Abstract
Background: Gastric carcinoma patients with peritoneal dissemination have an extremely poor prognosis. Attempting to improve regional control and decrease the risk of complications related to hyperthermic chemotherapy, we applied a new treatment modality using a combination of gastrectomy with postoperative intraperitoneal hyperthermo-chemotherapy (PIHC) using Thermotron RF-8. The purpose of this study was to evaluate the feasibility of PIHC in advanced gastric carcinoma patients with peritoneal seeding.
Patients and methods: Between March 2002 and April 2006, 20 gastric carcinoma patients with peritoneal dissemination were allocated to two groups in the patient's selection. The PIHC group (10 patients) received a 60-min PIHC with a cisplatin dose of 80 mg/m2 two weeks after surgery, and the control group (10 patients) received surgery alone. Thermotron RF-8 is a heating device that can raise temperatures in both superficial and deep-seated tumours using 8 MHz radiofrequency electromagnetic waves as a source of heat.
Results: No patients in either group had life-threatening complications. The most frequent nonhaematologic toxicity (grade 3) was nausea. The one-, two-, and three-year cumulative survival rates for the PIHC group were 60%, 48%, and 36%, respectively, whereas those for the control group were 40%, 10%, and 0%, respectively. The survival rates for the PIHC group were significantly higher than those for the control group.
Conclusion: Although this study was conducted non-randomly with a small number of patients, the PIHC group had a higher survival rate and better prognosis compared with the control group.
Introduction
Gastric carcinoma patients with peritoneal dissemination have an extremely poor prognosis. Clinically, these patients show short-term survival independent of traditional treatment. Even when cytoreduction is macroscopically complete, it is hardly ever successful because microscopic residues together with malignant cell spillage cause tumour recurrence. According to some cytologic reports, gastric carcinoma cells were detected at the time of laparotomy in the abdominal cavity even in patients who were clearly shown macroscopically to have no peritoneal metastases Citation[1], Citation[2]. Thus, it is important to extirpate peritoneal metastases and intraperitoneal free cancer cells in the abdominal cavity to improve the clinical outcome of advanced gastric cancer.
Intraperitoneal chemotherapy offers potential therapeutic advantages over systemic chemotherapy by generating a high local concentration of a drug. Pharmacological studies have shown that drug concentration in the peritoneal cavity following intraperitoneal administration is five to ten times higher than the systemic concentration Citation[3]. However, prophylactic intraperitoneal administration of anticancer drugs alone has not decreased the frequency of peritoneal recurrence after surgery Citation[4].
Many articles have been published demonstrating the remarkable effects of heat on tumour control when used in combination with radiation or some anticancer drugs. Hyperthermia is a promising treatment method for tumour control because of two different biological effects–direct hyperthermic cytotoxicity and a modulator of cisplatin cytotoxicity Citation[5], Citation[6]. Hyperthermic perfusion of the peritoneal cavity with cytoreduction surgery has been performed in the prophylaxis and treatment of peritoneal dissemination from gastric cancer Citation[7–11]. A randomized clinical trial showed that intraperitoneal heated chemotherapy had a prophylactic benefit against peritoneal recurrence of gastric cancer Citation[9]. However, cytoreduction with intraperitoneal hyperthermic chemoperfusion is known to carry a considerable risk of serious complications and related death Citation[12]. Attempting to improve regional control and decrease the risk of complications related to hyperthermic chemotherapy, we applied a new treatment modality using a combination of gastrectomy with postoperative intraperitoneal hyperthermic chemotherapy (PIHC) using Thermotron RF-8. Thermotron RF-8 is a heating device that can raise temperatures in both superficial and deep-seated tumours using 8 MHz radiofrequency electromagnetic waves as a source of h Citation[13], Citation[14].
The purpose of this study was to evaluate the feasibility of postoperative intraperitoneal hyperthermo-chemotherapy (PIHC) combined with surgery as a potential cure for peritoneal carcinomatosis and to determine whether PIHC helps reduce the mortality rate from gastric carcinoma. We report here data on ten advanced gastric cancer patients with peritoneal dissemination given PIHC.
