Abstract
Purpose: Type 1 diabetes (T1D) is an autoimmune disease in which the insulin producing β cells of the pancreatic islets are destroyed by cytotoxic T lymphocytes (CTLs). It has been demonstrated that the injection of complete Freund's adjuvant (CFA) can prevent disease onset in non-obese diabetic (NOD) mice. This effect has been attributed to CFA-enhanced natural killer (NK) cell mediated control of autoimmune CTLs. Fever-range whole body hyperthermia (FR-WBH) has also been shown to stimulate NK cell cytotoxicity. This led to the hypothesis that FR-WBH can prevent disease onset in NOD mice by a thermally regulated mechanism.
Methods: FR-WBH or mock treatment was administered weekly until the NOD mice reached 32 weeks of age. Blood glucose levels were monitored weekly, with measurements ≥33.5 mM indicating onset of diabetes, at which time the mice were euthanized for histological and cellular analyses.
Results: Weekly FR-WBH prevented the onset of T1D in NOD mice and this effect correlated with increased NK cell cytotoxicity and control of blood glucose concentration. Histological analysis revealed significantly fewer lymphocytes infiltrating the pancreatic islets of FR-WBH treated mice than those of untreated mice, suggesting a relationship between thermally induced protection of β cells and their ability to regulate blood glucose concentrations.
Conclusions: These studies show, for the first time, that mild systemic hyperthermia can prevent the generation of T1D in a clinically relevant mouse model. Further study of the thermally sensitive aspects of immunoregulation could lead to the development of heat-based therapies for the prevention or treatment of autoimmune diseases.
Introduction
Type 1 diabetes (T1D), also known as insulin-dependent diabetes mellitus or juvenile diabetes, is an autoimmune disease that results in the destruction of insulin producing β cells in the islets of Langerhans Citation[1]. Although T1D is not the most common form of diabetes in the US, the physiological effects of this disease tend to have a much greater impact upon patients’ lives than those of the more common adult-onset form of diabetes known as type II or non-insulin-dependent diabetes mellitus Citation[2]. Thus, the identification of safe new therapeutic strategies for prevention and treatment of T1D is of utmost importance for the eventual elimination of this disease or for the reduction of the severity of the symptoms associated with disease progression. While the study of patients with T1D has provided important insight into the mechanism of disease onset and progression, the identification of the non-obese diabetic (NOD) mouse, which spontaneously develops a clinically relevant form of T1D 60–80% of the time, has provided an important experimental model to test new preventative therapies Citation[3–5].
Since T1D is a disease resulting from irreversible β cell destruction by immune effector cells, slowing or blocking the ability of the immune system to cause autoimmune destruction as early as possible during disease progression would seem a logical strategy for fighting this disease. Thus, it seems paradoxical that an effective way of preventing the onset of autoimmune diabetes was demonstrated by the treatment of NOD mice with the potent immunological stimulator, complete Freund's adjuvant (CFA). A single injection of CFA was discovered to prevent the onset of hyperglycemia in NOD mice and greatly increases the lifespan of these mice without additional therapy Citation[6–9]. While none of the CFA treated mice developed hyperglycemia by one year of age, the majority of the untreated NOD mice developed diabetes and subsequently died within eight months Citation[6]. Even the adoptively transferred lymphocytes from the spleen and the pancreatic draining lymph nodes of normal male NOD mice, known to generate the diabetic state Citation[7], were markedly suppressed when the recipients were treated with CFA Citation[8]. Unfortunately, although these data on CFA are suggestive of exciting new avenues of treatment, use of CFA itself is not permitted in humans because it creates significant systemic toxicity and unacceptable inflammatory reactions.
