Abstract
Purpose: Two major questions were addressed: (1) Can fever-range whole body hyperthermia (FR-WBH) affect the number of perfused tumor blood vessels? (2) Can pre-treatment with FR-WBH improve accumulation or anti-tumor efficacy of doxorubicin or DOXIL (liposomal doxorubicin)?
Materials and methods: Perfused blood vessels were visualized by intravenous injection of the fluorescent dye (DiOC7(3)) and the number of labeled vessels in tumors and normal organs of unheated mice and those previously heated to 39.5°C for 6 hours were compared. Using three animal tumor models (one syngeneic murine model and two human tumor xenografts in SCID mice) we also compared tumor growth and amount of intratumoral doxorubicin (given as free drug or as DOXIL) in control mice or those given pre-treatment with FR-WBH.
Results: FR-WBH had no effect on the number of CD-31 labeled blood vessels. However, in tumors, but not in normal organs of the same animals, FR-WBH resulted in a significant increase in those blood vessels which could take up dye over a prolonged period of time after heating. There was also an increase in DOXIL uptake in the tumors of mice given FR-WBH prior to drug injection as well as enhanced therapeutic efficacy in all three tumor models.
Conclusions: FR-WBH increases the number of perfused blood vessels in tumors over a prolonged period following FR-WBH and thus may be useful for improving tumor targeting of cancer therapeutics. We discuss these data in relation to long-conserved thermoregulatory features in normal vasculature, which may be deficient in tumor vasculature.
Introduction
Heat treatment of tumors is a strategy that has been primarily explored for enhancing the tumor killing efficacy of radiation therapy, and recent clinical trials continue to provide very encouraging data regarding overall clinical benefit of this combination Citation[1–6], Citation[7]. The basis for the benefit of combining local hyperthermia and radiation is not entirely clear, however two possibilities have been proposed: either, (1) The direct cytotoxic effects of heat on tumor cells and intratumoral vascular structures that could synergize with the cytotoxic effects of ionizing radiatio Citation[8], Citation[9] particularly when the tumor temperatures reach 43°C and the tumor microenvironment is acidic and nutrient deprived Citation[10], Citation[11], or conversely, (2) The indirect effect of heat on tumor re-oxygenation (presumably from an increased volume of blood moving through the region to remove the excess heat) which could result in radiation-enhancing increases in intratumoral oxygen tension Citation[12–15], Citation[16–18]. Recent evaluations strongly support the latter mechanism of action since intratumoral temperatures high enough to cause direct thermal killing of either tumor cells or vascular endothelium are not as commonly achieved in the clinical setting as was previously believed, at least not for the duration of time needed Citation[9]. However, if tumor re-oxygenation is the major beneficial effect of thermal therapy directed locally at tumors, then it has also been recognized that current clinical practice with regard to the placement of hyperthermia treatment within the radiation fractionation is not optimal for exploiting the indirect effects of heating on tumor oxygenation Citation[9].
Local hyperthermia is also being evaluated in combination with chemotherapy and in triple therapy with both radiation and chemotherapy, although again, a rationale to support these approaches is incomplete. However, the observation that similar positive results are being obtained from both preclinical animal studies and clinical trials Citation[19–28] is a strong reason for continuing research on local heating in combination with radiation and/or chemotherapy. After reviewing published studies of combination therapy in terms of both tumor drug uptake and tumor growth delay, Kong and Dewhirst Citation[19] reported that in all 32 studies reviewed, local heating of tumors in combination with chemotherapy (either free drug or liposomally encapsulated drug) showed an enhanced therapeutic effect compared to either treatment modality alone.
Local hyperthermia for treatment of cancer is created by various physical means of focusing heat on a limited region of the body containing a tumor and it is not known if tumors outside of the heated field, (e.g. metastatic foci) are affected indirectly by heat treatments. Nevertheless, randomized clinical trials are suggesting a strong overall survival benefit from the use of local hyperthermia in combination with radiation in advanced cancer patients Citation[5] supporting the idea that there are positive systemic effects that are generated. Future studies that optimize scheduling of the heating to take advantage of radiation-enhancing oxygenation effects may further improve long-term tumor control. In the meantime, we and others Citation[29] have been exploring the potential usefulness of using whole body heating, which may improve the effects of heat treatment in patients with disseminated tumors. Previous work by others has evaluated whole body hyperthermia (WBH) protocols in which the patient is brought to relatively hot systemic temperatures (e.g. greater than 41.5°C) in combination with various chemotherapeutic agents Citation[30–31]; however, clinical gain was limited by increased chemotherapy-induced toxicity Citation[32]. As a result, Bull and co-workers pioneered a milder (i.e. 40°C) protocol in which heating was extended over a much longer period of time (6 hours) using a model in which rats bearing metastatic mammary tumors are immersed in warm water. They reported that combining this protocol with chemotherapy improved tumor control without increasing normal tissue toxicity Citation[33], Citation[34]. They also reported that application of mild WBH alone resulted in a significant decrease of axillary lymph node metastasis in the rat mammary tumor model Citation[34] and currently, this group is conducting Phase I/II clinical trials using mild whole body heating in combination with various chemotherapies.
Using a similar mild whole body hyperthermia protocol, (39.5°C for 4–6 hours, or ‘fever-like’ hyperthermia achieved using a warm air environment rather than warm water Citation[35–42], Citation[43]) our group confirmed the occurrence of delayed tumor growth using heat alone in some tumor models. In addition, upon examining tumors for clues to the underlying cellular mechanism for this thermally induced inhibition, we observed increased tumor cell apoptosis and accumulation of erythrocytes and leukocytes, including granulocytes, in the intratumoral vasculature and stromal microenvironment. Since this temperature range is achieved during natural febrile reactions in response to inflammation, it may be expected that systemic immunological effects at this temperature range could be triggered and indeed, important effects of fever-range hyperthermia on (1) leukocyte re-compartmentalization, and trafficking of T cells to lymph node high endothelial venules Citation[44], Citation[44–47], (2) LPS-induced acute inflammatory responses Citation[48], T lymphocyte activation and antigen-dependent immune responses that are mediated by T lymphocytes and dendritic cells Citation[36], Citation[40], Citation[49], Citation[50], (3) tumor-specific NK cell mediated cytotoxic activity Citation[37], Citation[51] and (4) induction of heat shock proteins in lymphocytes Citation[36], Citation[42] could collectively contribute toward enhanced systemic memory immune responses and longer-term immune control of tumor growth. However, whether there are changes in tumor blood vessel perfusion or oxygenation at these lower, physiologically relevant temperatures, has not yet been investigated.
During our early examinations of hematoxylin and eosin-stained paraffin sections of tumors isolated from mice at various time points after they were given FR-WBH, we consistently noticed that there appeared to be more stromal space visible around groups of tumor cells, and that blood vessels appeared to be more distinct and obvious. Often, there seemed to be an increase in the number of discernable erythrocytes and leukocytes within the vessels throughout the tumors isolated from heated mice in comparison to unheated mice. We observed that these changes in vessel appearance and size were apparent for several days following a single hyperthermia treatment Citation[37] although there could be considerable variation between tumors in animals given the same FR-WBH treatment, and regional differences across the same tumor in terms of the magnitude of these changes. However, since similar vascular changes did not occur in normal organs, (or at least did not persist past the time of heating when we collected the organs), this suggested that particular properties of tumor vasculature might make these vessels more vulnerable to mild increases in body temperature and that heat- induced, structural changes might persist for a longer period of time in tumors than in normal organs.