Patients and methods
Patients
From March 2002 to April 2006, 20 patients with peritoneal seeding, who had undergone cytoreduction surgery for gastric cancer, were chosen for this study. This study was not a randomized controlled study. The patients who agreed to have this hyperthermic therapy constituted the PIHC group, while those who refused this therapy constituted the control group. Twenty patients were candidates at the time of surgery. Ten patients agreed to PIHC, the other patients refused PIHC. The distribution of clinical characteristics in these patients is shown in .
Table I. Clinicopathologic features of patients.
Inclusion criteria were (1) age younger than 70 years, (2) peritoneal seeding of gastric carcinoma proven by biopsy of abdominal granulation or cytology with peritoneal lavage during laparotomy, (3) abdominal granulations (peritoneal seeding) less than 5 mm in greatest diameter (Lyon staging system; stage 1) Citation[15], (4) no signs of distant metastases, (5) not many scattered metastases to the distant peritoneum, (6) no presence of ascites, (7) a technically resectable primary tumour, (8) satisfactory cardiorespiratory status, and (9) no peritoneal dissemination on preoperative explorations. The exclusion criteria were (1) abdominal granulations (peritoneal seeding) more than 5 mm in greatest diameter (Lyon staging system; stage 2 or 3 or 4), (2) numerous metastases to the distant peritoneum, (3) the presence of ascites, (4) renal or myocardial failure, and (5) administration of systemic chemotherapy less than one month prior to inclusion. Before inclusion in the protocol, all patients underwent a preoperative clinical and biologic examination, cardiac ultrasound, abdominal and chest computed tomography, and biologic renal and haemostasis exploration. The protocol was approved by the local ethics committee and written informed consent was obtained from all patients after explaining surgical procedures, postoperative complication, quality of life after gastrectomy and the investigational nature of the study.
Cytoreductive surgery
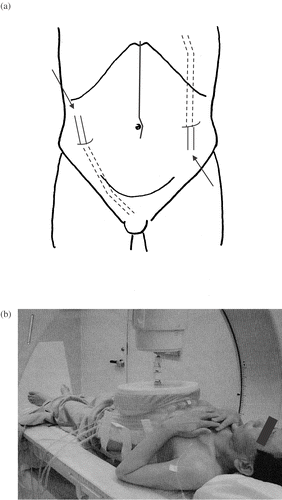
A midline abdominal incision from xiphoid to pubis was used. The entire peritoneal surface was inspected and palpated. Cytoreductive surgery consisted of radical resection of the stomach and all gross tumours with involved organs, peritoneum, or tissue that was deemed technically feasible and safe for the patients. Nodules less than 2 mm in diameter were fulgurated by electrocoagulation. The aim of cytoreductive surgery was to remove all macroscopic tumours, but peritonectomy as described by Sugarbaker was not carried out in this study Citation[16]. After cytoreductive surgery was completed, infusion catheters (22F silicone drainage) were placed percutaneously. Two infusion catheters were placed beneath the hemidiaphragma and in the Douglas pouch for infusion and drainage ().
Figure 1. (a) Two infusion catheters that were placed beneath the hemidiaphragma and in the Douglas pouch for infusion and drainage. (b) Thermotron RF-8, 8 MHz radiofrequency (RF) capacitive heating equipment. In general, two opposing 25 cm diameter electrodes were selected for heating. To prevent surface burning, a cooling pad was used during hyperthermia through which 0.9% saline cooled to 5°C was circulated.