Previous researchers have suggested that the effectiveness of CFA treatment in preventing diabetes is through CFA-induced stimulation of natural killer (NK) cell activity Citation[9], Citation[10]. NOD mice have impaired NK cell function due to modulations in the levels of NKG2D, an activating receptor, on the cell surface Citation[10]. This defect in activation in NK cells, which has also been found in patients with T1D, causes impaired NKG2D-dependent cytotoxicity and IFN-γ production Citation[10]. However, CFA treatment has been shown to stimulate IFN-γ secretion resulting in the accumulation of NK cells in the blood and is associated with a reduction of β cell antigen specific cytotoxic T lymphocytes (CTLs) Citation[9]. Further implication of NK cells as the target of CFA treatment was obtained by the observation that the preventative effect of CFA is lost when NK cells were depleted in NOD/SCID mice that received adoptively transferred autoreactive CTLs. However, when NK cells were injected into the same mouse model, the preventative effect was recovered Citation[9]. Together these data suggest that NK cell stimulation is responsible for the prevention of T1D induced by the immunological adjuvant CFA.
Research in our laboratory has revealed that raising the body temperature of normal and tumor-bearing mice to fever-range for several hours can act as an immunological adjuvant, enhancing various endpoints of the immune response. For example, fever-range temperatures (39.5–40°C) have been shown to enhance antigen specific immunity during a contact hypersensitivity response Citation[11]. Further, mild fever-range whole body hyperthermia (FR-WBH) has also been found to increase the activation associated migration of epidermal dendritic cells Citation[12], Citation[13], resulting in the enhanced ability of these cells to stimulate an adaptive immune response Citation[14]. LPS induced nitric oxide production by murine peritoneal macrophages is also enhanced by fever-range hyperthermia Citation[15]. More recently we have found that mild, fever-like thermal stress can also increase NK cell cytotoxic activity Citation[16]. This is further supported by the earlier observation that the anti-tumor effects of FR-WBH appear to be dependent on NK cells Citation[17]. Together these data have prompted us to test the hypothesis that raising body temperature could delay, or even prevent the onset of T1D in NOD mice. Since safe and effective strategies are needed for the prevention and treatment of humans with T1D, manipulation of body temperature within physiological limits could provide a feasible strategy for further evaluation.
Methods
Animals
Female NOD/MrkTac mice (Taconic Farms, Germantown, NY) were 6 weeks old upon arrival and were housed in microfilter cages (Lab Products, Maywood, NJ) in an air-conditioned and light-controlled (12 h/day) room. Mice had access to chow and water ad libitum in between treatments. The RPCI Institute Animal Care and Use Committee approved all experiments using these mice.
CFA preparation and injection
Equal volumes of complete Freund's adjuvant (CFA) (Sigma, St Louis, MO) and sterile, non–pyrogenic 0.9% saline were emulsified in a micromate interchangeable syringe with a 22-gauge needle, 100 μL of which was then injected once subcutaneously at the base of the mouse's tail at 8 weeks of age. Mice were continuously monitored for weight loss and any signs of morbidity as killed mycobacterium (a component of CFA) are known to induce other autoimmune syndromes in NOD mice Citation[5].
Fever-range whole body hyperthermia
Starting at 8 weeks of age, whole body hyperthermia (FR-WBH) was performed as previously described Citation[18] once a week, once every two weeks, or once a month. Briefly, mice were injected i.p. with 1.0 mL sterile, non-pyrogenic 0.9% saline before each FR-WBH treatment to prevent dehydration Citation[19]. Mice were then placed in preheated cages (5 mice/cage) without access to water (to circumvent the possibility of water-induced cooling) and transferred to an environmental chamber (Memmert model BE500, East Troy, WI), the temperature of which is adjusted to maintain the animals’ average core body temperature at 39.5°–40°C for 6–8 hours. Body temperatures were monitored using a microchip transponder (BMDS, Seaford, DE) implanted subcutaneously into the dorsal thoracic area one week prior to initial heating. After removal from the heated environmental chambers, mice were not observed immediately going to the water for a drink, thus supporting the efficacy of saline injections in preventing dehydration. Control and CFA-treated animals were handled similarly to hyperthermia-treated animals (i.e. receiving saline injection, placed in the dark, and kept without water), but remained at normothermic temperature.