In the present study we have specifically investigated the changes in tumor vasculature in response to FR- WBH using a syngeneic mouse tumor model, CT26 cells grown in BALB/c mice. Secondly, we have measured tumor uptake of free doxorubicin and liposomal doxorubicin (DOXIL) in mice pre-treated with FR-WBH and compared it to that achieved in mice maintained under normothermic conditions prior to drug injection. Using this same model system, we have compared the therapeutic efficacy of pre-treatment with FR-WBH followed by either DOXIL or doxorubicin with that achieved by the same drug treatments in animals maintained under normothermic conditions. Finally, we have examined whether pre-treatment with FR-WBH could enhance the therapeutic efficacy of DOXIL in two different human colon tumor xenografts in SCID mice (using the HT-29 cell line and a colon tumor derived from a patient's surgical specimen). These new data regarding the potential of FR-WBH to affect tumor vasculature and improve subsequent therapeutic efficacy of chemotherapy are discussed in relationship to the physiology of thermoregulation in normal tissue which could indirectly impact tumor blood vessel function.
Materials and methods
Animal and tumor models
Six- to eight-week-old female SCID (CB17/ICR Tac, CB17/CB17) and BALB/c mice were obtained from the animal facility in Roswell Park Cancer Institute or were purchased from Taconic Labs. The human colon cell line HT-29 (obtained from ATCC) and a patient's surgical specimen-derived human colon carcinoma from Roswell Park Cancer Institute, were each grown in SCID mice. A murine colon carcinoma CT26 (obtained from ATCC) was grown in its syngeneic host, BALB/c mice.
Tumor implantation and growth assessment
Murine (CT26) and human (HT-29) colon tumor cells were inoculated subcutaneously into BALB/c (105 cells in 0.1 ml) or SCID (106 cells in 0.1 ml) mice respectively. A patient's surgical specimen-derived colon tumor was also grown in SCID mice Citation[52] and dissected into small pieces approximately 2 mm in diameter. The tumor pieces were then implanted subcutaneously into 25 eight-week-old female SCID mice. The tumors were allowed to reach 4 to 5 mm in diameter before treatment. The tumors were measured with a Promax 6” digital caliper (Doall, Buffalo, NY) to determine the shortest diameter (A) and the longest diameter (B). The volume was then calculated by using the formula V = (A2B)/2. The relative tumor growth (Tr) was calculated for each tumor by dividing the volume at any given day after FR-WBH (Td) by the volume at the start of FR-WBH (T0).
FR-WBH treatment and administration of DOXIL or doxorubicin
FR-WBH was carried out as follows for each experiment Citation[42]. To prevent dehydration, 1 ml sterile saline was administered to all mice by intraperitoneal injection immediately prior to their being placed in preheated microisolator cages in a gravity convection oven (Memmert model BE500, East Troy, Wisconsin) or placed in a sham control chamber kept at room temperature. Temperature microchip transponders (14 × 2.2 mm) were inserted subcutaneously into the dorsal thoracic area of SCID/BALB/c ‘sentinel’ mice that were heated with experimental mice. Core temperatures of the sentinel mice were monitored at 30–45 min intervals using a temperature scanner (Biomedic Data System (BMDS), Seaford, Delaware) and the animals were maintained at 39.5°C (±0.2°C) for 6 hours. Doxorubicin (Sigma) or liposome-encapsulated doxorubicin, DOXIL, (kindly provided by Dr Gail Colbern at ALZA Corp. Mountain View, CA) was given weekly through tail vein injection within 10 minutes following FR-WBH treatment. Although we did not observe toxicity associated with either FR-WBH alone or after combination with drugs at the doses indicated, we checked for toxicity in each experiment by measuring mouse weight and observing general health and behavior.
Histopathological analysis
At the termination of each experiment or at the designated time points, animals were sacrificed and excised tumors and normal tissues (kidney, liver, heart) were either placed in cryomolds (Tissue-Tek) with embedding medium (OCT compound) and frozen in liquid nitrogen or fixed in 10% buffered formalin followed by paraffin embedding for study of histology or immunohistochemistry.
Immunohistochemical analysis
Frozen tissue sections were fixed in cold acetone for 10 minutes and air dried. The tissue sections were rinsed in phosphate-buffered saline (PBS) and endogenous peroxidase was then quenched in PBS containing 0.1% hydrogen peroxide for 20 minutes. Endogenous biotin was blocked with a biotin blocking system (DAKO Corp., Carpinteria, CA) and non-specific binding was blocked with 10% normal goat serum. Rat anti-mouse CD31 monoclonal antibody (PharMingen, San Diego, CA) was used for detection of vascular endothelial cells (2 µg/ml) and was allowed to react with the tissue sections overnight at 4°C in a humid chamber. The tissue sections were then reacted with 1 µg/ml of biotin-conjugated goat anti-rat Ig (PharMingen, San Diego, CA) for 30 minutes at room temperature. Between the antibody reactions, three 5-minute washes with PBS solution containing 0.1% Triton X-100 were performed. The sections were then incubated with the chromagen DAB and counterstained with hematoxylin.
Analysis of the density of functional vessels within tumors and normal organs
Functional blood vessels were imaged with the fluorescent dye 3, 3′diheptyloxacarbocyanine, DiOC7(3) Citation[53]. This dye was prepared by dilution in 75% DMSO (3.75 ml DMSO + 1.25 ml dH2O) to a final concentration of 0.4 mg/ml (2 mg/5ml 75% DMSO). The solution was stirred overnight, filtered, aliquoted and stored, protected from the light, at 4°C until use. The dye was administered to animals maintained under normothermia and to heated animals at various time points after FR-WBH at a final dose of 1.0 mg/kg (50 ul of DiOC7 solution) by tail vein injection and a minute later the animals were sacrificed and the tumors excised and frozen in liquid nitrogen. This dose has been shown to provide optimal visualization of tumor vasculature by preferentially staining cells immediately adjacent to blood vessels Citation[53] and thus only blood vessels which are functional (i.e. able to bring blood into the tumor) at the time of injection show the presence of fluorescent dye. Serial 6–8 µm sections were cut from different depths of each frozen tumor using a cryostat. Immediately following cryo-sectioning, the tumor sections were imaged for DiOC7(3) staining using an fluorescent microscope (ZEISS, Axioskop 2) equipped with a color digital camera (SPOT 2, Diagnostic Instruments, Sterling Heights, MI). DiOC7(3) emits green fluorescence when excited by blue light. We counted at least 25 randomly chosen fields per tumor section and at least 3 sections per tumor were used per time point. We also noted whether the counted field was on the tumor periphery or from a field deeper in the tumor.
Determination of drug uptake
Total doxorubicin concentration in tumors and normal organs was determined using fluorescence intensity measurements of the free drug in methanol. Mice were treated with doxorubicin or DOXIL as described above and tissues were collected at several time points up to 7 days post-injection. Note that the vascular compartment was not cleared prior to tissue collection. Doxorubicin was extracted from excised tissue using organic solvents using a procedure modified from that used by Robert Citation[54]. Briefly, tissue samples (0.2 g tissue/2 ml PBS) were homogenized and then incubated with equal volume of chloroform/isopropanol solution (3 : 1 ratio) for 2 hours at room temperature. The mixed solutions were centrifuged at 3000 rpm for 15 minutes, and the lower organic phases were collected and air dried. The dried materials were re-suspended in 200 µl of 70% methanol and loaded on to pre-prepared Sep-Pak C18 cartridges (Waters Corporation, Milford, MA) to extract doxorubicin. The cartridges were washed with 5 ml of 70% methanol and the eluates were discarded. The cartridges were then washed with 5 ml of 100% methanol. The first 1 ml of the eluate was discarded, and the remaining 4 ml of the eluates were collected and air dried. The dried materials were re-suspended in 40 µl of 100% methanol and the doxorubicin concentrations in the solutions were determined from the measured fluorescence intensity using a standard curve that was obtained using known concentrations of doxorubicin. This procedure was found to extract drug equally from tumors treated with DOXIL or doxorubicin and both forms of drug were added to tissue and used as standards.
