Abstract
Hyperthermic isolated limb perfusion with tumour necrosis factor alpha (TNF-α) and melphalan was repeatedly reported to achieve extraordinarily high clinical remission rates in advanced and non-resectable soft tissue sarcoma of the limbs, thus avoiding imminent mutilation or amputation for most of those patients. With the limb being isolated throughout the extracorporal perfusion, high doses of recombinant TNF-α as well as melphalan can be applied. Basically, TNF-α directly affects the vasculature of the tumour and induces a severe inflammation with consecutive deterioration of the tumour capillaries. Furthermore, TNF-α increases the tumour-selective uptake of melphalan into the tumour cells thus leading to synergy of antivascular targeted treatment and antineoplastic effects of highest dose chemotherapy supplemented by hyperthermia. Meanwhile, a lot of sarcoma centres in Europe adopted this technique and established referral programmes for patients with non-resectable soft tissue sarcomas of the limbs. Despite these programmes many patients still do not get offered hyperthermic ILP with TNF-α and melphalan as a treatment option and modality. This article summarizes multimodality in treatment of soft tissue sarcoma of the limbs and reviews the current status of melphalan-based ILP with TNF-α (TM-ILP) and its results, to enable comparison and critical consideration of other treatment options.
Soft tissue sarcoma of the limbs
Excluding primary malignant bone tumours such as osteosarcoma, multiple myeloma and Ewing-sar-coma, all other malignant mesenchymal neoplasms form the heterogeneous group of soft tissue sarcomas (STS). Due to the mesenchymal origin, STS occur in every region of the body, but the vast majority of sarcomas (>60%) are localized at the extremities. The primary goal of treatment is complete and radical resection of the tumour whereas margins of roughly 20 mm are being reported to be associated with optimal local tumour control Citation[1], Citation[2]. Simply, there are two categories of STS with regard to their localization and resectability: one category consists of superficially localized tumours which hence are commonly diagnosed in the early stages and rarely exceed a size of more than 5 cm. Most often, these sarcomas can be sufficiently treated by surgical resection with wide margins (20 mm) or margins at least several millimetres wide. In contrast to these tumours which are associated with a good overall prognosis, the other category consists of deep seated sarcomas which are also mostly high grade sarcomas. Because sarcomas do not make any specific symptoms, deep seated tumours commonly reach a significant extent before they are detected, causing non-specific symptoms such as swelling, palpable mass, pain, impairment of function and, rarely, nerve palsy. In contrast to superficially located tumours these deep seated sarcomas are associated with more complex surgical scenarios. There are a considerable number of patients where a radical resection in the sense of clear or even wide resection margins is not feasible. Several studies have clearly demonstrated that insufficient local tumour control increases the rate of local recurrence which negatively impacts on disease free survival and overall survival Citation[1–4]. But studies prior to those reports indicated that mutilating surgical procedures as compartmental resections and amputations–which are apparently the most radical surgical procedures in the sense of local tumour control–do not improve overall survival Citation[5–7]. This is most likely associated with the 40–70% risk of developing distant metastases independently from what is done in order to achieve sufficient local tumour control Citation[6], Citation[8]. Thus, limb sparing treatment has become the key element in the management of soft tissue sarcoma of the limbs.
Multimodality: Prerequisite for limb sparing treatment
Surgery
Even considering all current treatment options involving vascular repair, endoprosthetics and plastic or reconstructive surgery, there will be a significant number of patients where surgical resection alone remains insufficient regarding local tumour control. In patients who are marginal or even incompletely resected there might be a feasibility to perform re-resection which has repetitively been reported to enhance local control and disease free survival Citation[2–4].
Radiotherapy
The value of postoperative radiotherapy has clearly been demonstrated for sarcoma resections with clear but narrow margins according to several retrospective studies Citation[9–13] as well as according to prospective randomized trials Citation[14], Citation[15]. However, beginning of postoperative radiotherapy is frequently hindered by delayed wound healing following extensive surgery. Additionally, the extent of the irradiation field is significantly greater than in preoperative radiotherapy resulting in a considerable loss of function. Davis reported that with a significantly greater rate of fibrosis, joint stiffness and oedema in patients who underwent postoperative radiotherapy Citation[16]. These limitations and the fact that limb-sparing surgery is in general not inferior to amputations strongly enhanced the interest in neoadjuvant treatment options in order to reduce the tumour in size and vitality, thus enabling later resection without mutilation.
Preoperative radiotherapy offers the benefit of short intervals between radiotherapy and surgery, reduced irradiation fields compared to postoperative radiotherapy and a potentially reduced subsequent surgery related to downstaging. However, up to now there is no clear evidence from prospective randomized trials that the timing of radiotherapy does significantly impact on local tumour control Citation[17]. Focusing on the incidence of local recurrences Zagars and coauthors did not detect any differences between pre- and postoperative radiotherapy in their retrospective studies Citation[18]. Whereas late complications are more likely associated with postoperative radiotherapy Citation[16], Citation[18], Citation[19], are major wound complications seriously compromising the results of preoperative radiotherapy at the limbs Citation[16–18], Citation[20], Citation[21]. Thus, the value of preoperative radiotherapy remains unclear.
