug Deliv 2004; 11(3)169–183
- Visaria RK, Griffin RJ, Williams BW, Ebbini ES, Paciotti GF, Song CW. Enhancement of tumor thermal therapy using gold nanoparticle-assisted tumor necrosis factor-alpha delivery. Mol Cancer Ther 2006; 5(4)1014–1020
- Chao BH, He X, Bischof JC. Pre-treatment inflammation induced by TNF-alpha augments cryosurgical injury on human prostate cancer. Cryobiology 2004; 49(1)10–27
- Goel R, Swanlund D, Coad J, Paciotti GF, Bischof JC. TNF-α based Accentuation in Cryoinjury–Dose, Delivery and Response. Molecular Cancer Therapeutics 2007; 6(7)2039–2047
- Griffin RJ, Okajima K, Ogawa A, Song CW. Radiosensitization of two murine tumours with mild temperature hyperthermia and carbogen breathing. Int J Radiat Biol 1999; 75(10)1299–1306
- Griffin RJ, Williams BW, Wild R, Cherrington JM, Park H, Song CW. Simultaneous inhibition of the receptor kinase activity of vascular endothelial, fibroblast, and platelet-derived growth factors suppresses tumor growth and enhances tumor radiation response. Cancer Res 2002; 62(6)1702–1706
- Davison PM, Bensch K, Karasek MA. Isolation and growth of endothelial cells from the microvessels of the newborn human foreskin in cell culture. J Invest Dermatol 1980; 75(4)316–321
- Basilico R, Blomley MJ, Cosgrove DO, Liull JB, Broillet A, Bauer A, Bonomo L. The first phase I study of a novel ultrasound contrast agent (BR14): Assessment of safety and efficacy in liver and kidneys. Acad Radiol 2002; 9(Suppl 2)S380–S381
- Selby P, Hobbs S, Viner C, Jackson E, Jones A, Newell D, Calvert AH, McElwain T, Fearon K, Humphreys J, et al. Tumour necrosis factor in man: Clinical and biological observations. Br J Cancer 1987; 56(6)803–808
- Feyerabend T, Steeves R, Wiedemann GJ, Richter E, Robins HI. Rationale and clinical status of local hyperthermia, radiation, and chemotherapy in locally advanced malignancies. Anticancer Res 1997; 17(4B)2895–2897
- Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia 2005; 21(8)779–790
- Horsman MR, Siemann DW. Pathophysiologic effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Res 2006; 66(24)11520–11539
- Thompson JF, de Wilt JH. Isolated limb perfusion in the management of patients with recurrent limb melanoma: An important but limited role. Ann Surg Oncol 2001; 8(7)564–565
- van der Veen AH, Eggermont AM, Seynhaeve AL, van T, ten Hagen TL. Biodistribution and tumor localization of stealth liposomal tumor necrosis factor-alpha in soft tissue sarcoma bearing rats. Int J Cancer 1998; 77(6)901–906
- Li CY, Dewhirst MW. Hyperthermia-regulated immunogene therapy. Int J Hyperthermia 2002; 18(6)586–596
- Senzer N, Mani S, Rosemurgy A, Nemunaitis J, Cunningham C, Guha C, Bayol N, Gillen M, Chu K, Rasmussen C, Rasmussen H, Kufe D, Weichselbaum R, Hanna N. TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene: A phase I study in patients with solid tumors. J Clin Oncol 2004; 22(4)592–601
- Jang SH, Wientjes MG, Lu D, Au JL. Drug delivery and transport to solid tumors. Pharm Res 2003; 20(9)1337–1350
- Urano M, Kahn J, Kenton LA. The effect of cis-diamminedichloroplatinum(II) treatment at elevated temperatures on murine fibrosarcoma, FSa-II. Int J Hyperthermia 1990; 6(3)563–570
- Roemer RB. Engineering aspects of hyperthermia therapy. Annu Rev Biomed Eng 1999; 1: 347–376
- Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, Bentzen SM. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European Society for Hyperthermic Oncology. Int J Hyperthermia 1996; 12(1)3–20
- Farma JM, Puhlmann M, Soriano PA, Cox D, Paciotti GF, Tamarkin L, Alexander HR. Direct evidence for rapid and selective induction of tumor neovascular permeability by tumor necrosis factor and a novel derivative, colloidal gold bound tumor necrosis factor. Int J Cancer 2007; 120(11)2474–2480
- Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-alpha as a tumour promoter. Eur J Cancer 2006; 42(6)745–750
- Balkwill F. Tumor necrosis factor or tumor promoting factor?. Cytokine Growth Factor Rev 2002; 13(2)135–141