Abstract
Hyperthermic isolated limb perfusion (HILP) with melphalan and more recently isolated limb infusion (ILI) with melphalan +/− dactinomycin are common treatment modalities for both in-transit melanoma of the extremity and advanced extremity sarcoma. In order to further optimize treatment, future research should focus on selection of appropriate patients, verification of a technique that produces consistent results while maintaining acceptable toxicity, and development of novel strategies and agents. Development of these novel agents and strategies has potential to not only improve the efficacy of regional chemotherapy but may also help guide future strategies for systemic treatment.
Introduction
The techniques of delivering regional chemotherapy to an isolated extremity via hyperthermic isolated limb perfusion (HILP) and more recently isolated limb infusion (ILI) have been relatively effective limb-sparing treatment modalities for patients with in-transit melanoma or advanced sarcoma of the extremity. While no study has demonstrated a survival benefit for therapeutic HILP, evidence does suggest that a survival benefit exists for melanoma patients achieving a complete response (CR) to therapy possibly independent of regional nodal status Citation[1–5]. In order to further maximize the therapeutic effects of treatment and improve CR rates, several issues need additional consideration and expanded comprehensive evidence. Specifically, future efforts should focus on the identification of appropriate patients who will benefit from treatment, determination of a technique that produces consistent responses across similar populations, and the development of novel strategies and agents that can improve response. Clarifying these issues can optimize clinical response with the ultimate goal to improve survival while maintaining acceptable levels of toxicity.
Primary indications
HILP with melphalan has generally been the accepted treatment for patients with in-transit disease that is unresectable and for unresectable soft tissue sarcoma of the extremity. Additionally, HILP has been effective as a palliative treatment in selected stage IV melanoma patients with symptomatic advanced limb disease Citation[6]. There have been no randomized controlled trials comparing HILP with other treatment options for patients with measurable in-transit disease. Assessing the results of HILP for truly unresectable disease is difficult because often potentially resectable recurrent lesions are left in situ to monitor the effect of HILP. Complete response (CR) rates from HILP have been reported to be 40–82% and averaged out to be 54% in a retrospective meta-analysis Citation[7–10]. Modifications to the procedure as well as alternative agents to melphalan have been studied. The introduction of hyperthermia was based on evidence that hyperthermia can improve uptake of chemotherapy in tumor cells Citation[11]. Currently, mild hyperthermia is advocated as a balance between optimizing response while maintaining acceptable levels of toxicity Citation[12], Citation[13]. Additionally, several different drugs have been tried in regional perfusion treatments including cisplatin Citation[14–18], DTIC Citation[19], and nitrogen mustard Citation[20], with none being superior to melphalan. However, the addition of dactinomycin has been widely used in combination with melphalan for both HILP and ILI because of exceptionally good response rates (CR 73%) when the combination was administered by conventional HILP in a small number of patients without any apparent increase in toxicity Citation[21]. However, there are no randomized control trials comparing the combination of melphalan plus dactinomycin to melphalan alone for either HILP or ILI. Currently, both HILP and ILI are primarily utilized in the treatment of patients with in-transit melanoma of the extremity although other subsets of patients including those with sarcomas, and Merkel cell carcinomas may benefit from treatment.
Adjuvant treatment
Selection of patients likely to benefit from HILP in melanoma has been examined in several clinical trials. A large multicenter prospective randomized trial demonstrated no benefit on time to systemic metastases or survival in patients undergoing adjuvant HILP with melphalan after excision of their primary melanoma Citation[22]. This is not necessarily surprising as historically only 2–10% of extremity melanoma cases recur as in-transit metastasis Citation[23]. HILP by design has the theoretical benefit of treating in-transit disease but not systemic or nodal disease. Therefore, to establish the potential efficacy, a very large trial would be necessary to try and demonstrate a difference in the small number of patients who theoretically could benefit from the intervention. The authors’ conclusion was that in view of the lack of a significant survival benefit in addition to the considerable morbidity and cost of adjuvant HILP, this procedure after excision of high risk primary melanoma could not be recommended. However, this practice does not take into consideration those subpopulations of patients who might benefit the most from early regional treatment. This population would include those individuals at highest risk of developing in-transit disease such as patients with thicker primary tumors, ulceration, regional lymph node metastasis, and lower extremity location of primary tumor Citation[24–26]. While tumor thickness has traditionally been the most sensitive parameter for predicting the metastatic risk of cutaneous melanoma, other prognostic markers like the extent of lymphangiogenesis may provide more precise identification of patients at risk for recurrence Citation[27]. In addition, future methods like microarray analysis may identify particular pathway deregulations or other aspects of tumor biology that can more accurately predict patients who will develop in-transit disease and would thus benefit from prophylactic HILP or ILI. Treatment to potentially decrease the occurrence of in-transit disease is important as the presence of in-transit metastases is associated with poor prognosis with 5-year survival rates ranging from 12% to 37% Citation[28–30].
The value of HILP has also been examined in a prophylactic setting after excision of in-transit metastases. A randomized Phase III trial of 69 patients showed no overall survival benefit despite an improved disease-free survival Citation[31]. While this study is limited by a low number of patients, the cost and morbidity of the procedure precluded a conclusion for the recommendation of prophylactic HILP. However, with the introduction of isolated limb infusion, novel well tolerated agents, and possible strategies to predict response, the delivery of regional chemotherapy to patients with high-risk melanoma of the extremity after excision of primary or in-transit disease may prove beneficial and should be the focus of future clinical trials.
