Abstract
Purpose: The aim of this study was to investigate the anti-cancer effect of the novel vascular disrupting agent (VDA), combretastatin-A1-disodium-phosphate (OXi4503), when combined with mild hyperthermia and/or radiation.
Materials and methods: A C3H mammary carcinoma was grown subcutaneously in the rear right foot of female CDF1 mice, and treated when a volume of 200 mm3 was reached. OXi4503 was administered intra-peritoneally at variable doses. Hyperthermia was administered locally to the tumour-bearing foot using a thermostat-controlled water bath. Radiation treatment was performed locally using a conventional X-ray machine. Tumour response was assessed with either a tumour growth time or a tumour control assay.
Results: The optimal delay between administration of 50 mg/kg of OXi4503 and hyperthermia was found to be 3 hours. The linear relationship between tumour growth time (TGT) and heating time at a specific temperature resulted in slope values between -0.003 days/min and 0.09 days/min at temperatures between 40°C and 42.5°C. When combined with OXi4503 this was significantly increased to 0.008 days/min and 0.03 days/min at temperatures between 39.5°C and 41°C, respectively. Above 41°C, combined treatment did not result in significantly greater slope values. The radiation dose required to control 50% of the tumours (TCD50) was 52 Gy. Combining radiation with either heat treatment at 41.5°C for 1 hour or OXi4503 reduced the TCD50 to 47 Gy and 41 Gy, respectively. Combining radiation with heat and OXi4503 further reduced the TCD50 to 37 Gy.
Conclusions: OXi4503 is a highly potent VDA, which is capable of significantly enhancing the anti-cancer effect of mild hyperthermia. Mild temperature thermoradiosensitization was also enhanced.
Introduction
Tumour vasculature has several significant differences from normal vasculature. It is persistently angiogenic, chaotic, displays extensive branching and shunts and frequent transient stasis Citation[1]. The vessels lack many of the ‘protective’ mechanisms characterizing normal vessels yielding thin, dilated and consequently leaky walls Citation[2], Citation[3]. As a consequence most solid tumours contain significant fractions of hypoxic and acidic regions Citation[4], Citation[5]. Viable cells may still reside in these regions and are known to display increased resistance to conventional anticancer therapies especially radiation and chemotherapy Citation[3], Citation[6].
It has long been recognized that tumour vasculature is an attractive target for anticancer therapy Citation[7–9]. Small molecule vascular disrupting agents (VDAs), which utilize the physiological specifics of tumour vasculature have been studied extensively Citation[10–12] during the past two and a half decades. Predominant among these molecules are the so-called combretastatins – a family of drugs originally isolated from the African bush willow combretum caffrum Citation[13]. The current leading combretastatin VDA is the tubulin binding inhibitor combretastatin-A4-disodium-phosphate (CA4DP), which induces significant vascular damage at doses far below the maximum tolerated dose (MTD) Citation[14]. Administration of CA4DP causes almost complete depletion of tumour blood flow in a matter of minutes, and the stasis is maintained for approximately 24 hours depending on dose Citation[15].
The main mechanism of cell killing of most VDAs is secondary, since the vascular shutdown causes haemorrhagic necrosis of cells suffering fatal oxygen and nutrient deprivation. The cells most likely to suffer from this effect first are the already hypoxic cells often residing in the central tumour regions. Additional pre-clinical studies suggest that a compartment of viable cells typically persists in the tumour rim adjacent to normal tissu Citation[16], Citation[17]. In consequence, although capable of inducing massive tumour cell killing, the actual effect of VDAs on tumour growth time remains modest Citation[14], Citation[18], Citation[19]. Hence, VDAs are most often applied in combination with other anti-cancer therapies due to the potentially enhancing effect. Indeed, combining the two major cancer therapies – radiation and chemotherapy – with a VDA has revealed enhanced anti-cancer efficacy Citation[20].
Contrary to the case of radio- and chemotherapy cancer cells in hypoxic regions are known to exhibit increased sensitivity to the cytotoxic effect of heat Citation[21]. The increased sensitivity is a consequence of the associated effects of hypoxia, for example extracellular acidosis, rather than hypoxia per se Citation[22]. Hyperthermia is, however, only of clinical relevance when combined with other therapies Citation[23]. Traditionally, hyperthermia has been utilized to enhance the effect of radiation. Although different candidates for the mechanism of enhancement have been proposed Citation[24], Citation[25], it is irrefutable that radiation, under proper circumstances, can be significantly enhanced when combined with hyperthermia Citation[25]. VDAs are attractive as enhancers of hyperthermia since the induction of hypoxia increases the number of thermosensitive cells Citation[21], Citation[22]. Furthermore VDAs temporarily shut down blood flow which is the primary mechanism for tissue cooling Citation[26].