Hyperthermia
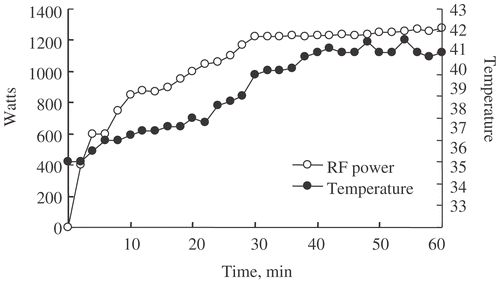
Two weeks after the gastrectomy, hyperthermia was started using 8-MHz radiofrequency capacitive heating equipment (Thermotron RF-8, Yamamoto Vinyter, Osaka, Japan) () Citation[17]. Pairs of electrodes with a cooling bolus were placed on the front and back of the patient's abdominal wall to load 8-MHz radiofrequency waves for each session. The power level which could be tolerated by the patients was generally between 800–1300 W. To prevent surface burning, a cooling pad was used during hyperthermia through which 0.9% saline cooled to 5°C was circulated. The temperature in the abdominal cavity was measured by microthermocouple through the infusion tube when possible. Influx passed into the abdominal cavity through the catheter. One litre of physiologic saline containing a dose of 80 mg/m2 cisplatin warmed to 37°C in a dry incubator was instilled as rapidly as possible into the peritoneal cavity via a catheter. The catheter was clamped for 6 hours after PIHC. A minimum temperature in the abdominal cavity of more than 40°C was attempted, and it was maintained for 60 minutes (). During the PIHC period, resuscitation with a sufficient quantity of intravenous fluid to avoid intravenous dehydration and 800 mg of cimetidine to minimize the renal tubule injury by cisplatin were administered to each patient. The infusion fluid was drained from the abdominal cavity after PIHC. All complications relating to surgery and PIHC were recorded. The same treatment was repeated every 2 weeks to a maximum of 4 cycles. If the patients refused further PIHC or unacceptable toxicity occurred, including intractable catheter-site infection, the PIHC procedure was stopped.
Follow-up and endpoint
All patients were seen at the outpatient clinic once a month for 2 years, and every 3 months thereafter. The follow-up consisted of physical examination and serum CEA and CA 19–9 every 3 months and an abdominal CT scan every 6 months. All patients received systemic chemotherapy with 5-FU (TS-1; 80 mg/m2/day, Taiho Pharmaceutical Co., Tokyo, Japan) at the outpatient clinic for 1 year 4 weeks after surgery. The follow-up period was defined as the time from the day of operation. The progression-free survival was the interval from the first day of operation to the time of progression.
Statistical analysis
Data were collected and analysed on a commercially available computer program (Statview 4.5, Abacus Inc., Berkeley, CA) and are expressed as mean, SE, median, and range. Statistical analysis was performed by the Mann-Whitney U test, the chi-square test, or the unpaired Student's t-test as appropriate. P values less than 0.05 were considered to be significant. Survival data for 20 patients were analysed by means of the Kaplan-Meier method, and the log rank test was used for the assessment of the differences between prognostic factors.
Results
Clinicopathologic characteristics of patients
Peritoneal carcinomatosis was diagnosed at laparotomy when it was found during treatment of the primary tumour. All patients who agreed to PIHC received the treatment. There was no difference between the PIHC group and control group in the performance status, the extent of gastric wall invasion, lymph node involvement, and histology of the primary tumour (). All patients had malignant granulations or intraperitoneal free cancer cells detected at the time of the laparotomy in both groups. Just after the laparotomy, intraperitoneal free cancer cells in the irrigated solution were detected in six of 10 patients in the PIHC group and in eight of 10 patients in the control group by the intraperitoneal cytologic examination. Both positive cytology and malignant granulations were detected in four of 10 patients in the PIHC group and six of 10 patients in the control group. One patient underwent distal gastrectomy and nine underwent total gastrectomy in the PIHC group. Two patients underwent distal gastrectomy and eight underwent total gastrectomy in the control group. All resection margins were free of carcinoma cells.
Adverse effects of PIHC
There were 18 hyperthermic treatments in 10 patients, with a median of 1.5 treatments per patient. Two patients refused further PIHC. The maximum number of treatments was four, and the minimum was one. No patients in either group had life-threatening complications, such as intestinal perforation or massive bleeding. In the PIHC group, transient leucopenia (National Cancer Institute-Common Toxicity Criteria, Grade 2) occurred in 30.0% of patients, 40.0% experienced prolonged drainage of fluid from the peritoneal cavity through drains, and 70.0% had appetite loss (National Cancer Institute-Common Toxicity Criteria, Grade 3). Most of these complications after PIHC could be managed conservatively without any significant sequelae.