Measurement of glucose concentration and determination of disease onset
To measure blood glucose concentrations, mice were placed in a plexiglass tail restraint in order to maintain access to a tail vein. Using a 27-gauge needle, a tail vein was punctured yielding a drop of blood. The blood was loaded into a Walgreens blood glucose test strip (Deerfield, IL) attached to a Walgreens® TrueTrack™ smart system in which blood glucose concentration (mmol/L) was measured. Normal blood glucose levels in mice average around 5–7 mM, however, as disease progresses mice develop hyperglycemia (>7 mM) which will fluctuate in its severity depending on the animal's diet and activity level. However, when blood glucose concentrations reach levels as high as 33.5 mM, it is associated with the inability to regulate glucose concentration, and thus can be considered complete disease progression. Therefore, mice with glucose readings ≥33.5 mM, which registered on the meter as ‘high’, were considered diabetic and were euthanized Citation[9]. Glucose concentrations were taken weekly at the same time and day every week to control for changes in feeding behavior.
Measurement of insulin concentrations
To measure serum insulin concentrations, 20 μL of blood was collected per mouse from the retro-orbital vein once a month from 8 weeks to 32 weeks of age. The blood was allowed to clot for 30 min and then centrifuged for serum collection. A rat/mouse insulin ELISA kit (LINCO Research, St Charles, MO) was used to calculate serum insulin concentrations using rat/mouse insulin standards provided by the kit.
Histology
When the mice became diabetic or at the end of an experiment, pancreas tissues were harvested and placed in zinc fixative overnight, then placed into 70% ethanol for 24 hours, embedded in paraffin, sectioned on a Finesse 325 microtome (5 μm), and stained using a standard hematoxylin and eosin staining protocol. The histology was examined at 40× magnification with a light microscope to confirm diabetes onset.
Isolation of NK cells and measurement of cytotoxic activity
NK cells were isolated two days post FR-WBH from total splenocytes of normothermic, CFA, and FR-WBH treated NOD mice by negative selection using a MACS® mouse NK cell isolation kit as per the manufacturer's directions (Miltenyi Biotec Inc., Auburn, CA). Purity of the NK cells was >95% as determined by flow cytometry using FITC anti-mouse pan-NK cell antibody (DX5; BD PharMingen, San Diego, CA). Purified NK cells were then utilized in a CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI) in which lytic activity against NK specific YAC-1 target cells (obtained from ATCC) was measured. Lactose dehydrogenase secretion was measured using a MRX 96 well spectrophotometer (Dynatech Laboratory, Orlando, FL) at 490 nm. Percentage cytotoxicity was determined using the formula: % cytotoxicity = 100 × [(experimental release − effector spontaneous release − target spontaneous release)/(target maximum release − target spontaneous release)].
Statistics
The Student's t-test was used to calculate statistical significance where indicated. A log-rank test was applied to compare survival curves. Data was determined to be statistically significant if the p-value was <0.05.
Results
Weekly FR-WBH prevents the onset of T1D in NOD mice
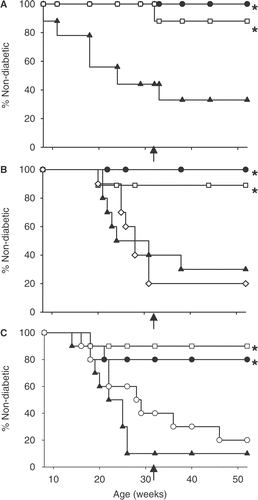
To determine whether FR-WBH could prevent the onset of T1D, female NOD mice were treated once a week with an 8-hour FR-WBH and compared to normothermic and CFA treated mice for incidence of diabetes onset as determined by a blood glucose level ≥33.5 mM and by the presence of islet infiltration. Since NOD mice are more susceptible to developing other autoimmune syndromes that can occur when exposed to killed mycobacterium Citation[5], which is a component of CFA, it was especially important to maintain continuous monitoring of the mice and confirm diabetes onset by both blood glucose concentrations and histology. At 32 weeks of age, none of the FR-WBH treated mice were diabetic (A). This is in contrast to the normothermic mice, of which over half were already diabetic at this age. Furthermore, even after termination of the weekly FR-WBH treatments, there was not a significant increase of diabetes incidence for up to 52 weeks of age suggesting the potential for long-term control by early treatment with weekly FR-WBH provided for a finite period of time.