Chemotherapy agents
Although melphalan has been the drug of choice for regional chemotherapy, the intravenous (IV) formulation of temozolomide (TMZ) has shown tremendous promise as a regional chemotherapy agent in preclinical models. TMZ is a DNA alkylating agent which is spontaneously converted to the active metabolite MTIC. In an animal model, regional infusion of TMZ was more effective than systemic therapy and frequently more effective than melphalan via regional infusion especially in tumors with low O6-alkylguanine-DNA alkyltransferase (AGT) activity Citation[32]. Treatment with TMZ can be further enhanced with concomitant presence of hyperthermia during temozolomide infusion Citation[33]. Another factor that makes ILI with TMZ a potentially promising approach is the ability to more accurately predict response. In recent work using a xenograft model of extremity melanoma, no correlation was identified between melphalan sensitivity and the GST/glutathione cellular detoxification pathway. In contrast, a strong correlation between the levels of AGT activity and percentage increase in tumor volume was noted for tumors treated with temozolomide; xenografts with low AGT activity showed much higher levels of response to regional TMZ Citation[34]. Regional therapy with TMZ may ultimately prove to be more effective than melphalan in certain subsets of tumors. However, TMZ has not been used as a regional chemotherapy agent in humans due to its unavailability as an intravenous formulation. The IV formulation is currently under FDA review with phase I regional trials planned for melanoma patients pending FDA approval using both ILI and HILP.
Immunotherapy with the addition of cytokines to HILP with melphalan has also been explored for the potential to augment anti-tumor activity. Tumor necrosis factor α (TNFα) has been studied as an agent for regional chemotherapy. The combination of melphalan plus TNFα in HILP has been reported to achieve higher CR rates than melphalan alone Citation[35], Citation[36], Citation[37]. A small randomized trial of 103 patients by Fraker et al. reported CR rates of 58% for the HILP with melphalan alone arm and 72% for HILP with melphalan plus interferon (INF) plus TNF- α Citation[38]. However, a recently completed larger randomized prospective phase III trial run by the American College of Surgeons Oncology Group (ACOSOG) which compared melphalan alone to melphalan plus TNF was stopped at its interim analysis by the data safety monitoring committee because no response advantage to TNF was identified and there were three times the number of grade IV adverse events in the TNF arm including two treatment related amputations Citation[39]. In addition, the single institution response rates of 80% using melphalan were not reproduced, with overall response rates in the ACOSOG trial being approximately 70% in both arms with only 25% of patients in each arm exhibiting a complete response. However, there was a trend toward more complete responders and more durable complete responses seen in the melphalan plus TNF group at the 6-month follow-up time point. Further exploration of the addition of TNF to melphalan in the reperfusion of patients who responded poorly to initial perfusion with melphalan alone may be appropriate.
In contrast to melanoma, the use of TNF appears to increase the effectiveness of melphalan when used in regional therapy for advanced extremity sarcoma. In 186 patients with locally advanced soft tissue sarcoma, major tumor responses were seen in 82% of patients after HILP with melphalan and TNF Citation[40]. Treatment also rendered the large sarcomas resectable in most cases Citation[40]. The study was conducted with modest systemic and loco regional toxicity. Thus the addition of TNF to melphalan has been advocated for use in regional therapy of advanced extremity sarcoma. Several single institution studies have subsequently reported similar high response rates Citation[41], Citation[42].
The cytokine interleukin 2 (IL-2) as well as the inflammatory mediator, histamine (Hi) have also been studied in combination with melphalan HILP in a rat experimental model. In a rat experimental model of melphalan-based HILP for lower limb soft tissue sarcoma, histamine appeared to augment tumor response with an overall response (OR) of 66% Citation[43]. Similarly, HILP with IL-2 plus melphalan resulted in an OR of 67% compared to an OR of 17% with melphalan alone in the same experimental model Citation[44]. The combination of histamine and IL-2 to melphalan HILP had no further benefit and was found to have an OR of 28%, less than each achieved alone when combined with melphalan HILP Citation[45]. As new agents and modifications to treatment with regional chemotherapy are studied for their potential to improve response, the emphasis on maintaining acceptable treatment associated toxicity should be maintained.
Treatment modifications to decrease toxicity: Isolated limb infusion
Complications from HILP are common. Significant regional toxicity with skin necrosis, deep tissue necrosis (compartment syndrome) and peripheral neuropathy can occur. Vascular catastrophe requiring arterial reconstruction or amputation is uncommon but may occur Citation[46]. In addition, approximately 1–3% of patients can suffer complications leading to amputation, 10–15% develop compartment syndrome, 30–40% develop lymphedema, and 5–8% can sustain long-term neuropathy Citation[46], Citation[47]. Repeated limb perfusions are technically difficult because of surgical scarring. The overall complication rate increases from 28% for a single procedure to 51% for a second procedure. As discussed previously, the addition of TNF was associated with three times the number of high grade adverse events and an observed CR in only 25% of patients in each study cohort. The disappointing results of this trial combined with the toxicity of HILP have led to the search for more effective, durable, and less toxic regional treatment strategies.