Survival
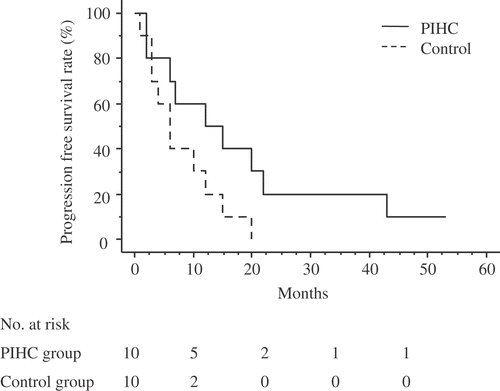
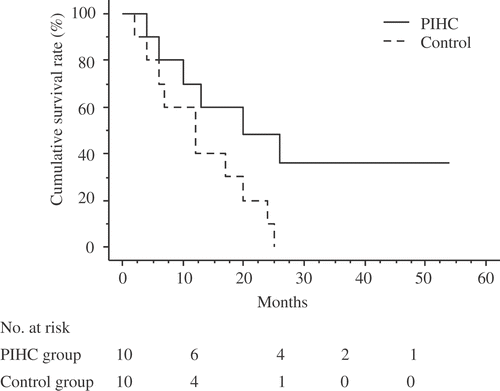
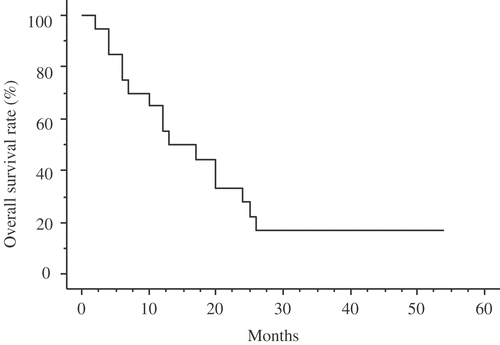
The median follow-up for the entire study group was 14.5 months, and their overall 1-year, 2-year, 3-year and 4-year survival rates were 55%, 27.8%, 16.7% and 16.7%, respectively. The Kaplan-Meier survival distribution is shown in . Among the 20 patients, progressive disease was confirmed in 19 patients (9 in the PIHC group, 10 in the control group), and 16 of these patients died during the course of this study. The progression-free 1-, 2-, and 3 year survival rates for the PIHC group were 50%, 20%, and 10%, respectively (). The progression-free 1- and 2-year survival rates for the control group were 20% and 0%, respectively (). The survival rates for the PIHC and control groups are shown in . One-, two-, and three-year cumulative survival rates for the PIHC group were 60%, 48%, and 36%, respectively, whereas those for the control group were 40%, 10%, and 0%, respectively. The survival rates for the PIHC group were significantly higher than those for the control group, with a statistical difference of P = 0.044 by the Kaplan-Meier method and the log rank test (). The median survival for patients in the PIHC group and in the control group was 18 and 12 months, respectively, after gastrectomy. There are three cases that lived for 30 months or more after PIHC. Two patients received 1 treatment and one patient received 2 treatments. There is not a significant difference between the number of treatments and their effects. The most frequent pattern of recurrence was peritoneal metastasis in both these groups of patients. The PIHC did not influence the pattern of recurrence.
Figure 3. Cumulative survival of gastric cancer patients in this study (the relationship between survival months and cumulative survival rate using the Kaplan-Meyer method). Survival was computed from the initial surgery performed at our institution.