Figure 1. Weekly FR-WBH prevents the onset of T1D. Female NOD mice were either left at normothermic temperatures (▴), injected with a single dose of CFA at 8 weeks of age (•), or heated for 6 (B and C) or 8 (A) hours once a week (□; A, B, C), once a month (⋄; B), or once every two weeks (○, C). Percent non-diabetic mice represent those mice with blood glucose levels that were <33.5 mM. Arrow indicates when FR-WBH treatments were terminated. N = 8-10 mice per group in each experiment; *, p ≤ 0.004 when compared to the normothermic group using a log rank statistical test.
Less frequent FR-WBH treatments do not prevent the onset of T1D
To determine whether a less frequent FR-WBH treatment could prevent the onset of T1D, a FR-WBH treatment schedule of once a month was first compared to the weekly FR-WBH treatment schedule for incidence of disease onset (B). For these experiments, the duration of FR-WBH treatment was shortened from 8 to 6 hours when it was discovered by Ostberg and colleagues that a 6 hour in vitro hyperthermia (39.5°C) was sufficient for enhancing NK cell cytotoxicity Citation[16]. Although it was shorter in duration, the weekly 6 hour FR-WBH treatments were able to significantly prevent diabetes onset. In contrast to the weekly FR-WBH treated mice, the percentage of monthly FR-WBH treated and normothermic non-diabetic mice were not significantly different from one another upon the termination of the FR-WBH treatments. Since monthly FR-WBH treatments did not prevent the onset of T1D, we tested administration of FR-WBH once every two weeks and found that although some disease control did occur, the difference was not statistically significant in comparison to control mice (C). However, in each experiment, weekly FR-WBH and CFA treatments were effective in preventing disease onset, confirming that weekly FR-WBH, administered during a period of 8 to 32 weeks of age, consistently prevents diabetes in NOD mice.
Blood glucose concentrations are regulated in weekly FR-WBH treated mice
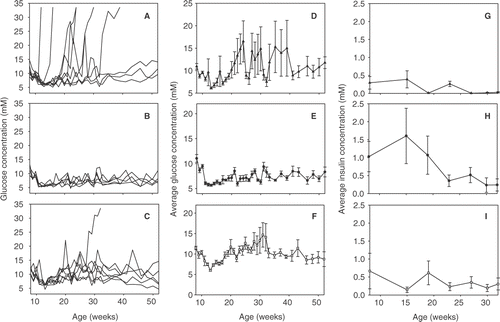
To better characterize the physiological effects of weekly FR-WBH treatments on NOD mice, weekly blood glucose concentrations of every mouse were examined. As expected, NOD mice kept at normothermic temperatures demonstrated the widest range of blood glucose levels from 5.1 mM to diabetic levels greater then 33.5 mM (A). However, since not every NOD mouse develops T1D at the exact same time, the average blood glucose concentration at any given time is lower than 33.5 mM (D). In contrast, NOD mice treated with CFA showed the tightest level of regulation with a blood glucose concentration range of only 4.7 to 13.2 mM (B and E). Blood glucose concentrations were also controlled in the weekly FR-WBH treated mice, although they displayed a greater variance than the CFA treated mice, from 4.7 to 25.4 mM, with the exception of one mouse that became diabetic (C and F). Blood glucose concentrations are regulated by insulin secreted from the β cells in the pancreas, therefore one explanation for the difference in blood glucose concentration ranges observed between the weekly WBH and CFA treated mice might relate to differences in the amount of insulin found in the blood. To test this, serum insulin levels were examined in the normothermic, CFA, and weekly FR-WBH treated NOD mice. There was no significant difference in insulin concentrations between the three groups. However, mice treated with CFA appeared to have the largest concentration of insulin (H) whereas weekly WBH treated NOD mice appeared to have pretty consistent insulin levels (I). This is in contrast to mice kept at normothermic temperatures, where insulin levels at the beginning of the experiments were similar to those seen in WBH treated mice, but were undetectable by week 19 (G), thus corresponding to the high incidence of diabetes in this group of mice.