Isolated limb infusion for recurrent extremity melanoma and unresectable sarcoma is a strategy attempting to deliver regional melphalan in an equally effective but less toxic fashion than HILP. ILI, developed in Australia at the Sydney Melanoma Unit, differs from HILP in that ILI circulates blood in an isolated extremity at a much slower rate than HILP and for a duration of only 30 minutes Citation[48]. In addition, during ILI the extremity is hypoxic which leads to marked acidosis in contrast to HILP where the pump oxygenator maintains the oxygenation and acid/base status of the extremity. ILI, which has not been as extensively studied as HILP, had in a preliminary report a single institution overall response rate of 85% with 41% being complete responders and 44% being partial responders Citation[49]. The mean time to recurrence for a complete responder was 12 months. During ILI, melphalan is given in conjunction with dactinomycin, and the treatment is reportedly associated with a lower complication rate than HILP.
The initial experience in this country has not been as promising as the Australian experience. A recent report from Memorial Sloan-Kettering Cancer Center reported a 23% CR with an overall response rate of 50% while the combined experience of both Duke and MD Anderson Cancer Center described a 33% complete response rate and a 67% overall response rate in a cohort of 41 patients Citation[50], Citation[51]. Furthermore, we have recently analyzed data in 58 patients undergoing ILI with melphalan and dactinomycin at Duke University over a four-year period. CR was 30%, the partial response (PR) was 14%, and there was no response in 56% of patients at 3 months Citation[52]. In comparison to the Duke experience with HILP (n = 59), a significantly better response rate at 3 months (CR 57%, PR 31%, NR 12%) was observed with perfusion. However, toxicity was significantly greater with HILP including 9 compartment syndromes and 2 amputations. One possible explanation for the discrepancy in response rates is the relatively low flow system of ILI compared to HILP may lead to lower levels of melphalan uptake by tumor cells although this has not been examined thoroughly. While ILI may not be as effective as HILP, the low toxicity and morbidity of ILI is an appealing option for patients as a first step in regional treatment reserving HILP for patients who are ILI failures or non-responders. In addition, the low toxicity allows for repeat ILIs without an apparent increase in toxicity. The potential to improve complete response with novel targeted agents and new chemotherapeutic agents may help further improve the therapeutic index of ILI making the technique the preferred treatment option for patients with in-transit melanoma the extremity.
Optimizing response
The ability to obtain a CR following HILP was the strongest predictor of survival in the Duke experience with HILP Citation[1]. Similarly, in a recent review of the long term results of HILP, complete response was a positive prognostic indicator for survival Citation[2] while the ability to achieve a CR in patients with a lower stage had an increased likelihood but was not statistically significant. In the SMU experience with ILI, median survival was longer in patients in whom a CR was achieved Citation[49]. The finding that CR is a predictor of survival may suggest that tumors which are chemosensitive have a more favorable tumor biology as compared to tumors which possess or have acquired chemotherapy resistance. Recent work has explored resistance to chemotherapy and has shown in a nude rat human xenograft model that targeting resistance pathways with novel agents can improve tumor response to ILI with melphalan Citation[53], Citation[54]. We have also begun work with systemic administration of targeted therapies that have a low toxicity profile in combination with melphalan via ILI.
One approach to overcoming regional chemotherapy resistance has been to give a modulator of drug resistance proteins systemically pre, peri, and post regional chemotherapy treatment with melphalan and more recently regional therapy with temozolomide (TMZ). Buthathione sulfixime (BSO), is an inhibitor of gamma-glutamyl-cysteine-synthetase (c-GCS). C-GCS is a rate-limiting enzyme in GSH synthesis. Elevated intracellular GSH levels are associated with increased resistance to melphalan Citation[55], Citation[56]. Using a melanoma infusion animal model, short-term systemic BSO therapy was found to potentiate the effects of regional melphalan without increasing toxicity Citation[57]. A phase I trial using a 3-day infusion of BSO around the time of a regional melphalan infusion has recently been approved by the Duke IRB and is designed to determine the efficacy of this approach in patients who have failed a previous melphalan-based therapy. In this trial, BSO will be given at its phase II dose and the melphalan will be dose escalated. Tumor biopsies before and during BSO therapy will attempt to document the degree of glutathione depletion before the regional melphalan treatment so that more can be learned about this type of treatment strategy.
We also examined whether modulation of resistance pathways would be an effective strategy for improving tumor response to other alkylating agents such as TMZ. O6-benzylguanine (O6BG) is an inhibitor of the DNA repair enzyme O6-alkylguanine-DNA alkyltransferase (AGT), the main resistance pathway for TMZ in melanoma. Using systemic O6BG with regional temozolomide via isolated limb infusion in an animal model has been shown to enhance tumor response Citation[34]. While this demonstrates the importance of tumor resistance mechanisms in determining response for regionally treated tumors, the use of O6BG will not be an option until after the regional TMZ ILI phase I trial is completed. This trial is currently in preparation at Duke University.
Another potential method of treatment to improve CR rates with regional chemotherapy is administration of targeted therapies that may disrupt various cell signaling pathways that may make the tumors more susceptible to the effects of cytotoxic chemotherapies. Chemoresistance in melanoma may be an intrinsic property of the tumor cell potentially related to one of a number of molecular and genetic changes that leads to deregulation of signaling pathways. Preliminary in vitro data suggests that the inherent chemoresistance of melanoma may be overcome by using traditional chemotherapy agents in combination with a targeted drug that inhibits selected signaling pathways. One promising targeted therapy has been with the N-cadherin antagonist ADH-1 (Exherin) which is a cyclic pentapeptide that disrupts N-cadherin binding interactions. The cadherins are a large supergene family of proteins that are involved in cell to cell adhesion. Malignant transformation in melanoma is characterized by a ‘switch’ from E-cadherin to N-cadherin and is associated with changes in intracellular signaling pathways leading to increased proliferation, survival and angiogenesis and decreased apoptosis. The use of systemic ADH-1 in conjunction with regional melphalan shows marked increases in tumor responses even in melphalan resistant tumors Citation[58]. A phase I dose escalation trial of systemic ADH-1 in combination with melphalan via ILI for patients who have AJCC Stage IIIB or IIIC extremity melanoma is currently open to accrual at Duke and MDACC.