Recently a new and more potent combretastatin derivative, combretastatin-A1-disodium-phosphate (OXi4503), has become available Citation[27]. OXi4503 resembles CA4DP in many respects also with regard to function Citation[28]. However, comparable levels of vascular shutdown are achieved at substantially lower doses of OXi4503 Citation[29] and stasis is maintained for longer time Citation[30]. Furthermore OXi4503 leaves a smaller rim of viable cells than in the case of CA4DP Citation[29]. The apparent damage inflicted on the well perfused tumour compartment suggests a direct mode of cell killing in addition to the secondary effect resulting from the cessation of blood flow. As a whole, OXi4503 has been demonstrated to be a more potent VDA than its close analogue, CA4DP. A detailed pharmacokinetic comparison between these two VDAs has not been able to identify a specific origin of difference in anti-cancer effect Citation[28]. However, it has tentatively been proposed that the in vivo conversion of OXi4503 into a highly reactive o-quinone Citation[28], may account for the direct cell killing.
Because of this increased anti-cancer effect OXi4503 has been proposed as a monotherapy when administered repeatedly Citation[30]. However, combining OXi4503 with other therapies holds greater potential. In this study we investigate whether the anti-cancer effect of mild hyperthermia and/or radiation is enhanced by OXi4503 as is known from its close analogue CA4DP Citation[31].
Materials and methods
Tumour model
All experiments were performed using our well-established C3H mammary carcinoma model which has been described in detail previously Citation[32]. Tumours were grown in the rear right foot of 10–14-week-old female CDF1 mice. Inoculation was performed by a subcutaneous injection of approximately 5–10 µl of minced tumour material between the third and fourth toes. Tumours were treated when they reached a volume of 200 mm3 approximately 2–3 weeks after inoculation. The tumour shape was assumed to be ellipsoidal and volume calculated as: V = d1d2d3π/6, where the d-values are the orthogonal ellipsoid diameters.
Ethics
All animal studies were approved by national authorities and in compliance with European standards. The mild temperatures applied required no anaesthetics. Following treatment animal health status was observed daily.
Combretastatin-A1-disodium-phosphate
OXi4503 was supplied in solid form by Oxigene Inc. (Watertown, MA, USA) and kept frozen in opaque containers. The drug was dissolved in isotonic NaCl and used within 24 hours. Solutions were prepared at 0.5 mg/ml, 1.25 mg/ml, 2.5 mg/ml and 5.0 mg/ml. The solution was administered intra-peritoneally as a single injection at a relative volume of 0.02 ml/g resulting in a final dose of 10 mg/kg, 25 mg/kg, 50 mg/kg and 100 mg/kg, respectively.
Hyperthermia
Heat treatment was performed locally by submerging the tumour-bearing foot in a circulating water bath (model TE 623, HETO, Birkerød, Denmark). The non-anaesthetized animal was restrained in a specially constructed Perspex jig Citation[33], with the tumour-bearing foot loosely attached to the jig. The jig was placed on a perspex plate covering the water surface and the tumour-bearing foot submerged through a hole in the plate. Based on data from previous studies the water temperature was set 0.2°C above the desired temperature in order to maintain the target temperature in the tumour centre.
Radiation
Radiation treatment was given as a single local dose using a conventional X-ray machine (240 kV, 15 mA, 2 mm Al filter, 1.1 mm Cu half-value layer, dose rate: 2.3 Gy/min; Philips Medical Systems, Best, The Netherlands). Before radiation the non-anesthetized animal was placed in a jig similar to that applied for heat treatment, and the tumour-bearing foot exposed and fixed in a similar manner. The tumour-bearing foot was placed in a thermostat-controlled water bath set at 25°C in order to promote a homogeneous radiation dose, and irradiated. A 1 cm thick lead shield was used to protect the rest of the animal.
Treatment response
Hyperthermia and VDA-treatment was assessed using a tumour growth time (TGT) assay. Following treatment of the 200 mm3 sized tumours, tumour volume was measured 5 times weekly (Monday to Friday), and the day at which 5 times the treatment volume (TGT5) was reached calculated. Radiation treatment was assessed by a local tumour control assay. Treated animals were observed once a week for 90 days following treatment. After the 90-day period the percentage of animals showing local tumour control was recorded for each treatment group and the radiation dose required to produce tumour control in 50% of the animals (TCD50) calculated.