Figure 2. Blood glucose concentrations are regulated in weekly FR-WBH treated NOD mice. Female NOD mice were left at normothermic temperatures (A, D, G), injected with a single dose of CFA (B, E, H), or heated for 8 hours once a week from 8-32 weeks of age (C, F, I). Blood glucose measurements were taken once a week for 52 weeks (A–F). Serum insulin measurements were taken once a month until the mice reached 8 months of age (G–I). NOD mice were considered diabetic when blood glucose levels were ≥33.5 mM. (A–C) Each line represents an individual mouse. N = 8–10 mice per group in each experiment.
A decrease in the lymphocytic infiltration of pancreatic islets is seen in mice given weekly FR-WBH
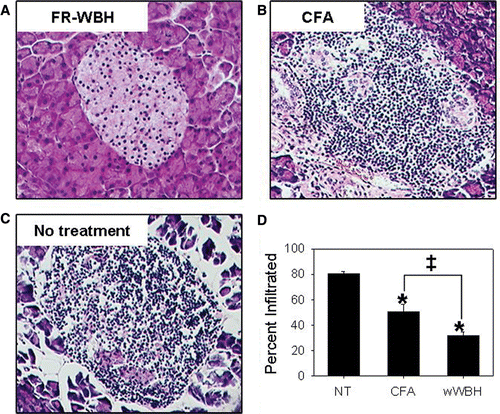
One of the hallmarks of T1D is the chronic destruction of the pancreatic islets by autoreactive CTLs. Thus we also examined the levels of islet lymphocyte infiltration in the pancreata of weekly FR-WBH treated, CFA treated, and normothermic NOD mice. As expected, normothermic NOD mice exhibited the highest percentage of islets with lymphocytic infiltrates (), with approximately 80% of the pancreatic islets showing heavy infiltration. CFA and weekly FR-WBH treated NOD mice demonstrated a significantly lower percentage of lymphocyte infiltrated pancreatic islets when compared to normothermic mice (). Most interestingly, weekly FR-WBH treated NOD mice had significantly less infiltration in their islets than CFA treated NOD mice. Together with the differences in glucose measurements mentioned above, this comparison of the pancreatic histology further suggests that differential protective mechanisms may be occurring in CFA versus FR-WBH treated mice.
Figure 3. Pancreatic islets of weekly FR-WBH treated NOD mice demonstrate significantly less lymphocytic infiltration than those of CFA treated mice. Hematoxylin and Eosin staining was performed on pancreatic tissue of normothermic (NT; C), CFA (B), and weekly FR-WBH (A) treated mice and representative images of non-infiltrated and infiltrated pancreatic islets are shown. The percent of lymphocyte infiltrated islets were then quantified in each treatment group. N = 39 age-matched mice in each group; *, p ≤ 0.0217 when compared to normothermic controls using an unpaired Student's t-test; ‡, p = 0.046 when comparing the CFA and weekly FR-WBH groups using an unpaired Student's t-test.
Weekly FR-WBH increases the cytotoxic activity of NK cells
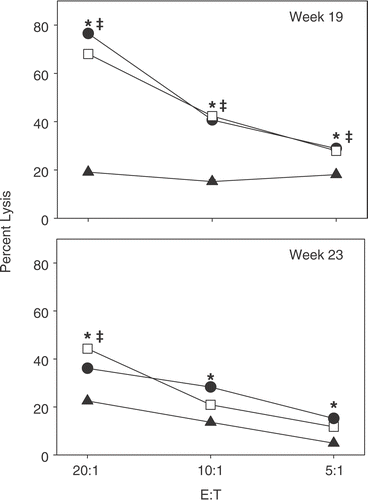
Because CFA has been postulated to prevent diabetes through an NK cell mediated mechanism Citation[9], we next examined the effect of weekly FR-WBH on the ability of splenic NK cells to lyse YAC-1 cells, a classic NK cell-sensitive target. NK cells from weekly FR-WBH and CFA treated NOD mice have significantly higher lytic activity then age-matched normothermic controls that have become diabetic (). Interestingly, the enhancing effect of weekly FR-WBH and CFA on NK cell activity appears to diminish with the age of the animal, as indicated by the decrease in fold enhancement when using splenic NK cells from 23-week-old NOD mice.