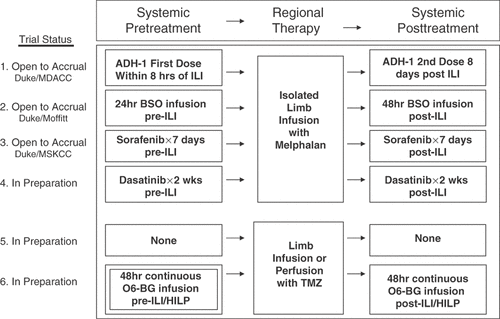
Other potential targets involve the B-raf and N-ras signaling pathways. Sixty percent of melanoma tumors harbor an activating mutation in B-raf kinase Citation[59].These genetic mutations lead to deregulation of multiple signaling pathways in the cell, but most noticeably increase activity of the MAP kinase signaling pathway which leads to increased proliferation and survival of tumor cells. As single agents, targeted therapies aimed at these proteins have been relatively unsuccessful in terms of producing tumor response. Interestingly, however, surprising responses were seen in a small number of clinical trials in melanoma where targeted therapy was utilized in conjunction with other chemotherapy agents Citation[60], Citation[61]. Initial clinical trials with the Bayer compound Sorafenib (Nexavar), a B-raf kinase inhibitor, suggest that as a single agent it may have limited activity. However, when used in combination with other chemotherapy agents like DTIC, sorafenib has shown some encouraging responses Citation[62]. In contrast, the combination of sorafenib with carboplatin plus paclitaxel for patients with advanced melanoma did not demonstrate improvements in any clinical endpoints Citation[63]. While these studies can potentially provide a basis for combinations that utilize regional chemotherapy, the ability to deliver regional chemotherapy at doses 10–15 times higher than what can safely be administered systemically may lead to higher levels of synergistic interaction and response. The interesting results from the trial of Sorafenib in combination with systemic DTIC has prompted a new chemosensitizing study using Sorafenib in patients with regionally advanced metatstatic melanoma. This trial, combining oral systemic Sorafenib before and after ILI with melphalan, has recently been IRB approved at Duke University and will also be carried out at MSKCC. Another potential target in melanoma is the src family of kinases. Stat3, a downstream target of src kinase, has been found to be activated in the majority (85%) of melanoma cell lines Citation[64]. Stat3 has been shown to not only promote tumor growth and survival but also appears to up regulate VEGF and promote tumor angiogenesis Citation[65]. Inhibition of src kinase blocked the growth of human melanoma cell lines with elevated Stat3 activity Citation[65] and induced apoptosis by inhibiting the expression of anti-apoptotic genes Bcl-xL and Mcl-1. Src inhibitors like Dasatinib (Sprycel) may also potentiate the effectiveness of traditional chemotherapy. A phase I trial combining Dasatinib with melphalan via ILI at Duke University is currently being planned. A summary of these phase I trials is shown in .
There are currently several other combination therapies currently under investigation for the treatment of metastatic melanoma which may also be applicable for combination with infusion chemotherapy where one can take advantage of the high dose of regionally administered chemotherapy. While the list of potential targets and agents is extensive, three particular agents are noteworthy. One possible targeted therapy agent is bevacizumab, a monoclonal antibody to vascular endothelial growth factor. This agent has shown survival benefits when used in combination with standard chemotherapies in patients with metastatic colorectal cancer Citation[66]. There also appears to be anti-tumor activity with combination therapy in metastatic breast and lung cancer Citation[67], Citation[68]. Most recently, bevacizumab in combination with irinotecan has shown promise in the treatment of recurrent glioblastoma multiforme, demonstrating a high response rate and an improvement in the 6 month progression-free survival Citation[69]. There have been a few case reports using bevacizumab in combination with chemotherapy for patients with metastatic melanoma that may suggest activity Citation[70], Citation[71]. In a recent phase II trial of bevacizumab alone versus bevacizumab plus IFN-α in 32 patients with metastatic melanoma, stable disease was achieved in 5 of 16 patients who received bevacizumab alone while there was one partial response observed in 1 of 16 patients treated with the combination Citation[72]. While more preliminary evidence is still needed, the addition of bevacizumab to ILI with melphalan may provide improved response rates. Another potential chemosensitizing targeted therapy that has been explored is the multikinase inhibitor imatinib mesylate. While the frequency of KIT mutations in melanoma is unclear Citation[73], Citation[74], data suggests that melanomas with a lentiginous growth pattern have frequent genetic alterations of KIT and these patients may gain a therapeutic benefit from imatinib in combination with another chemotherapy Citation[73], Citation[74]. Finally, STA-4783 is a drug that enhances the therapeutic index of paclitaxel. The combination demonstrated anti-tumor activity in patients with metastatic solid tumors Citation[75]. While combination therapies continue to be explored for metastatic melanoma, combination therapies utilizing ILI and HILP for in-transit melanoma also have potential to improve the therapeutic index of treatment.