Statistics
Interaction between heat treatment and OXi4503 was assessed using analysis of variance in a standard factor analysis. Heat dose response data was evaluated using standard linear regression. Slopes were compared using a Students t-test. The OXi4503 dose response was fitted using non-linear regression to a 3-parameter Gompertz function:where d is the dose, a is the asymptotic TGT value and b and c are growth and shape related parameters. Radiation dose response data was fitted using a generalized linear model with the logit link function, and the obtained TCD50 values compared across experiments using a χ2-test. All calculations were performed using Stata (Stata Corp. College Station, Texas, USA) except the evaluation of radiation dose response which was performed using in-house written software. A significance level of 5% was employed throughout.
Results
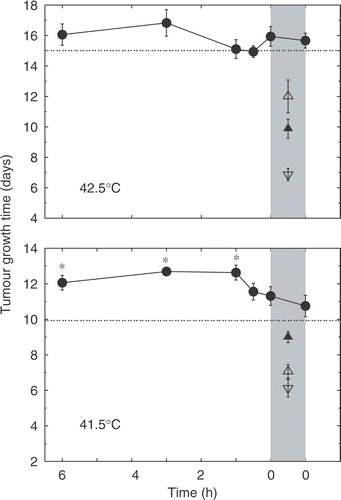
Time course assays were performed to establish the optimal time interval between administration of OXi4503 and heat treatment. Heat treatment was performed as a 1-hour session at either 41.5°C or 42.5°C (results shown in ). OXi4503 was administered as a single intra-peritoneal injection at a dose of 50 mg/kg at various intervals before or immediately after heat treatment. Heat treatment at 41.5°C showed only a modest increase in TGT with respect to non-treated tumours, whereas treatment with 50 mg/kg of OXi4503 alone showed a significant increase in TGT. When OXi4503 was administered between 1 and 6 hours prior to heat treatment the resulting TGT statistically significantly exceeded the additive level, with a maximal effect at the 3 hour interval; the 6, 3 and 1 hour intervals, however, were not significantly different from each other. At shorter time intervals or when OXi4503 was administered immediately after heat treatment, the TGT-values decreased with respect to the longer time intervals and no significant interaction was observed. Heat treatment alone at 42.5°C resulted in a marked increase in TGT exceeding the TGT resulting from treatment with OXi4503 alone. Combined treatment revealed, in agreement with the results for 41.5°C, that the optimal interval between OXi4503 administration and heat treatment was 3 hours. However, no significant interaction beyond the additive level was observed at any of the applied intervals.
Figure 1. Time course analysis showing the relationship between TGT5 and the delay between administration of OXi4503 and hyperthermia treatment. Heat treatment consisted of a 1-hour session at either 41.5°C or 42.5°C. OXi4503 was administered intra-perineally at a dose of 50 mg/kg at variable intervals with respect to heat treatment. Treatment groups contained an average of eight animals. Control group: ▽; OXi4503 alone: ▴; heat treatment alone: △; OXi4503 combined with heat: ○; expected additive effect of heat alone and OXi4503 alone: ·. Heating period is indicated by vertical greyed area and asterisks indicate TGT5 significantly different from additive level. Data points are indicated as mean ± standard error.
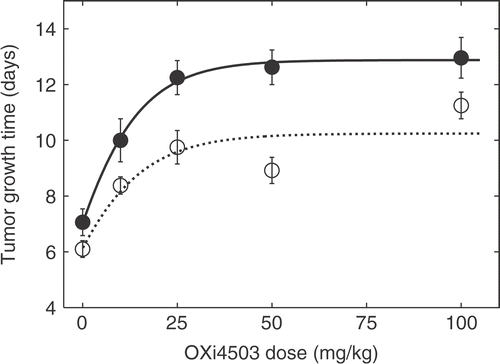
Tumour response to different doses of OXi4503 (10 mg/kg to 100 mg/kg) administered intra-peritoneally, either alone or combined with heat treatment, initiated 3 hours after the OXI4503 injection, is shown in . Heat treatment was performed as a 1 hour session at 41.5°C. A significant growth delay was observed at the lowest applied dose, both for OXi4503 alone and the combination treatment. At higher doses a plateau was reached corresponding to a ‘maximal’ TGT5-value of 10.2 days for OXi4503 alone and 12.9 days for the combination treatment.
Figure 2. Dose response assay for the effect of OXi4503 dose with and without subsequent heat treatment on TGT5. Heat treatment was performed as a 1-hour session at 41.5°C 3 hours after injection of OXi4503. Treatment groups contained an average of nine animals. OXi4503 alone: ○ OXi4503 combined with heat: ○; fitted curves are represented by solid and dashed lines. Data points are indicated as mean ± standard error.