Statistical considerations
Statistical significance was calculated using the Students’ t-test. All of the experiments were repeated at least twice (as indicated in the figure legends) except for the tumor growth experiments shown in , where we performed a comparative analysis using two different human colon tumor xenograft models only once, obtaining a similar result for each tumor. The number of animals per group or the number of fields analysed for fluorescence is also indicated in the legends.
Results
Analysis of the uptake of DiOC7(3) in tumor blood vessels following FR-WBH
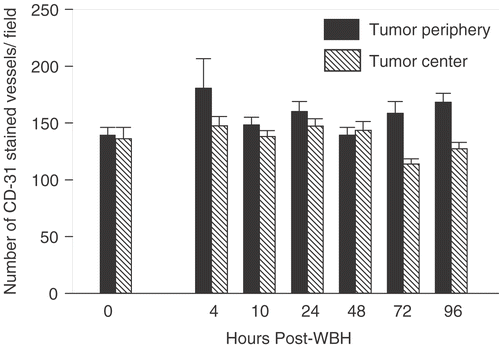
Our previous data Citation[37] and unpublished] suggested that FR-WBH causes prolonged, post-heating histological changes in tumor blood vessels but not those of normal organs, which led to the hypothesis that there is a tumor-selective, thermally induced increase in vascular accessibility to blood. Following FR-WBH, fewer vessels appeared collapsed and non-functional and instead more appeared patent, often filled with blood cells. To test this hypothesis, and to characterize more completely the vascular changes that are induced in tumors by FR-WBH, we examined the density of functional microvessels in tumors or normal organs of CT26-bearing BALB/c mice by quantifying the number of vessels accessible to a fluorescent dye, DiOC7(3), that labels vascular endothelium Citation[53]. Dye was injected into the tail vein at various time points after heating was completed (and body temperature had returned to normal), beginning 30 minutes after heat or sham treatment ended. In mice, we have previously determined that normal body temperatures are regained rapidly, within 10 minutes after removal of the animal from the heated chambers Citation[43]. We analysed blood vessels at both the tumor periphery and center following DiOC7(3) injection at multiple time points. We observed that pre-treatment with FR-WBH enhances the number of blood vessels containing DiOC7(3) in both the periphery and the center of the tumors in each of 3 separate experiments, although tumors differ from each other in terms of the overall pattern of distribution of perfused vessels. shows representative fluorescent images of control and heated tumors following in vivo perfusion of DiOC7(3) given at 2 hours post FR-WBH (similar results were obtained at 30 minutes, 2, 4, 10, 24 hours after heating; see ). A quantitative and kinetic analysis of the fluorescent vessel densities shows an increase in the number of DiOC7(3) labeled vessels in FR-WBH-treated tumors up to 48 hours post FR-WBH treatment especially at the tumor periphery, but increases in the number of labeled vessels in the center are also visible (); after this time point, the number of labeled vessels returns to control levels. While tumors vary both in comparison to each other, and within the same tumor, we consistently observed that a 2- to 4-fold increase in the number of functional tumor vessels is reached at 4–10 hours post FR-WBH treatment. We did a similar analysis of normal tissue (liver, kidney and heart) and found that the number of perfused vessels in normal organs is not changed by FR-WBH (data not shown), suggesting that FR-WBH does not affect perfusion of normal tissues, at least after animals are returned to normothermic conditions, when these experiments were conducted. To make sure that new blood vessel formation was not the cause of the changes in density of DiOC7(3)-positive blood vessels, we also did an analysis of the total number of vessels from 2 hours to 4 days following hyperthermia treatment by analysing immunohistochemically stained tumor sections of CT26 tumors in BALB/c mice using anti-CD31 antibodies (). Since CD31 identifies all vessels (whether perfused or not), the data confirms that FR-WBH has little or no effect on overall microvascular density, at least during the first 4 days after heating. However, the proportion of these vessels which was perfused increased following heating and persisted for 48 hours after animals were returned to room temperature. A double staining procedure in which the same section is analysed for the number of CD31-stained vessels and those that are perfused with dye would be necessary for a more complete analysis of the percentage change in perfused or non-perfused following FR-WBH.
Figure 1. Fluorescence photomicrographs of CT26 tumor sections from mice maintained under normothermic conditions or given pre-treatment with FR-WBH comparing the density of DiOC7 (3)-labeled blood vessels. DiOC7(3) was injected at various time points post sham (A) or FR-WBH (B) treatment. Shown here is representative data from the 2 hour post treatment time point demonstrating an increase in the number of perfused vessels in tumors removed from mice given pre-treatment with FR-WBH. The animals were sacrificed within 1 minute after the injection and tumors were quickly excised and frozen in liquid nitrogen. Arrows show the presence of blood vessels containing dye. These data are representative of 3 separate experiments, with 4 mice per group in each experiment.
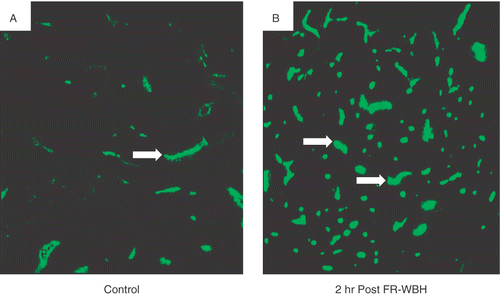
Figure 2. Time-dependent changes in functional vessel densities in CT26 tumor periphery and center following FR-WBH. The data demonstrate that pre-treatment with FR-WBH increases number of labeled vessels in both tumor periphery and center up to 48 hours post FR-WBH treatment. The highest functional vascular density in tumor periphery was reached at approximately 4 hours while that in tumor center peaked generally by 10 hours post-hyperthermia treatment. These data are representative of 3 separate experiments with 4 mice per group in each experiment. For each data point, we counted at least 25 randomly chosen fields per section at 5× magnification with at least 3 sections per tumor. Statistical significance (p < 0.05) was reached at all time points between 2 and 48 hours.
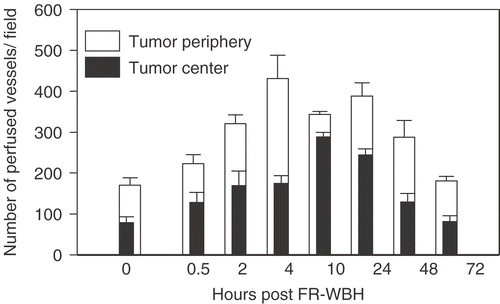
Figure 3. Analysis of the number of CD31-stained tumor vessels following FR-WBH treatment. Portions of the same tumors used in and were stained for the mouse endothelial cell marker, CD31. The plot of the number of CD31-stained vessels per field at different times after FR-WBH shows that there were no significant changes in the total numbers of tumor vessels in response to FR-WBH treatment. The data are representative of 3 separate experiments with 4 mice per group in each experiment. For each data point we counted at least 5 randomly chosen fields at 10× magnification with at least 3 sections per tumor.