In addition to exploring novel therapies, research is also being conducted into examining how gene expression signatures can optimize regional chemotherapy treatment in patients with in-transit malignant melanoma of the extremity. Specifically, recent work has demonstrated that gene expression signatures can predict response to various cytotoxic chemotherapeutic agents which may ultimately provide an opportunity to optimize the use of these drugs in patients Citation[76]. In a similar fashion to methods described previously Citation[76], Citation[77], a melphalan sensitivity signature using microarray gene expression data combined with in vitro drug response data in melanoma cell lines has been described. This signature is currently being validated using biopsy samples from patients prior to treatment with ILI with melphalan to test if the signature correlates to clinical response as a measure of melphalan sensitivity. Application of a gene signature like this one in the context of targeted agents can help optimize both regional and systemic therapeutic strategies in the future. For example, the gene signature obtained from a biopsy sample could not only predict tumor response to chemotherapies allowing for optimal initial chemotherapy selection in ILI but also identify deregulated pathways and mechanisms of resistance that can subsequently be targeted with appropriate agents. While further development of this technology is needed, the potential exists to improve outcomes by individualizing treatment strategies.
Conclusion
The delivery of regional chemotherapy to an isolated limb continues to be an important treatment for patients with in-transit melanoma of the extremity and unresectable soft tissue sarcoma. The focus of the majority of these approaches is on melanoma which is a more homogenous and common entity presenting as advanced disease in an extremity. There are multiple strategies and therapies currently being explored that have the potential to improve response to regional therapy. The regional model of advanced extremity melanoma, with its easy accessibility of tumor tissue allows a unique opportunity to define how to utilize targeted agents and correlated alterations in functional proteins with tumor response and drug pharmacokinetics. The novel regional infusion trials discussed in this chapter should help facilitate our comprehension of how targeted therapies should be optimally utilized to overcome chemotherapy resistance. Additionally, while these trials may augment the therapeutic index of regional chemotherapy, they may also serve as a basis to more logically direct the development of novel approaches to systemic treatment strategies. The fact that most of these approaches focus on melanoma does not preclude their potential application to other malignancies like sarcomas, Merkel cell carcinomas, and squamous cell carcinomas that may recur in a multifocal pattern in the extremity.
References
- Aloia TA, Grubbs E, Onaitis M, Mosca PJ, Cheng TY, Siegler H, Tyler DS. Predictors of outcome after hyperthermic limb perfusion: Role of tumor response. Arch Surg 2005; 140: 1115–1120
- Sanki A, Kam P, Thompson JF. Long-term results of hyperthermic, isolated limb perfusion for melanoma. Ann Surg 2007; 245: 591–596
- Grunhagen DJ, Brunstein F, Gravelan WJ, van Geel AN, de Wilt JH, Eggermont AM. One hundred consecutive isolated limb perfusions with TNF-alpha and melphalan in melanoma patients with multiple in-transit metastases. Ann Surg 2004; 240: 939–948
- Bryant PJ, Balderson GA, Mead P, Egerton WS. Hyperthermic isolated limb perfusion for malignant melanoma: Response and survival. World J Surg 1995; 19: 363–368
- Noorda EM, Vourenraets BC, Nieweg OE, van Geel AN, Eggermont AM, Kroon BR. Isolated limb perfusion for unresectable melanoma of the extremities. Arch Surg 2004; 139: 1237–1242
- Takkenberg RB, Vrouenraets BC, van Geel AN, Nieweg OE, Noorda EM, Eggermont AM, Kroon BR. Palliative isolated limb perfusion for isolated limb perfusion for advanced limb disease in stage IV melanoma patients. J Surg Oncol 2005; 91: 107–111
- Grunhagen DJ, de Wilt JHW, van Geel AN, Eggermont AMM. Isolated limb perfusion for melanoma patients-a review of its indications and the role of tumour necrosis factor-α. EJSO 2006; 32: 371–380
- Vrouenraets BC, Nieweg OE, Kroon BB. Thirty-five years of isolated limb perfusion for melanoma: Indications and results. Br J Surg 1996; 83: 1319–1328
- Di Filippo F, Calabro A, Giannarelli D, Carlini S, Cavalier F, Moscarelli F, Cavalier R. Prognostic variables in recurrent limb melanoma treated with hyperthermic antiblastic perfusion. Cancer 1989; 63: 2551–2561