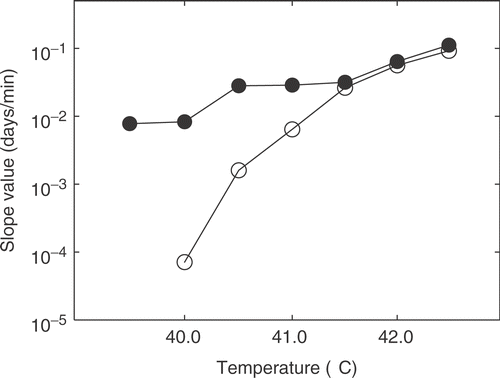
The linear relationship between heating time and TGT5 was assessed at different temperatures. This was done for temperatures ranging from 39.5°C to 42.5°C both using heat treatment alone and heat treatment 3 hours after an initial injection of 50 mg/kg of OXi4503. These results are shown in . Slope values are depicted and compared in . In the case of 39.5°C only a combined treatment was assessed since there is at best a flat response to heat treatment alone. Slope values ranging from −0.003 days/min to 0.09 days/min were obtained at temperatures ranging from 40°C to 42.5°C, respectively. Below 41°C, the slope values for heat treatment alone were not significantly different from 0. Combination treatment resulted in slope values between 0.008 days/min and 0.1 days/min for temperatures ranging from 39.5°C to 42.5°C, respectively. In the case of the combined treatments all slope values were significantly greater than 0. At temperatures below 41.5°C the slope values obtained for the combination were significantly greater than those obtained for heat treatment alone at the corresponding temperatures. At 41.5°C and above, the slope values were not significantly different and resulting response curves were parallel.
Figure 3. Heat dose response assay of the effect of temperature and heating time on TGT5 with and without injection of 50 mg/kg OXi4503 3 hours prior to heating. Treatment groups contained an average of ten animals. Heat alone: ○; heat combined with OXi4503: ○; heat combined with OXi4503 at 39.5°C: ▴; fitted curves indicated by lines. Data points are presented as mean ± standard error.
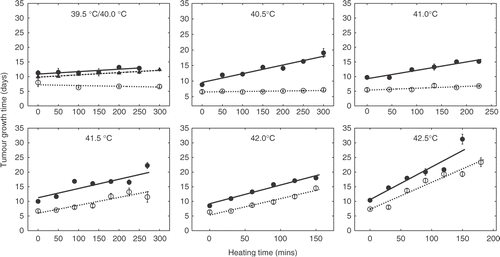
Figure 4. Relationship between applied temperature and fitted slope value (days/min) for TGT. Values were obtained from the data of . Heat alone: ○; heat combined with OXi4503: ○. The slope value obtained for heat alone treatment at 40°C was negative. In this case the plotted slope value has been added 1 standard error in order to obtain a positive value.
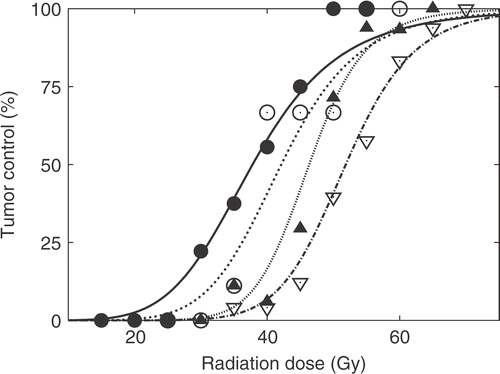
Hyperthermia-mediated modification of the radiation response when combining with OXi4503 is shown in . The applied endpoint was the radiation dose required to control 50% of the tumours (TCD50). Treating with radiation alone resulted in a TCD50-value (with 95% confidence interval) of 52 Gy (51 : 54). When radiation was combined with heat treatment at 41.5°C for 1 hour initated 4 hours after irradiating, this resulted in a small yet significant decrease (p = 0.046) in the TCD50 value to 47 Gy (45 : 50). Combining radiation with an injection of 50 mg/kg OXi4503 1 hour after radiation significantly decreased the TCD50 value to 41 Gy (38 : 45) (p < 0.05). Finally, when combining the three modalities in a schedule where OXi4503 was administered 1 hour after radiation, and heat treatment (41.5°C for 1 hour) 3 hours later a TCD50-value of 37 Gy (34 : 41) was obtained. Although significantly different from radiation combined with heat alone (p < 0.05) this was not significantly different from radiation combined with OXi4503 alone (p = 0.18).