Discussion
The diagnosis of peritoneal dissemination due to gastric cancer has a dismal prognosis. There is no standard treatment for patients with peritoneal dissemination. Palliative treatments to remove the primary lesion of the peritoneal carcinomatosis have always resulted in rapidly recurring tumour growth within the abdominal cavity. Effective complementary treatment is therefore required to eliminate minimal tumour residue. However, systemic chemotherapy is a poor treatment for peritoneal metastasis, although a small advantage has been reported for gastric cancer patients with carcinomatosis who are undergoing systemic chemotherapy Citation[18]. There have been some encouraging reports published regarding the efficacy of cytoreduction surgery and intraperitoneal hyperthermic chemotherapy in the treatment of peritoneal dissemination due to gastric cancer Citation[3], Citation[7–9], Citation[11]. Some reports have shown that cytoreduction surgery and intraperitoneal hyperthermic chemotherapy can and do produce long-term survivors among gastric cancer patients with peritoneal carcinomatosis Citation[3], Citation[7–9,], Citation[11], Citation[19]. The concept of this treatment indicates that cytoreductive surgery should remove gross disease and that intraperitoneal chemotherapy will eliminate microscopic residual disease. In the present study, PIHC significantly improved the survival rate in gastric cancer patients with peritoneal dissemination. The aim of PIHC is to damage cancer cells and peritoneal micrometastases directly by heating and exposure to an anticancer drug. The important factors in this therapy are the temperature and duration of the hyperthermia. The temperature range of 40.5°C to 42.5°C can increase the tissue blood flow and lead to a substantial thermal enhancement of the cytologic chemotherapeutic agents Citation[20]. During PIHC, the entire peritoneal surface can be heated homogenously with a large volume (1 litre saline) of hyperthermic fluid by RF-8.
True clinical hyperthermia is defined as the use of temperatures of 41°C and higher. The temperature used in this study was lower than that in the other reports Citation[3], Citation[4], Citation[7–9]. The cytotoxic effect of hyperthermia is not only temperature dependent but is also related to the exposure time and time-relation to other therapies. Hyperthermia shows a synergism with certain cytotoxic drugs (e.g. mitomycin and cisplatin). Increased cell-membrane permeability at higher temperatures can increase drug uptake by tumour tissue Citation[21]. This synergism can already occur at temperatures as low as 39–41°C (mild hyperthermia) with some cytotoxic drugs, such as cisplatin, mitomycin, and melphalan Citation[21].
There is considerable evidence that cytoreduction combined with hyperthermic chemotherapy prolongs survival and can potentially cure peritoneal carcinomatosis of gastric cancer Citation[7–9], Citation[11]. However, this does not necessarily mean that all patients suffering from peritoneal carcinomatosis due to gastric cancer benefit. A patient having the microscopic type of peritoneal dissemination is the best candidate for PIHC. The experimental evidence strongly suggested that hyperthermic chemotherapy might be effective against small disseminations rather than grossly disseminated lesions. A study by Fujimoto and Kim also reported unsatisfactory results against numerous peritoneal disseminations Citation[8], Citation[22]. Furthermore, Jones et al. reported the most limiting factor in the clinical use of intraperitoneal chemotherapy is the restrictive penetration depth of the drugs used in tumour tissue (1–3 mm) Citation[23]. In an attempt to increase the treatment efficacy of PIHC, this study focused on patients with a small tumour burden, with the aim of optimizing the regimen and technique of chemotherapy. Cytoreductive surgery was performed to reduce tumour bulk in an effort to overcome the limited penetration of drugs into the tissue. It is important to note that patients in the reported study were selected using strict criteria; therefore, there is an important selection bias in this trial. However, this select group (inclusion limited to the patients without several scattered metastases to the distant peritoneum and peritoneal dissemination on preoperative explorations) may achieve improved survival results with an acceptable morbidity rate. When the primary tumour is not amenable to resection or when the resection of peritoneal disseminations does not allow a sufficient reduction in tumour volume, PIHC does not seem to be indicated as the gain in terms of survival is minimal.