Effect of FR-WBH on DOXIL accumulation in tissues
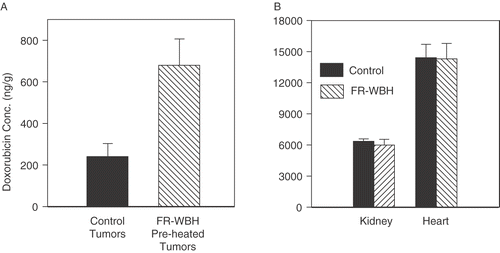
Since there has been considerable previous work on the use of local, higher temperature heating to enhance simultaneous delivery of therapeutic drugs Citation[19–28] including liposomally encapsulated doxorubicin Citation[19–21] to tumors, we wondered whether the sustained, selective increase in the number of perfused blood vessels in tumors following FR-WBH could help increase the intratumoral accumulation of DOXIL while not further increasing its concentration within normal organs. Employing the same tumor model used for the perfusion data described above, we injected DOXIL in animals given pre-treatment with FR-WBH or maintained under normothermic conditions, and measured the total drug concentrations in tumor and in normal tissues by extraction of the drug from tissues and measurement of the fluorescence intensity of doxorubicin in the extracts. Our results show that at 4 hours after drug administration DOXIL accumulation in the tumor increases 2–3 fold (; p < 0.01) in animals given pre-treatment with FR-WBH. Importantly, although under normothermic conditions normal organs had considerably higher accumulation of drug (25–50 fold) than did tumor tissues (consistent with known differences in drug uptake between tumors and normal organs; see Discussion), no additional increase in drug accumulation in normal tissues () was induced by FR-WBH, consistent with the lack of increased perfusion noted above for normal organs.
Figure 4. Pre-treatment with FR-WBH results in an increase in doxorubicin concentration in tumors but not normal tissues. The concentration of doxorubicin is significantly increased (p < 0.01) from baseline in tumors previously treated with FR-WBH (A) but not in kidney or liver (B) when it is analysed 4 hours after injection of DOXIL. Note that the drug concentrations within kidney and liver are considerably higher at baseline than in tumors (with no treatment), but this concentration shows no further increase as a result of pre-treatment with FR-WBH. The error bars indicate standard error of the mean. Data representative of 2 separate experiments with at least 9 mice per group.
Kinetics of tumor accumulation of DOXIL and free doxorubicin
To investigate further the effect of thermally induced tumor vascular changes in CT26 tumors on the uptake of chemotherapeutic drugs, we measured the temporal changes in tumor content of both free and liposomal doxorubicin (DOXIL) up to 7 days post drug administration with or without a pre-treatment with FR-WBH. Our results () show that pre-treatment with FR-WBH only slightly enhances accumulation of free doxorubicin at early time points (up to 2 hours) but subsequently, there were similar concentrations in both heated and unheated mice. The peak tumor content of free doxorubicin occurs at 1 hour after drug administration and the drug is no longer detectable by 24 hours post-injection. In contrast, the uptake of DOXIL appears significantly increased by prior heating. The doxorubicin content in tumors from animals receiving DOXIL reaches a peak at 24 hours after drug administration and even after 7 days post-injection, the drug concentration in tumor is still higher in the heated tumors than in controls. This data is summarized in giving the area under the curve (AUC) for the two forms of doxorubicin in normothermic control mice and their hyperthermia treated counterpart. This difference in the retention of free and liposomal doxorubicin may have a significant effect on the therapeutic efficacy of the two formulations of the same drug when used following FR-WBH treatment. To test this possibility, we next investigated the anti-tumor efficacy of either free doxorubicin or DOXIL in combination with FR WBH. using the murine CT26/BALB/c model.
Figure 5. Kinetics of doxorubicin accumulation in tumors from mice given either free doxorubicin or DOXIL at various time points following treatment with FR-WBH. Pre-treatment with FR-WBH generally enhances the accumulation of DOXIL in tumors (the 24 hr time point is statistically significant; p = 0.027) in comparison to the tumors from the animals in the control group, while only a slight increase in the uptake of free doxorubicin was observed at the earliest time points. DOXIL accumulation in tumors reaches a peak at approximately 24 hours post-injection following FR-WBH treatment and after 5 days, the DOXIL concentration in tumors remained at a level similar to that at 2 hours. Data obtained from 3 mice per group. The error bars indicate standard error of the mean. This experiment was repeated 2 additional times comparing the 0 and 24 hr time points only and in each case, the accumulation of DOXIL was increased significantly from that seen in controls, while the accumulation of free doxorubicin was not significantly different from controls.
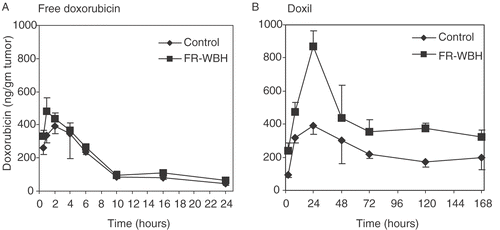
Table I. Doxorubicin availability in tumors estimated from the area under the curve (AUC) over 24 hours following free doxorubicin or DOXIL administration 1 hour after FR-WBH treatment. The data is calculated from the accumulation kinetics presented in .
Pre-treatment with FR-WBH enhances the anti-tumor efficacy of DOXIL in a murine tumor model
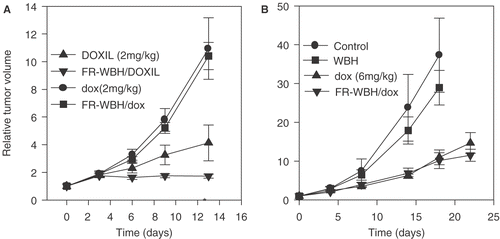
BALB/c mice bearing the CT26 tumor were treated with either DOXIL or doxorubicin alone or following FR-WBH. As shown in , DOXIL alone inhibited tumor growth while a dose of 2 mg/kg free doxorubicin did not in this particular tumor model. Furthermore, we observed a significant improvement in tumor growth inhibition by the pre-treatment with FR-WBH followed by DOXIL compared with DOXIL alone. The data in is one of two separate experiments each showing that pre-treatment with FR-WBH significantly enhances the anti-tumor efficacy of DOXIL (p < 0.005). We also examined the effect of the FR-WBH on the therapeutic effect of free doxorubicin and found that FR-WBH had no enhancing effect on the therapeutic efficacy of free doxorubicin using this tumor model. Since the dose of free doxorubicin used in this experiment is suboptimal, we next carried out an experiment to determine whether FR-WBH enhanced the effect of free drug when used at a known effective dosage (6 mg/kg). The results () indicate that although this higher dose of free doxorubicin by itself does result in tumor growth inhibition, pre-treatment with FR-WBH again did not further enhance this therapeutic effect.
Figure 6. Therapeutic effects of liposomal doxorubicin (DOXIL) and free doxorubicin on CT26 tumor growth with and without pre-treatment with FR-WBH. Tumor growth profiles are plotted for animals that were treated with: (A) DOXIL and free doxorubicin (dox) at 2 mg/kg given weekly (injected within 10 minutes of either sham treated control animals or animals pre-treated with FR-WBH) or (B) a higher dose (6 mg/kg) of free doxorubicin with and without pre-treatment with either sham or FR-WBH. Control tumor growth and growth of tumors in mice given FR-WBH alone are also shown in (B). Tumors in animals that received the sequential treatment of FR-WBH and DOXIL and had the least growth over a period of 13 days and this difference is statistically different from DOXIL alone as indicated with an asterisk. Tumor growth in animals treated with free doxorubicin at 2 mg/kg or at its known effective dose (6 mg/kg) was not enhanced by pre-treatment by FR-WBH. Representative data from two separate experiments with 5 mice per group.