Figure 4. FR-WBH treated NOD mice exhibit greater NK cell cytotoxic activity then normothermic NOD mice that have become diabetic. When normothermic NOD mice became diabetic, splenic NK cells were harvested from the diabetic mouse (▴), as well as age matched non-diabetic, weekly FR-WBH treated (□), or CFA treated (•) mice, and analyzed for lytic activity against NK specific YAC-1 targets. In the case of the FR-WBH treated mice, the glucose measurements and harvesting of splenocytes/cytotoxicity assays were carried out two days after a WBH treatment. Samples collected at 19 (top) and 23 (bottom) weeks of age were examined in triplicat wells to determine mean % lysis +/− S.D., with error bars hidden by symbols. Using an unpaired Student's t-test, p < 0.05 when weekly FR-WBH (‡) and CFA (*) treated splenic NK cells were compared to normothermic controls. Data represent 3 separate experiments at each age.
Discussion
The studies reported here demonstrate that a weekly application of mild, FR-WBH is as efficient as CFA in preventing the onset of T1D in pre-diabetic NOD mice. Our experiments show that NOD mice treated with weekly FR-WBH demonstrate less pancreatic islet destruction in addition to more tightly regulated blood glucose concentrations than mice given no heat treatments. Together these data demonstrate for the first time that weekly mild WBH treatments help preserve the pancreatic islets in great enough numbers to halt the progression of T1D. Since we observed no toxicity associated with weekly heat treatments, thermal therapy could be a viable option to simulate the preventative effects associated with CFA for diabetic patients.
While further study is needed to fully understand the thermally-driven underlying mechanisms associated with prevention of T1D by FR-WBH, at least part of the effect may involve the observed increase in NK cell cytotoxic activity in comparison to that of age-matched, diabetic NOD mice kept under normothermic conditions. However, this enhancement in cytotoxicity diminishes over time (possibly due to either an age-related decline in activity or through thermal tolerance mechanisms) even though control of disease continues, suggesting that long-term activation of NK cells is not needed to prevent diabetes onset. These findings further suggest that the preventative mechanisms of CFA and weekly FR-WBH may both be mediated through similar effects on the function of NK cells. Lee et al. hypothesized that NK cells regulated the autoimmune response by directly affecting the β cell-specific CTLs Citation[9]. They suggested that CFA induces dendritic cell presentation of mycobacterial antigen to NKT cells via CD1 mediated mechanisms Citation[20], activating the NKT cells to secrete IFN-γ, which in turn stimulates NK cells Citation[21]. These stimulated NK cells can then block the expansion of β cell-specific CTLs Citation[22], thus reducing the number of CTLs, and therefore preventing β cell destruction. However, while Lee and colleagues hypothesize that NK cells are mediating the inhibitory mechanism of CFA Citation[9], their research and our work presented here does not eliminate the possible direct role of other regulatory immune cells such as NKT and regulatory T cells in providing the suppressive benefits of CFA or hyperthermia treatment. Therefore, it will be important to determine via immunohistochemistry whether NK, NKT, or regulatory T cells are present in higher numbers at the sites of infiltration in our FR-WBH treated mice and to deplete these cell types individually to help define what mechanism FR-WBH utilizes in preventing diabetes onset.
In the NK cell depletion experiments by Lee et al., they utilized anti-asialo-GM-1, an antibody that has historically been used to specifically deplete NK cells. However, asialo-GM1 can also be found on NKT cells and a small subset of regulatory T cells Citation[23], both of which are known to have the potential to directly regulate specific CTL responses Citation[5]. Further research on the role of NK cells in the prevention of T1D by both CFA and weekly FR-WBH needs to be performed using an antibody specific to NK cells in order to offer further proof that NK cells are indeed the cells causing the inhibitory effect. In addition, studies examining β cell-specific CTL activity, as well as CTL numbers and/or the general composition of the islet infiltrate will be important for future study.