The rationale behind the choice of drugs for hyperthermia is based on the intraperitoneal pharmacokinetics of the agent. The most frequently used regimens are cisplatin alone, mitomycin alone, or a combination of both Citation[24]. No standard dose has been defined for most regimens because the variables differ between reports Citation[24]. As for the choice of the drugs in chemotherapy, drugs which are cooperative with hyperthermia should be chosen, and cisplatin has proved to be both highly effective for digestive tract carcinoma, while also possessing an effective cooperative action with hyperthermia Citation[25–27]. In this study we used cisplatin at a dose of 80 mg/m2 for PIHC because of Japanese medical insurance. The 80 mg/m2 of cisplatin, which is the widely accepted dosage in Japan, is therefore considered to be suitable for PIHC. There have been many reports that have described that anticancer drugs demonstrate a synergic effect with hyperthermia and/or the mechanism of hyperthermic enhancement Citation[28–31]. Cisplatin was the most relevant drug for various forms of malignancies. Its combination with hyperthermia causes an increase in intracellular drug accumulation and DNA-platinum adducts Citation[30]. An inhibition of DNA repair was noted in cisplatin-treated cells Citation[31]. These studies suggest that hyperthermic chemotherapy will be effective for lesions that are refractory to the conventional chemotherapy. Taxane family of antineoplastic agents has activity in a variety of neoplasms. Docetaxel is a relatively new cytotoxic agent that produced semisynthetically from the needles of Taxus baccata, and it is the second member of the taxane class of cytotoxic agents to reach clinical use Citation[32]. Marchettini et al. reported that intraperitoneal administration of docetaxel attains a significantly higher drug concentration in the peritoneal cavity for a long time, as compared with systemic administration Citation[33]. Several reports have demonstrated that intraperitoneal administration of docetaxel is effective in the control of peritoneal dissemination of gastric cancer Citation[34–36]. These results suggest the potential of intraperitoneal docetaxel administration to treat peritoneal dissemination of gastric cancer. Docetaxel may be the most advantageous drug for intraperitoneal administration.
The adverse effect was an important factor in influencing the result of peritonectomy and intraperitoneal hyperthermic chemotherapy. Peritonectomy with intraperitoneal hyperthermic chemotherapy is a major undertaking and associated with a high incidence of postoperative complications as well as procedure-related mortality. Although continuous hyperthermic peritoneal perfusion has proven to be an effective procedure, several complications may occur, such as leakage of intestinal anastomosis, intestinal perforation, bone marrow suppression, and renal failure Citation[7]. In this study the treatment-related toxicities or complications were not remarkable. Systemic toxicity included grade 2 leucopenia and grade 3 nausea in this study. Therefore, it is reasonable to assume that this complication was not caused by the hyperthermic effect, but by the chemotherapeutic agents, and that it could be prevented by sufficient fluid supply and more careful chemotherapy. Moreover, four patients experienced prolonged drainage of fluid from the peritoneal cavity in this study. It was thought that peritoneal lavage with normal saline solution itself has an irritating effect on peritoneum. Shido et al. also reported that peritoneal damage by hyperthermic peritoneal perfusion is not caused by the hyperthermia but by the peritoneal perfusion with saline solution containing anticancer drugs Citation[37].
There have been no studies of intraperitoneal hyperthermic chemotherapy with RF-8 for the prevention of peritoneal recurrence of gastric cancer after gastrectomy. This study represents the first trial of the adjuvant application of intraperitoneal hyperthermic chemotherapy with RF-8. For clinicians to accept hyperthermic chemotherapy as a conventional therapy, more evidence must be drawn from the prospective study, and a definite evaluation of the prognostic factors is needed. Furthermore, to obtain clinical relevance, hyperthermia has some problems that must be resolved (i.e. specific apparatus to maintain a stable temperature distribution and cost of therapy).
In conclusion, cytoreductive surgery plus PIHC is effective for preventing and treating peritoneal metastasis in far-advanced gastric carcinoma patients. We recognize that in the application of PIHC, the target is free cancer cells in the peritoneal cavity and peritoneal small or micro metastases.
Conflict of interest statements
E. Mochiki, M. Shioya, H. Sakurai, H. Andoh, T. Ohno, R. Aihara, T. Asao and H. Kuwano hereby declare they are not engaged in any financial or personal relationship with other people or organizations that could inappropriately influence their work.