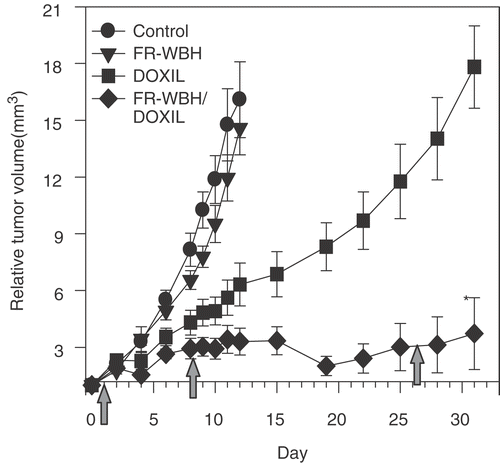
A second experiment was carried out to confirm and extend the observed ability of pre-treatment with FR-WBH to enhance the anti-tumor efficacy of DOXIL and to investigate the effects of multiple heat treatments (). In this case FR-WBH and/or DOXIL were given on days 1, 8 and 26. We observed growth inhibition by DOXIL alone and tumor regression in the FR-WBH/DOXIL combination. Additionally, significant tumor growth inhibition was sustained by the combination treatment for the duration of the experiment.
Figure 7. Effect of multiple FR-WBH treatments followed by DOXIL on murine colon-26 tumor growth in BALB/c mice. FR-WBH was administered on day 1, 8 and 26 as indicated by the arrows in the figure. DOXIL (2 mg/kg) was given weekly within 10 minutes after the completion of FR-WBH. Tumors in both the control and WBH treated groups grew rapidly and mice were terminated on day 12. In contrast, tumors treated with DOXIL alone showed noticeable growth delay, the greatest inhibition of tumor growth being achieved by the combination therapy and this enhancement of the efficacy of DOXIL by FR-WBH is statistically significant. The error bars represent standard error of the mean. Representative data from two separate experiments with 5 mice per group.
Effect of FR-WBH on DOXIL efficacy against human colon tumor xenografts in SCID mice
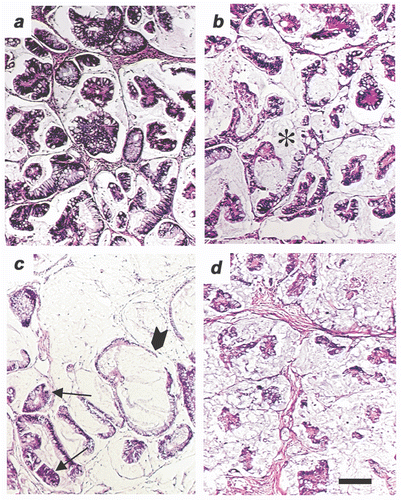
We also conducted an analysis of FR-WBH followed by DOXIL in SCID mice engrafted with human colon tumor xenografts derived from either subcutaneous injection of HT-29 human colon cancer cells or following implantation with an actual patient's colon carcinoma passaged as a xenograft Citation[52]. A significant tumor growth inhibition (p < 0.01) was seen in the groups given pre-treatment with FR-WBH followed by DOXIL therapy in comparison to groups given DOXIL treatment alone in both SCID-human xenograft models examined (). Histological analysis of the tumors from the experiment shown in using the patient colon-derived carcinoma shows some disruption of normal tumor structure and organization by FR-WBH alone, and tumor destruction by DOXIL alone. However, there was a more uniform destruction of tumor cell nests by FR-WBH and DOXIL () which may account for the more sustained tumor control observed.
Figure 8. Effects of pre-treatment with FR-WBH followed by DOXIL treatment on the growth of human colon carcinomas in SCID mice. FR-WBH was given as indicated by arrows in the figure followed by DOXIL treatment (2 mg/kg). Two different tumors were studied in these experiments with similar results obtained: (A) human colon cell line HT-29; and (B) a patient-derived colon carcinoma (No. 9934-5p). The tumor growth inhibitions by DOXIL alone and by sequential FR-WBH and DOXIL treatment are significantly different (p < 0.01 at the time points marked by an asterisk (*).
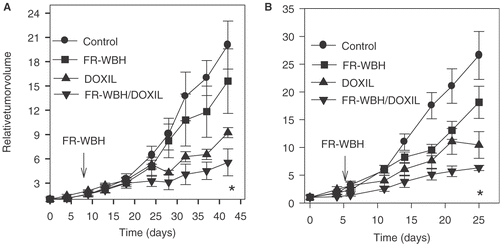
Figure 9. Histological analysis of a patient-derived colon tumor pre-treated with FR-WBH followed by DOXIL treatment. (A) A tumor section from a non-treated SCID mouse bearing a patient-derived colon carcinoma (9934-5P) showing the acinar histology that is typical of a colon adenocarcinoma. (B) Section from a tumor in an animal treated with FR-WBH alone revealing some changes in the organization in the tumor; the stroma (*) space appears to occupy a greater volume with occasional blood vessels becoming more visible. (C) Section from a tumor in an animal treated with DOXIL alone. In some areas, the tumor structure appears normal (arrow) and in other areas, the tumor cells are largely destroyed by the DOXIL treatment (arrowhead). (D) Section from a tumor in an animal pre-treated with FR-WBH followed by DOXIL treatment showing significant destruction of tumor cells throughout the section and revealing a greater volume of the interstitial space and stromal elements. Each figure was prepared from the patient- derived tumors collected on Day 25 in the experiment shown in . Bar equals 100 um.
Discussion
This study examined the effects of mild systemic heating on the subsequent proportion of perfused tumor blood vessels and their ability to receive DOXIL. Here we have demonstrated that while FR-WBH treatment does not change the total (anatomical) number of CD31-positive vascular structures within tumors, it results in an increase in the tumor vessels that are accessible to a tail vein injected dye for some time after heating is completed, correlating well to an increase in blood filled vessels we observed earlier Citation[37] and unpublished data]. We did not see these post-heating effects in normal organs, either in terms of visualization of perfused vessels nor in uptake of DOXIL. Since the physiologically relevant temperature range we utilized has long been known to profoundly affect normal blood flow patterns within the body in order to preserve core body temperature, Citation[55–57]; and see further discussion of thermoregulation below] we speculate that the post-heating, tumor-selective effects documented here are directly related to abnormal histological properties of tumor blood vessels Citation[58–60] and their inability to respond normally and reversibly to systemic changes in body temperature.
Previous laboratory or clinical research on the effects of hyperthermia in combination with chemotherapy has generally employed overlapping delivery of heat and drugs, whereas here we have evaluated post-heating effects on tumor vascular function and drug uptake. In several previous laboratory studies, it was shown that local hyperthermia applied for a shorter duration at higher temperatures can increase blood flow and oxygenation in tumors, although a number of other studies have reported a reduction in tumor blood flow and resulting tumor oxygenation as a result of heating Citation[12–17]. These differences could be a result of the temperature heterogeneity actually reached across tumors under local heating conditions where some hotter regions could result in cell death and vascular collapse rather than increased perfusion. In our studies, in which temperature across the tumor (and adjacent tissues) would be expected to be more uniform under the conditions imposed by the whole body heating approach, we observed a post-heating increase in the access of dye to the interior of tumors as visualized at the level of individual vessels. Whether this effect correlates to increased vascular flow is not yet known, but at the very least, it appears to help with the accumulation of molecules such as DOXIL.