Figure 5. The effect of heat treatment on the radiation dose when combined with OXi4503. Tumour control is plotted as a function of radiation dose. Radiation treatment was performed as a single local dose to the tumour-bearing foot. OXi4503 was administered 1 hour after completing the radiation. Heat treatment was administered at 41.5°C for 60 min and performed 3 hours after the injection of OXi4503. Radiation alone: ▽; radiation with heat: ▴; radiation with OXi4503 alone: ○; radiation with OXi4503 and heat: ○.
Discussion
Several studies have demonstrated that VDAs can be used to enhance the anti-cancer effect of hyperthermia and radiatio Citation[20], Citation[34]. A common feature is that the VDA-based enhancement of these therapies is highly dependent on timing and schedule for each separate treatment. Blood flow is one of the primary mechanisms for dissipating heat. Furthermore, in vitro studies have demonstrated that hypoxic and acidic cells are more sensitive to hyperthermia Citation[22]. Hence, it seems plausible that the enhancement of heat by VDAs can be attributed to increased heat delivery caused by reduced cooling, as well as an increase associated with reduced oxygen and nutrient delivery in the number of cells which are sensitive to the cytotoxic effect of heat. Indeed, several studies have shown that in order to obtain maximal enhancement the VDA must be administered within a few hours prior to the heat treatment Citation[20] correlating well with the timescale in which vascular shutdown is most prominent. Such an enhancement of hyperthermia has been demonstrated for a number of different VDAs, including the cytokine inducing small molecule VDAs, viz. flavone acidic acid (FAA) Citation[35] and 5,6 dimethylxantenone-4-acidic acid (DMXAA) Citation[36], as well as the tubulin binding inhibitor CA4DP Citation[31], Citation[37].
A number of studies have shown that an enhanced anti-tumour response can be obtained when VDAs and radiation are combined Citation[20]. The importance of timing and sequence between the two modalities has been investigated when using the VDAs arsenic trioxide Citation[38], DM Citation[39], Citation[40], CA4DP Citation[41] and ZD6126 Citation[42–44]. Generally, the greatest benefit was found when the VDA was administered shortly after irradiation. The mechanism for this enhancement is not clear. Radiation predominantly targets dividing cells, whereas VDAs will most probably inflict damage to already poorly oxygenated and consequently slowly dividing or quiescent cells. It seems plausible that the enhanced effect is merely facilitated by the two different therapies targeting two distinct cell populations, but the possibility that some mode of interaction occurs between the two modalities cannot be ruled out Citation[20].
Finally, including VDAs into the already well-established combination of hyperthermia and radiation has been investigated in preclinical studies. In general, the timing issues discussed above can be directly extrapolated to this trimodality scheme, so that radiation is followed by administration of a VDA followed by heat treatment. This approach has been shown to have increased anti-cancer effect using FAA Citation[35], DMXAA Citation[45] and CA4DP Citation[31].
The aim of the present study was to assess the new and more potent combretastatin derivative OXi4503 in combination with hyperthermia and radiation. Comparisons by dose between CA4DP and OXi4503 have shown that OXi4503 is approximately four times as effective as CA4DP in inducing vascular damage Citation[29]. Furthermore, it was shown that OXi4503 induces actual cell killing exceeding that induced by CA4DP by an order of magnitude, even when administered at a dose 1/10 of that of CA4DP Citation[29].
The interaction between OXi4503 and temperature was revealed to be highly dependent on the interval between injection and heat treatment (, ). When heat treatment was administered as 41.5°C for 1 hour a significant increased effect was observed when OXi4503 was injected between 1 and 6 hours before heat treatment with a maximum enhancement at the 3 hour interval. At shorter intervals the enhancement effect disappeared. However, at 42.5°C no significant enhancement was observed at any interval. The observed optimal interval agrees qualitatively with earlier results Citation[30] which show that OXi4503-induced reduction in tumour blood flow occurs on the scale of a few hours.
Tumour response to CA4DP-dose when assessed by growth delay assays show only modest effects Citation[31]. In contrast to this result we found that even a dose of 10 mg/kg OXi4503 was sufficient to induce a significant growth delay (see ). Above 25 mg/kg the response is essentially flat within the range of applied doses. We tentatively attribute this to an insignificant increase in damage to the compartment of viable and dividing tumour cells with increasing doses of OXi4503, while the compartment of poorly oxygenated and slowly dividing tumour cells have been almost completely eradicated even at the lower dose levels. The fact that statistically significant growth delays are achieved using OXi4503 as a monotherapy could be attributed to the primary kill of tumour cells that has previously been hypothesized Citation[28]. The combination of OXi4503 and hyperthermia results in a greater intrinsic growth rate and a greater value of the plateau, however, the dose response still fits the Gompertz-growth model very well. Although an OXi4503 dose of 50 mg/kg was not significantly more effective than 25 mg/kg, we still chose to apply the former in subsequent studies. This was motivated both by previous experience and because the dose of 50 mg/kg was more within the range of doses applied in other studies using OXi4503.