- Klaase JM, Kroon BBR, van Geel AN, Eggermont AM, Franklin HR, Hart AA. Prognostic factors for tumor response and limb recurrence-free interval in patients with advanced melanoma of the limbs treated with regional isolated perfusion with melphalan. Surgery 1994; 115: 39
- Clark J, Grabs AJ, Parsons PG, Smithers BM, Addison RS, Roberts MS. Melphalan uptake, hyperthermic synergism and drug resistance in a human cell culture model for the isolated limb perfusion of melanoma. Melanoma Res 1994; 4: 365–370
- Minor DR, Allen RE, Alberts D, Yei-Mei Peng, Tardelli G, Hutchinson J. A clinical and pharmacokinetic study of isolated limb perfusion with heat and melphalan for melanoma. Cancer 1985; 55: 2638
- Benckhuijsen C, Kroon BBR, van Geel AN, Wieberdink J. Regional perfusion treatment with melphalan for melanoma in a limb: An evaluation of drug kinetics. Eur J Surg Oncol 1988; 14: 157
- Skene AL, Bulman AS, Williams TR, Thomas JM, Westbury G. Hyperthermic isolated limb perfusion with melphalan in the treatment of advanced malignant melanoma of the lower limb. Br J. Surg 1990; 7: 765
- Fletcher WS, Pommier R, Small K. Results of cisplatin hyperthermic isolation perfusion for stage IIIA and IIIAB extremity melanoma. Melanoma Res 2004; Suppl.1: 17–19
- Hoekstra HJ, Schraffordt Koops H, de Vries LG, van Weerden TW, Oldhoff J. Toxicity of hyperthermic isolated limb perfusion with cisplatin for recurrent melanoma of the lower extremity after previous perfusion treatment. Cancer 1993; 72: 1224–1229
- Thompson JF, Gianoutsos MP. Isolated limb perfusion for melanoma: Effectiveness and toxicity of cisplatin compared with that of melphalan and other drugs. World J Surg 1992; 16: 227
- Krementz ET, Ryan RF. Chemotherapy of melanoma of the extremities by perfusion: Fourteen years’ clinical experience. Ann Surg 1972; 175: 900
- Bonenkamp JJ, Thompson JF, de Wilt JH, Doubrovsky A, de Faria Lima R, Kam P. Isolated limb infusion with fotemustine after dacarbazine chemosensitisation for inoperable loco-regional melanoma recurrence. EJSO 2004; 30: 1107–1112
- Briele HA, Djuric M, Jung DT, Mortell T, Patel MK, Das Gupta TK. Pharmacokinetics of melphalan in clinical isolation perfusion of the extremities. Cancer Res 1985; 45: 1885–1889
- Thompson JF, Hunt JA, Shannon KF, Kam PC. Frequency and duration of remission after isolated limb perfusion for melanoma. Arch Surg 1997; 132: 903–907
- Koops HSKH, Vaglini M, Suciu S, Kroon BB, Thompson JF, Gohl J, Eggermont AM, Di Filippo F, Krementz ET, Ruiter D, Lejeune FJ. Prophylactic isolated limb perfusion for localized, high-risk melanoma: Results of a multicenter randomized phase III trial. J Clin Oncol 1998; 16: 2906–2912
- Balch CM, Houghton AN, Peters LJ. Cutaneous melanoma. Cancer: Principles and practice of oncology4th ed, VT DeVita, S Hellman, SA Rosenberg. JB Lippincott, Philadelphia 1993; 1612
- Wong JH, Cagle LA, Kopald KH, Swisher SG, Morton DL. Natural history and selective management of in transit melanoma. J Surg Oncol 1990; 44: 146–150
- Leon P, Daly JM, Synnestvedt M, Schultz DJ, Elder DE, Clark WH, Jr. The prognostic implications of microscopic satellites in patients with clinical stage I melanoma. Arch Surg 1991; 126: 1461–1468
- Day CL, Jr, Harrist TJ, Gorstein F, Sober AJ, Lew RA, Friedman RJ, Pasternack BB, Kopf AW, Fitzpatrick TB, Mihm MC, Jr. Malignant melanoma: Prognostic significance of ‘microscopic satellites’ in the reticular dermis and subcutaneous fat. Ann Surg 1981; 194: 108–112
- Dadras SS, Lange-Asschenfeldt B, Velasco P, Nguyen L, Vora A, Muzikansky A, Jahnke K, Hauschild A, Hirakawa S, Mihm M, Detmar M. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Modern Pathology 2005; 18: 1232–1242
- Cascinelli N, Bufalino R, Marolda R, Belli F, Nava M, Galluzzo D, Santinami M, Levene A. Regional non-nodal metastasis of cutaneous melanoma. Eur J Surg Onc 1986; 12: 175–180
- Calabro A, Singletary SE, Balch CM. Patterns of relapse in 1001 consecutive patients with melanoma nodal metastases. Arch Surg 1989; 124: 1051–1055
- Zogakis TG, Bartlett DL, Libutti SK, Liewehr DJ, Steinberg SM, Fraker DL, Alexander HR. Factors affecting survival after complete response to isolated limb perfusion in patients with in-transit melanoma. Ann Surg Oncol 2001; 8: 771–778
- Hafstrom L, Rudenstam CM, Blomquist E, Ingvar C, Johsson PE, Lagerlot B, Lingholm C, Ringborg V, Westman G, Ostrup L. Regional hyperthermic perfusion with melphalan after surgery for recurrent malignant melanoma of the extremities. Swedish Melanoma Study Group. J Clin Oncol 1991; 9: 2091–2094
- Ueno T, Ko SH, Grubbs E, Yoshimoto Y, Augustine C, Abdel-Wahab Z, Cheng T, Abdel-Wahab O, Pruitt SK, Friedman HS, Tyler DS. Modulation of chemotherapy resistance in regional therapy: A novel therapeutic approach to advanced extremity melanoma using intra-arterial temozolomide in combination with systemic O6-benzylguanine. Mol Cancer Ther 2006; 5: 732–738