In an earlier study we were able to achieve a significantly greater concentration of adoptively transferred NK cells in tumors after FR-WBH, presumably through thermally enhanced vascular access to tumors Citation[37], although other thermally regulated immunological mechanisms affecting homing of NK cells to the tumor microenvironment or their cytotoxicity may certainly contribute Citation[51]; see also Citation[44–47] for data showing thermally enhanced homing and adhesion of T lymphocytes to lymph node vascular endothelium]. In the present study we compared the effect of the hyperthermia-induced tumor vascular changes on the subsequent uptake and the therapeutic efficacy of free and liposomal doxorubicin in murine and human tumor models. While systemic chemotherapy is a standard treatment for recurrent or metastatic cancer, it is difficult to achieve effective doses of toxic drugs in the tumor microenvironment without simultaneously causing extensive damage to normal organs, which have a much greater vessel density and perfusion. Consistent with this well known problem, our data () reveals that doxorubicin concentration in tumor (delivered using liposomes) is approximately 50-fold less than levels in the heart at 4 hours post drug administration. However, we demonstrated here that pre-treatment with FR-WBH resulted in an increased accumulation of DOXIL in tumors while there was no further increase in concentrations in normal tissue (), indicating that the FR-WBH may provide a window of opportunity to selectively increase drug uptake in the tumor without further increasing normal tissue toxicity. Since the vascular fraction is increased in these tumors (and the vasculature was not cleared before analysis of doxorubicin) it is possible, however, that a portion of the increased concentration in the tumors reflects this increased blood content. However, since this increase was only observed following DOXIL administration and not after administration of free drug, this suggests that the additional blood volume cannot be the only factor contributing to the observed increase. However, experiments in which the vascular compartment is cleared before analysis of tissue doxorubicin content need to be carried out to specifically address the question of whether more drug actually gets out of the blood and into the tumor.
The use of liposomal encapsulation of doxorubicin has been demonstrated to be therapeutically effective while reducing normal tissue toxicity in animal models and clinical trials Citation[61–66]. In our study, the anti-tumor effect of DOXIL eventually became ineffective following initial treatments; however, we observed a long-term inhibition of the growth of tumors in mice treated with the combination of FR-WBH followed by DOXIL (), indicating the potential of this sequential therapy to overcome at least some tumor resistance related to limited drug uptake. An enhanced efficacy of chemotherapy when used in an overlapping manner with mild whole body treatment has previously been noted by Bull and colleaguesCitation[36], Citation[37], Citation[39]. However, a difference between the previous studies and those conducted here is seen in the scheduling of drug dose. In our study, drug was not added until after 6 hours of heating was completed. Using the post-heating protocol tested here on the CT26 tumor model, there was generally no significant difference in the long-term accumulation of free doxorubicin in tumors () or its anti-tumor efficacy although we found a significant difference in the accumulation and anti-tumor efficacy of DOXIL after heating ( and ). Since the increased perfusion of tumor vasculature following FR-WBH is maintained over a period of hours and DOXIL has been shown to have a much longer life in the circulatory system as compared to free drug Citation[61–65], it is likely that the liposomes have an extended time to accumulate in tumors as compared to free drug after heating. Further, even though our protocol appears to increase the number of perfusible blood vessels, some of these vessels may end blindly Citation[59], Citation[60], causing larger particles to pack together or be trapped and eventually release their contents within these leaky cavities, causing increased tumor destruction. The intratumoral delivery of smaller-sized free drug (doxorubicin) may be increased when combined simultaneously with heat treatment, but during a post-heating phase (as studied here) it may be able to pass through the endothelial spaces, diffuse through the intratumoral spaces and exit the tumors through other draining vessels. These assumptions concerning the increased tumor accumulation of DOXIL are consistent with a previous report Citation[19] that local hyperthermia increases the extravasation of liposomes from tumor vasculature. However, it is important to note that we compared free drug with DOXIL on only one tumor model (the transplantable CT26 tumor model) and additional tumor models would need to be analysed before any meaningful conclusions could be drawn concerning the effects of FR-WBH on accumulation of different drug formulations.
Physiological aspects of systemic temperature regulation may play a large part in the underlying mechanism(s) contributing to the increase in the proportion of perfused tumor blood vessels noted here. Body temperature elevation (including the temperature studied here) occurs under physiological conditions such as fever or exercise, and even more commonly in response to environmental temperature elevations. To maintain basal body temperature within normal ranges following temporary increases in heat content, the body uses a long-conserved and powerful set of regulatory responses collectively termed ‘thermoregulation’ Citation[55]. Following even minor changes in body temperature, heat sensing thermoreceptors result in systemic changes in blood flow and pressure patterns designed to enable rapid removal of excess heat Citation[55–57]. While there has been little previous study of the thermoregulatory capacity of tumor blood vessels, we do know that as a result of abnormal angiogenesis, tumor blood vessels often lack the normal coating of smooth musculature and associated autonomic nerve endings that control both patency of vessels and blood pressure seen in normal tissues. Also, tumor blood vessels are known to be leaky and are often extremely convoluted with blind ends, or are collapsed in comparison to those in normal tissues Citation[58–60]. These abnormalities, together with the resultant effects of abnormal fluid transfer (including high interstitial fluid pressure in the tumor microenvironment Citation[58]), can result in compression or complete collapse of vessels leading to inefficient and uneven delivery of oxygen, therapeutic drugs, or even perhaps host immune effector cells into the interior of tumors. We speculate that a tumor present within a thermoregulating animal (such as one undergoing the FR-WBH conditions used in this study), whose blood supply is in series with normal vascular beds, could be exposed to profound changes in the pressure and flow of blood moving toward it and through it. Various barriers to the flow of blood present in at least some existent tumor vessels may be overcome under these conditions. However, unlike normal tissue, tumors are unable to reverse these effects rapidly following removal of the heating because of their abnormal vessel structure and resultant lack of autonomic control. While much further study is necessary, the data shown here suggest the possibility that systemic changes in blood flow and pressure, occurring over the course of several hours of thermoregulation, can result in the forced re-profusion of some tumor blood vessels which were previously collapsed or non-functional providing an opportunity to improve delivery of therapeutic molecules or cells.
It is important to note that while local heating protocols currently used in cancer patients are designed to focus the heat delivery to the tumor, the surrounding normal tissue may eventually become heated as blood drains the tumor, and if the heating occurs over a long enough interval it is likely that regional or systemic temperatures will rise, causing a similar set of thermoregulatory processes to occur as described above. However, we can assume that whole body heating will affect a larger number of normal, systemic thermoregulatory responses much sooner than local heating, including distant as well as proximal blood vessels, engendering a large volume of blood movement in the body, and thus there could be significant differences in extent to which tumor blood vessels are affected by the two approaches. Overall, FR-WBH could affect parameters such as drug delivery, as presented here, but could also affect the efficacy of radiation therapy (through increased oxygenation) and adoptive or vaccine-based immunotherapy, (which requires vascular delivery of immune effector cells from distant parts of the body) or delivery of other therapeutic reagents such as nanoparticles; thus, additional mechanistic studies comparing various heating protocols are needed as soon as possible. In conclusion, these studies reveal a differential response of tumor vasculature to physiological thermoregulation that could, with additional study, be utilized to selectively enhance tumor targeting of therapies in cancer patients.
Conclusions
In this study we have observed an enhanced therapeutic effect when FR-WBH treatment is given prior to the administration of DOXIL, an effect that may be related to a tumor-selective, thermally induced increase in the number of perfused, dye-accessible blood vessels. This treatment is not associated with an increase in drug accumulation in normal tissues. These data support the use of FR-WBH as an adjuvant therapy, one which could facilitate the improved vascular delivery of molecules such therapeutic liposomes, antibodies and nanoparticles to tumor sites as well as adoptively transferred cells.