At moderate temperatures (40.5°C – 42.5°C) the enhancement of hyperthermia by CA4DP appears to be independent of applied temperature Citation[31]. This is contrasted by our results for OXi4503 (). We found that significant enhancement was observed only at temperatures where the heat treatment alone resulted in flat response curves (<41.5°C). Although CA4DP was administered at half the OXi4503 dose, and 0.5 hours before heat treatment Citation[31], while OXi4503 was administered 3 hours before heating, it appears that thermo-enhancement by OXi4503 is predominant at low temperatures whereas thermotherapy combined with CA4DP results in an enhancement over the entire probed temperature scale. A possible explanation for this different interaction with thermotherapy for the two VDAs could be the greater anticancer effect of OXi4503. Growth delay is mainly achieved by causing significant cell death in the proliferating tumour compartments, whereas cell killing in the quiescent tumour regions has little effect on TGT Citation[46]. With CA4DP, sustantially larger doses (in the range of 250 mg/kg Citation[31]) compared to OXi4503 are required to produce a significant growth delay. As previously mentioned, OXi4503 alone causes a significant growth delay. It appears that a large fraction of the cells which would only be sensitized by CA4DP are killed by OXi4503 alone. A remaining fraction is however ‘only’ sensitized, and subsequently killed by mild hyperthermia (<41.5°C). At temperatures which are sufficient to cause a growth delay alone, these cells appear to be killed by the effect of heat alone. In consequence, it appears that in the case of OXi4503 and hyperthermia above 41°C a regimen of cells receive a lethal treatment twice – which obviously does not reflect on TGT. At the CA4DP-doses (25 mg/kg) previously applied in similar studies Citation[31] no significant growth delay was achieved using CA4DP alone, leaving a greater portion of tumour cells to be killed by the combined effect of heat and CA4DP. Hence, we propose that an effect similar to that of OXi4503 should occur for CA4DP, only at a higher temperature.
Studies investigating the interaction between radiation, mild hyperthermia and a VDA have previously been performed for the same tumour model using either CA4DP Citation[31], FAA Citation[35] or DMXAA Citation[45]. In those studies, combining radiation with either FAA or DMXAA produced TCD50-values similar to what we have reported for OXi4503 in identical timing schemes. When combining CA4DP with radiation – albeit for a slightly different yet for CA4DP optimal timing scheme – only a modest decrease in TCD50 was obtained (48 Gy (46 : 51)). However, combining either DMXAA or FAA with both radiation and heat in identical schedules as that presented here, produced TCD50 values of 30 Gy (26 : 35) and 28 Gy (22 : 35), respectively. In the case of CA4DP, using an interval of 30 min between radiation and CA4DP and an additional delay of 30 min before heat treatment (41.5°C/60 min), the TCD50 was found to be 33 Gy (31 : 37). These results are somewhat different (although not statistically significant) from what has been presented here, where the nominal reduction in TCD50 for radiation with both OXi4503 and heat equals the summed reduction for radiation with OXi4503 and radiation with heat. Although no conclusive statement can be made from the current results, this suggests that the direct cell killing apparently associated with OXi4503 plays a role in this case as well, so that a regimen of cells receive a lethal treatment twice. Further studies to elucidate the precise mode of action of OXi4503 are highly warranted.
In conclusion, it has been demonstrated that OXi4503 not only shows anti-tumour activity alone, but is effective at enhancing tumour response to heat with or without radiation. It is also currently undergoing preliminary clinical evaluation Citation[47]. The combination of hyperthermia and radiation has already undergone extensive clinical evaluation and shown to be an effective therapy Citation[25]. However, many studies failed to show any benefit of this heat and radiation combination, which is generally believed to be the result of a failure to adequately heat tumours to effective temperatures; while mild hyperthermia temperatures of around 40.5°C–41.5°C are easier to obtain clinically, such temperatures are not very efficient at enhancing radiation damage and temperatures of around 42.5°C–43°C are required Citation[25]. Our present study clearly demonstrates that when OXi4503 is included in the treatment scheme, mild hyperthermia temperatures produce effects comparable to those obtained with the ‘more clinically relevant’ temperatures. This suggests that clinical treatments combining hyperthermia and radiotherapy could benefit from including a VDA in the treatment schedule Citation[25].