- Ko SH, Ueno T, Yoshimoto Y, Yoo JS, Abdel-Wahab O, Abdel-Wahah Z, Chu E, Pruitt SK, Friedman HS, Dewhirst MK, Tyler DS. Optimizing a novel regional chemotherapeutic agent against melanoma: Hyperthermia-induced enhancement of temozolomide cytotoxicity. Clin Cancer Res 2006; 12: 289–297
- Yoshimoto Y, Augustine CK, Yoo JS, Zipfel PA, Selim MA, Pruitt SK, Friedman HS, Ali-Osman F, Tyler DS. Defining regional infusion treatment strategies for extremity melanoma: Comparative analysis of melphalan and temozolomide as regional chemotherapeutic agents. Mol Cancer Ther 2007; 6: 1492–500
- Lienard D, Lejeune, Delmotte J, Renard N, Ewalenco P. High doses of TNFα in combination with IFN-gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Onc 1982a; 10: 52
- Lienard D, Lejeune F, Ewalenko I. In transit metastases of malignant melanoma treated by high doses of TNFα in combination with Interferon-gamma and melphalan in isolation perfusion. World J Surg 1992b; 16: 234
- Lienard D, Eggermont AM, Schraffordt KH, Kroon BR, Towse O, Hiemstra S, Schmitz P, Clarke J, Steinmann D, Rosenkaimer F, Lejeune FJ. Isolated perfusion of the limb with high-dose tumour necrosis factor-alpha (TNF-α), Interferon gamma (IFN-γ) and melphalan for melanoma stage III. Results of a multi-centre pilot study. Melanoma Res 2004; 4Suppl.1: 21–26
- Fraker DL, Alexader HR, Ross M, Bartlett DL, Tyler DS, Libutti LS, Boodie A, Briele H, Karakousis G. A phase III trial of isolated limb infusion perfusion for extremity melanoma comparing melphalan alone versus melphalan plus tumor necrosis factor (TNF-α) plus Interferon-gamma (IFN). Ann Surg Onc 2002; 9: S8
- Cornett WR, McCall LM, Petersen RP, Ross MI, Briele HA, Noyes RD, Sussman JJ, Kraybill WG, Kane JM, Alexander HR, et al. Prospective randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone versus melphalan plus tumor necrosis factor: American College of Surgeons Oncology Group Trial Z0020. J Clin Oncol 2006; 24: 4196–4201
- Eggermont AM, Schraffordt, Koops H, Klausner M, Kroon BB, Schlay PM, Lienard D, van Geel AN, Hoekstra HJ, Meller I, Nieweg OE, et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter European experience. Ann Surg 1996; 756–764: 764–765, discussion
- Grunhagen DJ, de Wilt JH, Graveland WJ, Verhoet S, van Geel AN, Eggermont AM. Outcome and prognostic factor analysis of 217 consecutive isolated limb perfusions with tumor necrosis factor-alpha and melphalan for limb-threatening soft tissue sarcoma. Cancer 2006; 106: 1776–1784
- Lejeune FJ, Pujol N, Lienard D, Mosimann F, Raffoul W, Genton A, Guillou L, Landry M, Chassot PG, Chiolero R, Bischof-Delaloye A, et al. Limb salvage by neoadjuvant isolated limb perfusion with TNFalpha and melphalan for nonresectable soft tissue sarcoma of the extremities. Eur J Surg Oncol 2000; 26: 669–678
- Brunstein F, Hoving S, Seynhaeve AL, van Tiel ST, Guetens G, de Bruijn EA, Eggermont AM, ten Hagen TL. Synergistic antitumor activity of histamine plus melphalan in isolated limb perfusion: Preclinical studies. J Natl Cancer Inst 2004; 96: 1603–1610
- Hoving S, Brunstein F, Ann de Wiel-Ambagtsheer G, van Tiel ST, de Boeck G, de Bruijn EA, Eggermont AM, ten Hagen TL. Synergistic antitumor response of interleukin 2 with melphalan in isolated limb perfusion in soft tissue sarcoma-bearing rats. Cancer Res 2005; 65: 4300–4308
- Brunstein F, Hoving S, Aan de Wiel-Ambagtsheer G, de Bruijn EA, Guetens G, Eggermont AM, ten Hagen TL, et al. Decreased response rates by the combination of histamine and IL-2 in melphalan-based isolated limb perfusion. Cancer Immunol Immunother 2007; 56: 573–580
- Taber SW, Polk HC, Jr. Mortality, major amputation rates, and leukopenia after isolated limb perfusion with phenylalanine mustard for the treatment of melanoma. Ann Surg Oncol 1997; 4: 440–445
- Vrouenraets BC, Klaase JM, Nieweg OE, Kroon BBR. Toxicity and morbidity of isolated limb perfusion. Semin Surg Oncol 1998; 14: 224–231
- Thompson JF, Kam PC, Waugh RC, Harman CR. Isolated limb infusion with cytotoxic agents: A simple alternative to isolated limb perfusion. Semin Surg Oncol 1998; 14: 238–47
- Lindner P, Doubrovsky A, Kam PCA, Thompson JF. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Onc 2002; 9: 127–136
- Brady MS, Brown K, Patel A, Fisher C, Marx W. A phase II trial of isolated limb infusion with melphalan and dactinomycin for regional melanoma and soft tissue sarcoma of the extremity. Ann Surg Onc 2006; 13: 1123–1129
- Zager JS, Gershenwald JE, Aldrink J, Lin E, Cormier JN, Cheng T, Yoo J, Lee JE, Tyler DS, Ross MI. Isolated limb infusion for locally recurrent and in-transit extremity melanoma: A combined institutional initial experience. Ann Surg Oncol 2006; S13: 84