Acknowledgements
This work was supported by the National Institute of Health (CA71599 and CA094045) and the Roswell Park Cancer Institute Alliance Foundation. The authors would like to thank Rose Pitoniak and John Schueckler for their expert assistance with perfusion and tumor growth experiments and Jeanne Prendergast and Diane Thompson for their assistance in lab management throughout this project.
References
- van der Zee J, González González D, van Rhoon GC, van Dijk JDP, van Putten WLJ, Hart AAM. Comparision of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumors: A prospective, randomized, multicentre trial–Dutch Deep Hyperthermia Group. Lancet 2000; 355: 1119–1125
- Harima Y, Nagata K, Harima K, Ostapenko VV, Tanaka Y, Sawada S. A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma. Int J Hyperthermia 2001; 17: 97–105
- Sharma S, Sandhu AP, Patel FD, Ghoshal S, Gupta BD, Yadav NS. Side-effects of local hyperthermia: Results of a prospectively randomized clinical study. Int J Hyperthermia 1990; 6: 279–285
- Sneed PK, Stauffer PR, McDermott MW, Diederich CJ, Lamborn KR, Prados MD, Chang S, Weaver KA, Spry L, Malec MK, Lamb SA, Voss B, Davis RL, Wara WM, Larson DA, Phillips TL, Gutin PH. Survival benefit of hyperthermia in a prospectively randomized trial of brachythermy boost +/− hyperthermia for glioblastoma multiforme. J Radiat Oncol Biol Phys 1998; 40: 287–295
- Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 2005; 23: 3079–3085
- Thrall DE, Prescoot DM, Samulski TV, Rosner GL, Denman DL, Legorreta RL, Dodge RK, Page RL, Cline JM, Case BC, Evans SM, Oleson JR, Dewhirst MW. Radiation plus local hyperthermia and whole-body hyperthermia in canine sarcomas. Int J Oncol Biol Phys 1996; 34: 1087–1096
- Falk MH, Issels RD. Invited review: Hyperthermia in oncology. Int J Hyperthermia 2001; 17: 1–18
- Corry PM, Armour EP. The heat shock response: Role in radiation and cancer therapy. Int J Hyperthermia 2005; 21: 769–778
- Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia 2005; 21: 779–790
- Brady TJ, Gerweck LE. Relationship of changes in pH and energy status to hypoxic cell fraction and hyperthermia sensitivity. Int J Radiat Oncol Biol Phys 1990; 18: 1429–1435
- Engin K, Leeper DB, Thistlethwaite AJ, Tupchong L, McFarlane JD. Tumor extracellular pH as a prognostic factor in thermoradiotherapy. Int J Radiat Oncol Biol Phys 1994; 29: 125–132
- Reinhold HS, Endrich B. Tumor microcirculation as a target for hyperthermia. Int J Hyperthermia 1986; 2: 111–137
- Song CW. Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Res 1984; 44: 4721s–4730s
- Horsman MR, Overgaard J. Can mild hyperthermia improve oxygenation?. Int J Hyperthermia 1997; 13: 141–147
- Endrich B, Vaupel P. The role of microcirculation in the treatment of malignant tumors: Facts and fiction. Blood perfusion and Microenvironment of Human Tumors: Implications for Clinical Radiooncology, M Molls, P Vaupel. Springer Verlag, Berlin 2000; 19–40, (Brady LW, Heilmann H-P, Molls M, Series editors. Medical Radiology: Radiation Oncology)
- Song CW, Park HJ, Lee CK, Griffin R. Implication of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia 2005; 21: 761–767
- Shakil A, Osborn JL, Song CW. Changes in oxygenation status and blood flow in a rat tumor model by mild temperature hyperthermia. Int J Rad Oncol Biol Phys 1999; 43: 859–865
- Thrall DE, Larue SM, Pruitt AF, Case B, Dewhirst MW. Changes in tumor oxygenation during fractionated hyperthermia and radiation therapy in spontaneous canine sarcomas. Int J Hyperthermia 2006; 22: 365–373
- Kong G, Dewhirst MW. Hyperthermia and liposomes. Int J Hyperthermia 1999; 15: 345–370
- Kong G, Anyarambhatia G, Petros WP, Braun RD, Colvin OM, Needham D, Dewhirst MW. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: Importance of triggered drug release. Cancer Res 2000; 60: 6950–6957
- Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res 2001; 61: 3027–3032
- Ohtsubo T, Igawa H, Saito T, Matsumoto H, Park H, Song CW, Kano E, Saito H. Enhancement of cell killing by induction of apoptosis after treatment with mild hyperthermia at 42°C and cisplatin. Radiation Res 2001; 156: 103–109
- Kouloulias VE, Dardoufas CF, Kouvaris JR, Gennatas CS, Polyzos AK, Gogas HJ, Sandilos PH, Uzunoglu NK, Malas EG, Vlahos LJ. Liposomal doxorubicin in conjunction with reirradiation and local hyperthermia treatment in recurrent breast cancer: A Phase I/II trial. Clin Cancer Res 2002; 8: 374–382
- Jones EL, Samulski TV, Dewhirst MW, Alvarez-Secord A, Berchuck A, Clarke-Pearson D, Havrilesky LJ, Soper J, Prosnitz LR. A pilot Phase II trial of concurrent radiotherapy, chemotherapy, and hyperthermia for locally advanced cervical carcinoma. Cancer 2003; 98: 277–282
- Jones EL, Prosnitz LR, Dewhirst MW, Marcom PK, Hardenbergh PH, Marks LB, Brizel DM, Vujaskovic Z. Thermochemoradiotherapy improves oxygenation in locally advanced breast cancer. Clin. Cancer Res 2004; 10: 4287–4293
- Alvarez Secord A, Jones EL, Hahn CA, Petros WP, Yu D, Havrilesky LJ, Soper JT, Berchuck A, Spasojevic I, Clarke-Pearson DL, Prosnitz LR, Dewhirst MW. Phase I/II trial of intravenous DOXIL and whole abdomen hyperthermia in patients with refractory ovarian cancer. Int J Hyperthermia 2005; 21: 333–347
- Hahn CA, Jones EL, Blivin JL, Sanders LL, Yu D, Dewhirst MW, Alverez-Secord A, Prosnitz LR. Prospective assessment of quality of life in ovarian cancer patients receiving whole abdomen hyperthermia and liposomal doxorubicin. Int J Hyperthermia 2005; 21: 349–357
- Westermann AM, Jones EL, Schem BC, van der Steen-Banasik EM, Koper P, Mella O, Uitterhoeve AL, de Wit R, van der Velden J, Burger C, et al. First results of triple-modality treatment combining radiotherapy, chemotherapy, and hyperthermia for the treatment of patients with stage IIB, III, and IVA cervical carcinoma. Cancer 2005; 104: 763–770
- Rowe-Horwege RW. Hyperthermia, systemic encyclopedia of medical devices and instrumentation, JG Webster. John Wiley and Sons, Inc. 2006
- Bull JM, Cronau LH, Newman BM, Jabboury K, Allen SJ, Ohno S, Smith T, Tonnesen A.S. Chemotherapy resistant sarcoma treated with whole body hyperthermia (WBH) combined with 1-2-bis(2-chloroethyl)-1-nitrosourea (BCNU). Int J Hyperthermia 1992; 8: 297–304
- Robins HI, Cohen JD, Schmitt CL, Tutsch KD, Feierabend C, Arzoomanian RZ, Alberti D, D’Oleire F, Longon W, Heiss C, et al. Phase I clinical trial of carboplatin at 41.8°C whole-body hyperthermia in cancer patients. J Clin Oncol 1993; 11: 1787–1794