References
- Nakajima T, Harashima S, Hirata M, Kajitani T. Prognostic therapeutic values of peritoneal cytology in gastric cancer. Acta Cytol 1978; 22: 225–229
- Kiyasu Y, Kaneshima S, Koga S. Morphogenesis of peritoneal metastasis in human gastric cancer. Cancer Res 1981; 41: 1236–1239
- Yonemura Y, Ninomiya I, Kaji M, Sugiyama K, Fujimura K, Sawa T, Katayama K, Tanaka S, Hirono Y, Miwa K, et al. Prophylaxis with intraoperative chemohyperthermia against peritoneal recurrence of serosal invasion-positive gastric cancer. World J Surg 1995; 19: 450–455
- Levi JA, Fox RM, Tattersall MH, Woods RL, Thompson D, Gill G. Analysis of a prospectively randomized comparison doxorubicin versus 5-fluorouracil, doxorubicin, and BCNU in advanced gastric cancer: Implication for future studies. J Clin Oncol 1986; 4: 1348–1355
- Overgaard J. Effect of hyperthermia on malignant cells in vivo. Cancer 1977; 39: 2637–2646
- Hettinga JVE, Konings AWT, Kampinga HH. Reduction of cellular cisplatin resistance by hyperthermia–A review. Int J Hyperthermia 1997; 13: 439–457
- Fujimoto S, Shrestha RD, Kokubun M, Kobayashi K, Kiuchi S, Konno C, Ohta M, Takahashi M, Kitsukawa Y, Mizutani M, Chikenji T, Okui K. Positive results of combined therapy of surgery and intraperitoneal hyperthermic perfusion for far-advanced gastric cancer. Ann Surg 1990; 212: 592–596
- Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Isawa E, Sumida M, Ohkubo H. Improved mortality rate of gastric carcinoma patients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined surgery. Cancer 1997; 79: 884–891
- ujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer 1999; 85: 529–534
- Xu DZ, Zhan YQ, Sun XW, Cao SM, Geng QR. Meta-analysis of intraperitoneal chemotherapy for gastric cancer. World J Gastroenterol 2004; 18: 2727–2730
- Yonemura Y, Kawamura T, Bandou E, Takahashi S, Sawa T, Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg 2005; 92: 370–375
- Stephens AD, Alderman R, Chang D, Edwards GD, Esquivel J, Sebbag G, et al. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol 1999; 6: 790–796
- Abe M, Hiraoka M, Takahashi M, Ono K, Nohara H. Clinical experience with microwave and radiofrequency thermotherapy in the treatment of advanced cancer. Natl Cancer Inst Monogr 1982; 61: 411–414
- Abe M, Hiraoka M, Takahashi M, Egawa S, Matsuda C, Onoyama Y, Morita K, Kakehi M, Sugahara T. Multi-institutional studies on hyperthermia using an 8-MHz radiofrequency capacitive heating device (Thermotron RF-8) in combination with radiation for cancer therapy. Cancer 1986; 58: 1589–1595
- Gilly FN, Cotte E, Brigand C, Monneuse O, Beaujard AC, Freyer G, Glehen O. Quantitative prognostic indices in peritoneal carcinomatosis. Eur J Surg Oncol. 2006; 32: 597–601
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995; 221: 29–42
- Abe M, Hiraoka M. Localized hyperthermia and radiation in cancer therapy; Review. Int J Radiat Biol 1985; 47: 347–359
- Tahara M, Ohtsu A, Narikazu B, et al. Sequential methotrexate and 5-fluorouracil therapy for gastric cancer patients with peritoneal dissemination: a retrospective study. Gastric cancer 2001; 3: 212–218
- Hall JJ, Loggie BW, Shen P, Beamer S, Case LD, MaQuellon R, Geisinger KR, Levine EA. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J Gastrointestinal Surg 2004; 8: 454–463
- Son CW, Shakil A, Osborn JL, Iwata K. Tumor oxygenation is increased by hyperthermia at mild temperatures. Int J Hyperthermia 1999; 15: 79–107