Some differences between the preventative effects of CFA and weekly FR-WBH are seen in our data on blood glucose levels and histological studies in NOD mice, suggesting that there are differences in the progression of β cell destruction and degree of glucose regulation in these two preventative treatments. While these differences could be related to dose and scheduling issues, it is likely that CFA's preventative effect begins at the time of injection, before lymphocyte infiltration has occurred. This may preserve more of the pancreatic islet composition at earlier stages of disease progression as demonstrated by the more tightly regulated blood glucose concentrations. However, over time, the antigen supply of the oil emulsion of CFA may have decreased significantly enough so that CFA increasingly fails to prevent lymphocytic infiltration into the pancreatic islets to the same degree observed with weekly FR-WBH treatments. The findings that weekly FR-WBH treated NOD mice have less lymphocytic infiltration than CFA treated NOD mice would suggest that weekly FR-WBH treated mice should be producing more insulin therefore causing more cells to take up glucose from the blood. However, this is not observed, as analysis of blood glucose concentrations revealed a wider range of variation with FR-WBH as compared to the CFA administration, although the glucose concentrations remained within a normal range for both treatments. The variation seen in the glucose concentrations could also be a result of diet and exercise. To control for the animals’ feeding behavior, the glucose concentrations were measured at the same time and day for each group of mice. Although variations in glucose concentrations were seen in FR-WBH treated, CFA treated, and normothermic groups, the most severe spikes in glucose concentrations were seen in the normothermic mice, and correlated with the onset of disease. Furthermore, while not statistically significant, FR-WBH treated NOD mice display a trend toward lower levels of insulin when compared to the CFA treated group. Since the FR-WBH mice are not becoming diabetic (as determined by their glucose readings) it is possible the FR-WBH may be affecting insulin activity in a manner that allows for better glucose regulation. Lastly, since weekly FR-WBH, but not FR-WBH administered once a month or once every two weeks, prevented the onset of T1D, it appears that a more frequent heat treatment is required for the preventative effect. Although our work addressed preventative rather than treatment efficacy of WBH, the association between weekly FR-WBH and prevention of T1D as well as its ability to increase NK cell activity provides additional support for development of therapies targeting NK cells even in later stages of T1D. Future work will address the therapeutic potential of FR-WBH using the NOD mouse model.
Unlike CFA, FR-WBH adjuvant therapy is a non-toxic approach to stimulating a regulatory immune response that may be administered to individuals with very early stage disease or even those patients exhibiting only signs of hyperglycemia. Indeed, FR-WBH has been utilized in multiple pre-clinical models (including BALB/c, C57BL/6, and SCID mice) Citation[18], Citation[24] and in several clinical trials without demonstrating any severe side effects Citation[25], Citation[26]. In addition, with the advent of autoantibody-based detection kits against insulin, doctors will be able to diagnose disease well before clinical signs are apparent. Therefore, although much more work must be done to understand thermally regulated immune reactions, and to optimize treatment feasibility for patients (e.g., examining FR-WBH treatments that are shorter in duration or involve fewer total number of weekly treatments), clinical incorporation of mild heating protocols could be extremely beneficial in the future for both prevention and treatment of patients with T1D.
Acknowledgements
This research was supported by grants from the National Institute of Health (NIH; CA94045, CA71599, CA098852). The authors thank Dr. Bonnie Hylander, Melissa Grimm, and Lingwen Zhong for their scientific insight, helpful discussions, and expert review of this manuscript. We would like to thank Ann Miller and Rebecca Vu for their assistance in analysing data and Jeanne Prendergast and Diane Thompson for their laboratory management and technical assistance.