- Beasley GM, Petersen RP, Yoo J, McMahon N, Aloia T, Petros W, Tsung G, Sanders C, Pruitt S, Seigler H, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: A well tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Onc 2007, submitted
- Grubbs EG, Ueno T, Abdel-Wahab O, Cheng TY, Pruitt SK, Colvin OM, Friedman HS, Tyler DS. Modulation of resistance to regional chemotherapy in the extremity melanoma model. Surgery 2004; 136(2)210–218
- Grubbs EG, Abdel-Wahab O, Cheng TY, Abdel-Wahab Z, Peterson B, Pruitt SK, Colvin OM, Friedman HS, Tyler DS. In-transit melanoma: The role of alkylating-agent resistance in regional therapy. J Am Coll Surg 2004; 199: 419–427
- Green J, Vistica D, Young R, Hamilton TC, Rogan AM, Ozols RF. Potentiation of melphalan cytotoxicity in human ovarian cancer cell lines by glutathione depletion. Cancer Res 1984; 44: 5427–5431
- Suzukake K, Vistica B, Vistica D. Resistant tumor cells and its relationship to intracellular glutathione content. Biochem Pharmacol 1983; 32: 165–167
- Grubbs E, Ueno T, Abdel-Wahab O, Cheng TY, Pruitt SK, Colvin OM, Friedman HS, Tyler DS. Modulation of resistance to regional chemotherapy in the extremity melanoma model. Surgery 2004; 136: 210–218
- Tyler DS, Yashimoto Y, Grubbs E, Yoo J, Zipfel P, Selim A, Augustine C. Novel strategies to overcome chemoresistance in regional melanoma therapy by systemic modulation of tumor proteins. Mel Res 2006; 16(Suppl.1)S100
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417: 949–954
- Eisen T, Ahmad T, Gore M, Marais R, Gibbens I, James MG, Schwartz B, Bergaminin L. Phase 1 trial of Bay 43-9006 (Sorafenib) combined with DTIC in metastatic melanoma patients. Proc ASCO 2005; 23: 712S, Abstract 7508
- Flaherty KT, Brose M, Schuchter L, Tuveson D, Lee R, Schwartz B, Lathia C, Weber B, O’Dwyer P. Phase I/II trial of Bay 43-9006, carboplatin and paclitaxel demonstrates preliminary antitumor activity in the expansion cohort of patients with metastatic melanoma. Proc ASCO 2004; 22: 7507
- Lorigan P, Corrie P, Chao D, Nathan P, Ahmad T, Marais R, Burk K, Erlandsson F, Gore M, Eisen T. Phase II trial of sorafenib combined with dacarbazine in metastatic melanoma patients. Proc ASCO 2006; 24, No. 18S
- Agarwala SS, Keilholz U, Hogg D, Robert C, Hersey P, Eggermont A, Grabbe S, Gonzalez R, Patel K, Hauschild A. Randomized phase III study of paclitaxel plus carboplatin with or without sorafenib as second-line treatment in patients with advanced melanoma. Proc ASCO 2007; 25: 8510
- Niu G, Bowman T, Huang M, Shivers S, Reintgen D, Daud A, Chang A, Kraker A, Jove R, Yu H. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene 2002; 21: 7001–7010
- Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002; 21: 2000–2008
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–2342
- Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF, Gaudreault J, Damico LA, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non–small cell cancer. J Clin Oncol 2004; 22: 2184–2191
- Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 2005; 23: 792–799
- Vredenburgh JJ, Desjardins A, Herndon JE, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Wagner M, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 2007; 25: 4722–4729
- González CM, Viteri S, Garrán C, Nieto Y, Aristu J, Pomz M, Algarra SM. Response of resistant melanoma to a combination of weekly paclitaxel and bevacizumab. 2007; 9: 119–120
- Terheyden P, Hofmann MA, Weininger M, Brocker EB, Becker JC. Anti-vascular endothelial growth factor antibody bevacizumab in conjunction with chemotherapy in metastasising melanoma. Journal Cancer Res 2007; 133: 897–901
- Varker KA, Biber JE, Kefauver C, Jensen R. A randomized phase II trial of bevacizumab with or without daily low-dose interferon alfa-2b in metastatic malignant melanoma. Ann Surg Oncol 2007; 14: 2367–2376
- Curtin JA, Busam K, Pinkel D, Bastian B. Somatic activation of KIT distinct subtypes of melanoma. J Clin Onc 2006; 24: 4340–4346
- Wyman K, Atkins B, Prieto V, Eton O, McDermott DF, Hubbard F, Byrnes C, Sanders K, Sosman JA. Multicenter phase II trial of high-dose imatinib mesylate in metastatic melanoma. Cancer 2006; 106: 2005–2011
- Berkenblit A, Eder JP, Jr, Ryan DP, Seiden MV, Tatsuta N, Sherman ML, Dahl TA, Dezube BJ, Supko JG. Phase I clinical trial of STA-4783 in combination with paclitaxel in patients with refractory solid tumors. Clin Cancer Res 2007; 13: 584–590
- Bild AH, Guang Y, Chang JT, Potti A, Chasse D, Joshi MB, Harpole D, Jr, Lancaster JM, Berchuck A, Olson JA, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 2006; 439: 353–357
- Potti A, Mukherjee S, Petersen R, Dressman HK, Bild A, Koontz J, Kratzke R, Watson MA, Kelley M, Ginsburg GS, et al. A genomic strategy to refine prognosis in early-stage non-small-cell lung cancer. NEJM 2006; 355: 570–580