Acknowledgements
Ms Inger Marie Horsman and Dorthe Grand are acknowledged for expert technical assistance. This study was made possible by funding from the Danish Cancer Society and the Danish Cancer Research Foundation.
References
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res 1989; 49: 6449–6465
- Gillies RJ, Schornack PA, Secomb TW, Raghunand N. Causes and effects of heterogeneous perfusion in tumors. Neoplasia 1999; 1: 197–207
- Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol 2004; 14: 198–206
- Moulder JE, Rockwell S. Tumor hypoxia: Its impact on cancer therapy. Cancer Metast Rev 1987; 5: 313–341
- Vaupel P, Harrison L. Tumor hypoxia: Causative factors, compensatory mechanisms, and cellular response. Oncol 2004; 9: 4–9
- Vaupel P, Thews O, Hoeckel M. Treatment resistance of solid tumors: Role of hypoxia and anemia. Med Oncol 2001; 18: S243–259
- Chaplin DJ, Dougherty GJ. Tumour vasculature as a target for cancer therapy. Br J Cancer 1999; 80: S57–64
- Denekamp J. Vascular attack as a therapeutic strategy for cancer. Cancer Metast Rev 1990; 9: 267–282
- Siemann DW, Warrington KH, Horsman MR. Targeting tumor blood vessels: An adjuvant strategy for radiation therapy. Radiother Oncol 2000; 57: 5–12
- Gaya AM, Rustin GJ. Vascular disrupting agents: A new class of drug in cancer therapy. Clin Oncol (Royal College of Radiologists (Great Britain)) 2005; 17: 277–290
- Thorpe PE. Vascular targeting agents as cancer therapeutics. Clin Cancer Res 2004; 10: 415–427
- Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer 2005; 5: 423–435
- Pettit GR, Cragg GM, Singh SB. Antineoplastic agents, 122. Constituents of Combretum caffrum. J Nat Prod 1987; 50: 386–391
- Chaplin DJ, Pettit GR, Hill SA. Anti-vascular approaches to solid tumour therapy: Evaluation of combretastatin A4 phosphate. Anticancer Res 1999; 19: 189–195
- Tozer GM, Prise VE, Wilson J, Cemazar M, Shan S, Dewhirst MW, et al. Mechanisms associated with tumor vascular shut-down induced by combretastatin A-4 phosphate: Entravital microscopy and measurement of vascular permeability. Cancer Res 2001; 61: 6413–6422
- Dark GG, Hill SA, Prise VE, Tozer GM, Pettit GR, Chaplin DJ. Combretastatin A-4, an agent that displays potent and selective toxicity toward tumor vasculature. Cancer Res 1997; 57: 1829–1834
- Li L, Rojiani A, Siemann DW. Targeting the tumor vasculature with combretastatin A-4 disodium phosphate: Effects on radiation therapy. Int J Rad Oncol Biol Phys 1998; 42: 899–903
- Chaplin DJ, Hill SA. The development of combretastatin A4 phosphate as a vascular targeting agent. Int J Rad Oncol Biol Phys 2002; 54: 1491–1496
- Horsman MR, Ehrnrooth E, Ladekarl M, Overgaard J. The effect of combretastatin A-4 disodium phosphate in a C3H mouse mammary carcinoma and a variety of murine spontaneous tumors. Int J Rad Oncol Biol Phys 1998; 42: 895–898
- Horsman MR, Siemann DW. Pathophysiologic effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Res 2006; 66: 11520–11539
- Overgaard J, Nielsen OS. The role of tissue environmental factors on the kinetics and morphology of tumor cells exposed to hyperthermia. Ann N Y Acad Sci 1980; 335: 254–280
- Overgaard J, Bichel P. The influence of hypoxia and acidity on the hyperthermic response of malignant cells in vitro. Radiol 1977; 123: 511–514
- Dahl O, Overgaard J. Hyperthermia. Oxford Textbook of Oncology, R Souhami, I Tannock, P Hohenberger, J Horiot. Oxford University Press, Oxford 2001; 511–525
- Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia 2005; 21: 779–790
- Horsman MR. Overgaard J. Hyperthermia: A potent enhancer of Radiotherapy. Clin Oncol 2007, In press
- Horsman MR. Tissue physiology and the response to heat. Int J Hyperthermia 2006; 22: 197–203