References
- Yoon J-W, Jun H-S. Autoimmune destruction of pancreatic beta cells. Am J Therapeutics 2005; 12: 580–591
- Aly T, Devendra D, Eisenbarth GS. Immunotherapeutic approaches to prevent, ameliorate, and cure type I diabetes. Am J Therapeutics 2005; 12: 481–490
- Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Kikutani H. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu 1980; 29: 1–13
- Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol 1992; 51: 285–322
- Anderson MS, Bluestone JA. The NOD mouse: A model of immune dysregulation. AnnRev Immunol 2005; 23: 447–485
- Sadelain MWJ, Qin H-Y, Lauzon J, Singh B. Prevention of Type I diabetes in NOD mice by adjuvant immunotherapy. Diabetes 1990; 39: 1–6
- Ulaeto D, Lacy PE, Kipnis DM, Kanagawa O, Unanue ER. A T cell dormant state in the autoimmune process of nonobese diabetic mic treated with complete Freund's adjuvant. PNAS 1992; 89: 3927–3931
- Qin H-Y, Sadelain MWJ, Hitchon C, Lauzon J, Singh B. Complete Freund's adjuvant-induced T cells prevent the development and adoptive transfer of diabetes in nonobese diabetic mice. J Immunol 1993; 150: 2072–2080
- Lee I-F, Qin H, Trudeau J, Dutz J, Tan R. Regulation of autoimmune diabetes by complete Freund's adjuvant is mediated by NK cells. J Immunol 2004; 172: 937–942
- Ogasawara K, Hamerman JA, Hsin H, Chikuma S, Bour-Jordan H, Chen T, Pertel T, Bluestone JA, Carnaud C, Lanier LL. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity 2003; 18: 41–51
- Ostberg JR, Gellin C, Patel R, Repasky EA. Regulatory potential of fever-range whole body hyperthermia on Langerhans cells and lymphocytes in an antigen-dependent cellular immune response. J Immunol 2001; 167: 2666–2670
- Ostberg JR, Repasky EA. Emerging evidence indicates that physiologically relevant thermal stress regulates dendritic cell function. Cancer. Immunol Immunother 2006; 55: 292–298
- Ostberg JR, Patel R, Repasky EA. Regulation of immune activity by mild (fever-range) whole body hyperthermia: Effects on epidermal Langerhans cells. Cell Stress Chaperones 2000; 5: 458–461
- Ostberg JR, Kabingu E, Repasky EA. Thermal regulation of dendritic cell activation and migration from skin explants. Int J Hyperthermia 2003; 19: 520–533
- Pritchard MT, Li A, Repasky E. Nitric oxide production is regulated by fever-range thermal stimulation of murine macrophages. J Leukocyte Biol 2005; 78: 630–638
- Ostberg JR, Dayanc BE, Yuan M, Oflazoglu E, Repasky EA. Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent upon NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells. J Leukocyte Biol 2007; 82: 1322–1331
- Burd R, Dziedzic TS, Xu Y, Caligiuri MA, Subjeck JR, Repasky EA. Tumor cell apoptosis, lymphocyte recruitment and tumor vascular changes are induced by low temperature, long duration (fever-like) whole body hyperthermia. J Cell Physiol 1998; 177: 137–147
- Pritchard MT, Ostberg JR, Evans SS, Burd R, Kraybill W, Bull JM, Repasky EA. Protocols for simulating the thermal component of fever: Preclinical and clinical experience. Methods 2004; 32: 54–62
- Shen RN, Hornbeck NB, Shidnia H, Wu B, Lu L, Broxmeyer HE. Whole body hyperthermia: A potent radioprotector in vivo. Int J Radiat Oncol, Biol, Phys 1991; 20: 525–530
- Thurnher M, Ramoner R, Gastl G, Radmayr C, Bock G, Herold M, Klocker H, Bartsch G. Bacillus Calmette-Guerin mycobacteria stimulate human blood dendritic cells. Int J Cancer 1997; 70: 128–134
- Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol 1999; 163: 4647–4650
- Zitvogel L. Dendritic and natural killer cells cooperate in the control/switch of innate immunity. J Exp Med 2002; 195: F9–F14
- Slifka MK, Pagarigan RR, Whitton JL. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J Immunol 2000; 164: 2009–2015
- Xu Y, Choi J, Hylander B, Sen A, Evans S, Kraybill W, Repasky EA. Fever-range whole body hyperthermia increases the number of perfused tumor blood vessels and therapeutic efficacy of liposomally encapsulated doxorubicin. Int J Hyperthermia 2007; 23: 513–527
- Kraybill W, Olenki T, Evans S, Ostberg J, O'Leary K, Gibbs F, Repasky EA. A phase I study of fever-range whole body hyperthermia (FR-WBH) in patients with advanced solid tumors: Correlation with mouse models. Int J Hyperthermia 2002; 18: 253–266
- Rowe-Horwege RW. Hyperthermia, systemic encyclopedia of medical devices and intrumentation. John Wiley and Sons, Inc, New York 2006