- Ohno S, Strebel FR, Stephens LC, Siddik ZH, Baba H, Makino M, Khokhar AR, Bull JM. Haematological toxicity of carboplatin and cisplatin combined with whole body hyperthermia in rats. Brit J Cancer 1993; 68: 469–474
- Sakaguchi Y, Makino M, Kaneko T, Stephens LC, Strebel FR, Danhauser LL, Jenkins GN, Bull JM. Therapeutic efficacy of long duration-low temperature whole body hyperthermia when combined with tumor necrosis factor and carboplatin in rats. Cancer Res 1994; 54: 2223–2227
- Matsuda H, Strebel FR, Kaneko T, Danhauser LL, Jenkins GN, Toyota N, Bull JM. Long duration mild whole-body hyperthermia of up to 12 hours in rats: Feasibility, and efficacy on primary tumour and axillary lymph node metastases of a mammary adenocarcinoma: Implications for adjuvant therapy. Int J Hyperthermia 1997; 13: 89–98
- Hughes CS, Repasky EA, Bankert RB, Johnson RJR, Subjeck JR. Effects of hyperthermia on spectrin expression patterns of murine lymphocytes. Rad Res 1987; 112: 116–123
- Di YP, Repasky ER, Subjeck JR. Distribution of HPS70, protein kinase C, and spectrin is altered in lymphocytes during a fever-like hyperthermia exposure. J Cell Physiol 1997; 172: 44–54
- Burd R, Dziedzic TS, Xu Y, Caligiuri MA, Subjeck JA, Repasky EA. Tumor cell apoptosis, lymphocyte recruitment and tumor vascular changes are induced by low temperature, long duration (fever-like) whole body hyperthermia. J Cell Physiol 1998; 177: 137–147
- Repasky EA, Tims E, Prichard M, Burd R. Characterization of mild whole-body hyperthermia protocols using human breast, ovarian, and colon tumors grown in severe combined immunodeficient mice. Infect Dis Obstet Gynecol 1999; 7: 91–97
- Ostberg JR, Ertel BR, Lanphere JA. An important role for granulocytes in the thermal regulation of colon tumor growth. Immunol Invest 2005; 34: 259–272
- Wang XY, Ostberg JR, Repasky EA. Effect of fever-like whole-body hyperthermia on lymphocyte spectrin distribution, protein kinase C activity, and uropod formation. J Immunol 1999; 162: 3378–3387
- Ostberg JR, Repasky ER. Comparison of the effects of two different whole body hyperthermia protocols on the distribution of murine leukocyte populations. Int J Hyperthermia 2000; 16: 29–43
- Ostberg JR, Kaplan KC, Repasky ER. Induction of stress proteins in a panel of mouse tissue by fever-range whole body hyperthermia. Int J Hyperthermia 2002; 18: 551–562
- Pritchard MT, Ostberg JR, Evans SS, Burd R, Kraybill W, Bull JM, Repasky EA. Protocols for simulating the thermal component of fever: Preclinical and clinical experience. Methods 2004; 32: 54–62
- Wang WC, Goldman LM, Schleider DM, Subjeck JR, Repasky EA, Evans SS. Fever-range hyperthermia enhances L-selectin-dependent adhesion of lymphocytes to vascular endothelium. J Immunol 1998; 160: 961–969
- Evans SS, Bain D, Wang WC. Fever-range hyperthermia stimulates α4B7 integrin-dependent lymphocyte-endothelial adhesion. Int J Hyperthermia 2000; 16: 45–59
- Evans SS, Wang WC, Bain MD, Burd R, Ostberg JR, Repasky EA. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood 2001; 97: 2727–2733
- Chen Q, Fisher DT, Clancy KA, Gauguet JM, Wang WC, Unger E, Rose-John S, von Adrian UH, Baumann H, Evans SS. Fever-range hyperthermia promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanim. Nat Immunol 2006; 7: 1299–1308
- Ostberg JR, Taylor SL, Baumann H, Repasky EA. Regulatory effects of fever-range whole body hyperthermia on the LPS-induced acute inflammatory response. J Leuk Biol 2000; 68: 815–820
- Ostberg JR, Gellin C, Patel R, Repasky ER. Regulatory potential of fever-range whole body hyperthermia on Langerhans cells and lymphocytes in an antigen-dependant cellular immune response. J Immunol 2001; 167: 2666–2670
- Ostberg JM, Repasky EA. Emerging evidence indicates that physiologically relevant thermal stress regulates dendritic cell function. Cancer Immunol Immuntherapy 2006; 55: 92–95
- Ostberg JR, Dayanc BE, Yuan M, Oflazoglu E, Repasky ER. Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent upon NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells. J Leukoc Biol 2007. 82, (In Press)
- Naka T, Hylander BL, Rustum YM, Widmer MB, Repasky EA. Effects of tumor necrosis factor-related Apoptosis-inducing ligand alone and in combination with chemotherapeutic agents on patients’ colon tumors grow in SCID mice. Cancer Res 2002; 62: 5800–5806
- Trotter MJ, Chaplin DJ, Olive PL. Use of carbocyanine dye as a maker of functional vasculature in murine tumours. Brit J Cancer 1989; 59: 706–709
- Robert J. Extraction of anthrocyclins from biological fluids for HPLC evaluation. Clin Pharm Ther 1980; 22: 234–252
- Guyton AC, Hall JE. Body temperature, temperature regulation, and fever, AC Guyton, JE Hall. Elsevier, Philadelphia, PA 2006; 889–901
- Boulant JA. Hypothalamic neurons. Mechanisms of sensitivity to temperature. Ann NY Acad Sci 1998; 856: 108
- Romanowsky AA. Thermoregulation: Some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Research 2007; 292: R37–R46
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nature Rev Cancer 2006; 6: 583–592
- Dvorak HF. How tumors make bad blood vessels. Amer Soc Invest Path 2003; 162: 1747–1557
- Jain RK. Barriers to drug delivery in solid tumors. Scientific American 1994; 271: 58–65
- Gabizon A, Shiota R, Papahadjopoulos D. Pharmacokinetics and tissue distribution of doxorubicin encapsulated in stable liposomes with long circulation times. J Natl Cancer Inst 1989; 81: 1484–1488
- Gabizon A, Martin F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin: Rationale for use in solid tumors. Drugs 1997; 54(Suppl 4)15–21
- Huang SK, Stauffer PR, Hong K, Guo JWH, Phillips TL, Huang A, Papahadjopoulos D. Liposomes and hyperthermia in mice: Increased tumor uptake and therapeutic efficacy of doxorubicin in sterically stabilized liposomes. Cancer Res 1994; 54: 2186–2191
- Vaage J, Barbera-Guillem E, Abra R, Huang A, Working P. Tissue distribution and therapeutic effect of intravenous free or encapsulated liposomal doxorubicin on human prostate carcinoma xenografts. Cancer 1994; 73: 1478–1484
- Vaage J, Donovan D, Loftus T, Working P. Tumor uptake of doxorubicin in polyethylene glycol-coated liposomes and therapeutic effect against a xenografted human pancreatic carcinoma. Brit J Cancer 1997; 75: 482–486
- Ranson M, Carmichael J, O’Bryne K, Stewart S, Smith D, Howell A. Treatment of advanced breast cancer with sterically stabilized liposomal doxorubicin: results of a multicenter phase II trial. J Clin Oncol 1997; 15: 3185–3191