- Storm FK. Clinical hyperthermia and chemotherapy. Radiol Clin N America 1989; 27: 621–627
- Kim JY, Bae HS. A controlled clinical study of serosa-invasive gastric carcinoma patients who underwent surgery plus intraperitoneal hyperthermo-chemo-perfusion (IHCP). 2001, 4: 27–33
- Jones RB, Myers CE, Guarino AM, et al. High volume intraperitoneal chemotherapy (“belly bath”) for ovarian cancer. Pharmacologic basis and early results. Cancer Chemother Pharmacol 1978; 5: 1607–1612
- Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemotherapy. Lancet Oncol 2004; 5: 219–228
- Takahashi I, Emi Y, Hasuda S, Kakeji Y, Maehara Y, Sugimachi K. Clinical application of hyperthermia combined with anticancer drugs for the treatment of solid tumors. Surgery 2002; 131: S78–S84
- Hettinga JVE, Konings AWT, Kampinga HH. Reduction of cellular cisplatin resistance by hyperthermia–A review. Int J Hyperthermia 1997; 13: 439–457
- Sugimachi K, Kuwano H, Ide H, Toge T, Saku M, Oshiumi Y. Chemotherapy combined with or without hyperthermia for patients with oesophageal carcinoma: A prospective randomized trial. Int J Hyperthermia 1994; 10: 485–493
- Wallner KE, Li GC. Effect of drug exposure duration and sequencing on hyperthermia potentiation of mitomycin C and cisplatin. Cancer Res 1987; 47: 493–496
- Wallner KE, Degregorio MW, Li GC. Hyperthermia potentiation of cis-diamminedichloroplatinum (II) cytotoxicity in Chinese hamster ovary cells resistant to the drug. Cancer Res 1986; 46: 6242–6245
- Ohno S, Siddik ZH, Kido Y, Zwelling LA, Bull JMC. Thermal enhancement of drug uptake and DNA adducts as a possible mechanism for the effect of sequencing hyperthermia on cisplatin-induced cytotoxicity in L1210 cells. Cancer Chemother Pharmacol 1994; 34: 343–350
- Hettinge JV, Lemstra W, Meijer C, Dam WA, Uges DRA, Konings AWK. Mechanism of hyperthermia potentiation of cisplatin action in cisplatin-sensitive and–resistant tumor cells. Br J Cancer 1997; 75: 1735–1743
- Diaz JF, Andreu JM. Assembly of purified GDP-tubulin into microtubules induced by taxol and taxotere: reversibility, ligand stoichiometry, and competition. Biochemistry. 1993; 32: 2747–2755
- Marchettini P, Stuart A, Mohamed F, Yoo D, Sugarbaker PH. Docetaxel: pharmacokinetics and tissue levels after intraperitoneal and intravenous administration in a rat model. Cancer Chemother. Parmacol 2002; 49: 499–503
- Morgan RJ, Jr, Doroshow JH, Synold T, Lim D, Shibata S, Margolin K, Schwarz R, Leong L, Somlo G, Twardowski P, et al. Phase I trial of intraperitoneal docetaxel in the treatment of advanced malignancies primarily confined to the peritoneal cavity: Dose-limiting toxicity and pharmacokinetics. Clin Cancer Res 2003; 9: 5896–5901
- Yonemura Y, Endou Y, Bando E, Kuno K, Kawamura T, Kimura M, Shimada T, Miyamoto K, Sasaki T, Sugarbaker PH. Effect of intraperitoneal administration of docetaxel on peritoneal dissemination of gastric cancer. Cancer Lett 2004; 210: 189–196
- Yonemura Y, Bandou E, Sawa T, Yoshimitsu Y, Endou Y, Sasaki T, Sugarbaker PH. Neoadjuvant treatment of gastric cancer with peritoneal dissemination. Eur J Surg Oncol 2006; 32: 661–665
- Shido A, Ohmura S, Yamamoto K, Kobayashi T, Fujimura T, Yonemura Y. Dose hyperthermia induce peritoneal damage in continuous hyperthermic peritoneal perfusion?. World J Surg 2000; 24: 507–511