- Pettit GR, Lippert JW III,. Antineoplastic agents 429. Syntheses of the combretastatin A-1 and combretastatin B-1 prodrugs. Anticancer Drug Des 2000; 15: 203–216
- Kirwan IG, Loadman PM, Swaine DJ, Anthoney DA, Pettit GR, Lippert JW III,, et al. Comparative preclinical pharmacokinetic and metabolic studies of the combretastatin prodrugs combretastatin A4 phosphate and A1 phosphate. Clin Cancer Res 2004; 10: 1446–1453
- Salmon HW, Siemann DW. Effect of the second-generation vascular disrupting agent OXi4503 on tumor vascularity. Clin Cancer Res 2006; 12: 4090–4094
- Sheng Y, Hua J, Pinney KG, Garner CM, Kane RR, Prezioso JA, et al. Combretastatin family member OXI4503 induces tumor vascular collapse through the induction of endothelial apoptosis. Int J Cancer 2004; 111: 604–610
- Murata R, Overgaard J, Horsman MR. Combretastatin A-4 disodium phosphate: A vascular targeting agent that improves that improves the anti-tumor effects of hyperthermia, radiation, and mild thermoradiotherapy. Int J Rad Oncol Biol Phys 2001; 51: 1018–1024
- Overgaard J. Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumor and its surrounding normal tissue in vivo. Int J Rad Oncol Biol Phys 1980; 6: 1507–1517
- Horsman MR, Sampson LE, Chaplin DJ, Overgaard J. The in vivo interaction between flavone acetic acid and hyperthermia. Int J Hyperthermia 1996; 12: 779–789
- Horsman MR, Murata R. Combination of vascular targeting agents with thermal or radiation therapy. Int J Rad Oncol Biol Phys 2002; 54: 1518–1523
- Horsman MR, Murata R, Overgaard J. Improving local tumor control by combining vascular targeting drugs, mild hyperthermia and radiation. Acta Oncol 2001; 40: 497–503
- Murata R, Overgaard J, Horsman MR. Potentiation of the anti-tumour effect of hyperthermia by combining with the vascular targeting agent 5,6-dimethylxanthenone-4-acetic acid. Int J Hyperthermia 2001; 17: 508–519
- Eikesdal HP, Bjerkvig R, Raleigh JA, Mella O, Dahl O. Tumor vasculature is targeted by the combination of combretastatin A-4 and hyperthermia. Radiother Oncol 2001; 61: 313–320
- Kim JH, Lew YS, Kolozsvary A, Ryu S, Brown SL. Arsenic trioxide enhances radiation response of 9L glioma in the rat brain. Rad Res 2003; 160: 662–666
- Murata R, Siemann DW, Overgaard J, Horsman MR. Improved tumor response by combining radiation and the vascular-damaging drug 5,6-dimethylxanthenone-4-acetic acid. Rad Res 2001; 156: 503–509
- Wilson WR, Li AE, Cowan DS, Siim BG. Enhancement of tumor radiation response by the antivascular agent 5,6-dimethylxanthenone-4-acetic acid. Int J Rad Oncol Biol Phys 1998; 42: 905–908
- Murata R, Siemann DW, Overgaard J, Horsman MR. Interaction between combretastatin A-4 disodium phosphate and radiation in murine tumors. Radiother Oncol 2001; 60: 155–161
- Siemann DW, Rojiani AM. Enhancement of radiation therapy by the novel vascular targeting agent ZD6126. Int J Rad Oncol Biol Phys 2002; 53: 164–171
- Siemann DW, Rojiani AM. The vascular disrupting agent ZD6126 shows increased antitumor efficacy and enhanced radiation response in large, advanced tumors. Int J Rad Oncol Biol Phys 2005; 62: 846–853
- Wachsberger PR, Burd R, Marero N, Daskalakis C, Ryan A, McCue P, et al. Effect of the tumor vascular-damaging agent, ZD6126, on the radioresponse of U87 glioblastoma. Clin Cancer Res 2005; 11: 835–842
- Murata R, Horsman MR. Tumour-specific enhancement of thermoradiotherapy at mild temperatures by the vascular targeting agent 5,6-dimethylxanthenone-4-acetic acid. Int J Hyperthermia 2004; 20: 393–404
- Frindel E, Malaise EP, Alpen E, Tubiana M. Kinetics of cell proliferation of an experimental tumor. Cancer Res 1967; 27: 1122–1131
- Patterson DM, Rustin GJ. Vascular damaging agents. Clinical Oncol (Royal College of Radiologists (Great Britain)) 2007; 19: 443–456