Chemotherapy
Preoperative chemotherapy has been shown to induce objective remissions in a limited number of patients but did not improve resectability Citation[4], Citation[22–24]. Furthermore, preoperative chemotherapy has failed to demonstrate any benefit in the prospective randomized trial for stage II/III sarcomas in terms of progression-free survival and overall survival Citation[4].
By reason of the radiation-sensitizing effects of doxorubicin and ifosfamide preoperative chemotherapy plus irradiation was investigated and recently reported to be promising in a phase I study Citation[25]. Disease-free survival and overall survival were also superior in a historical control Citation[26] and in the just recently published prospective phase II trial Radiation Therapy Oncology Group (RTOG) Citation[27]. But a rate of substantial systemic toxicities and a considerable number of major local complications necessitates further evaluation of this regimen Citation[20], Citation[23]. The results of the just recently published prospective randomized phase III study on the combination of preoperative chemotherapy with hyperthermia are indicating improved disease-free survival and overall survival in stage II and stage III sarcomas Citation[28]. Although the majority of tumours consisted of resectable soft tissue sarcomas of the limbs, it is the only prospective neoadjuvant trial with adequate power which indicates improved surgical resectability and not significantly increased regional toxicity compared to other treatment modalities.
Besides the evidence that multimodal treatment strategies are a prerequisite to enable limb-sparing surgery, it has also clearly been demonstrated that side effects and complications of those treatment concepts are substantial in number and character, thus significantly impairing vantages of these treatment concepts Citation[19], Citation[20].
Isolated limb perfusion with TNF-α and melphalan: Effectiveness
Regional application of highly dosed chemotherapy seems to be reasonable to control local tumour. As a matter of principle, highest concentrations of chemotherapeutic agents can be achieved since isolation of the tumour-bearing limb is feasible. Via clamping and cannulation of the major artery and vein with placement of a tourniquet proximal to the cannula tips and via usage of an extracorporal rotor pump plus oxygenator, isolated limb perfusion (ILP) is technically established since decades Citation[29]. However, local remission rates have been reported to be very disappointing with solely use of melphalan, doxorubicin and cisplatin Citation[30–33]. It was the advent of recombinant tumour necrosis factor alpha (TNF-α) which made a breakthrough in local tumour control of advanced limb sarcomas. While the systemic use of TNF-α is not indicated due to its severe inflammatory characteristics Citation[34], Citation[35] it can be applied in high doses in the setting of sufficient isolation during limb perfusion. The role of TNF-α in the setting of melphalan-based ILP (TM-ILP) was investigated by Lejeune and co-workers who observed impressive remissions in a pilot study on 19 patients with melanoma or sarcoma of the limbs Citation[36]. These encouraging results were underlined by experimental data Citation[36], Citation[37] and led to several multicentre trials which reported extraordinarily high rates of local responses in advanced limb sarcomas Citation[36], Citation[38–41].
TNF-α: Tumour-specific and vasoactive anti-cancer agent
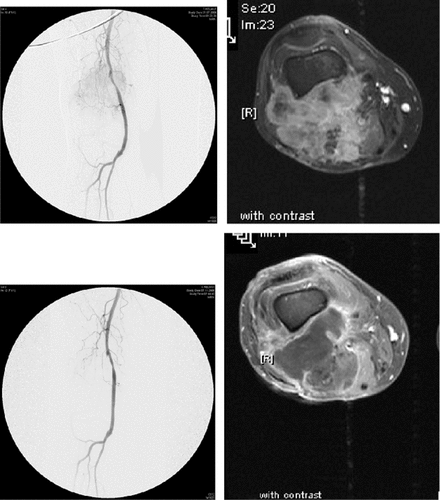
Although Posner reported a decade ago on minimal or missing response in ILP with sole use of TNF-α Citation[42] further research by the group of Lejeune and Eggermont made clear that TNF-α is the key element in ILP. Early experimental data demonstrated an immediate and severe intratumoral activation of endothelial cells by TNF-α which induces a strong up-regulation of endothelial leukocyte adhesion molecule 1 (ELAM-1) as well as vascular cell adhesion molecule 1 (VCAM-1). That is in turn followed by adhesion of polymorphonuclear cells and severe alteration of endothelium with subsequent haemorrhage and necrosis Citation[37], Citation[43]. Interestingly, the authors detected only a slight increase of adhesion molecules in healthy tissue and thus only a very slight inflammation at the vasculature of healthy tissue and skin. That strongly demonstrated that the tumour vasculature is the primary target for TNF-α (). Recent laboratory studies strengthened these findings and clearly demonstrated a deterioration of the vascular structure of the tumour, extravasation of erythrocytes and severe haemorrhage within a 30-minute time frame Citation[44]. A clinical example demonstrating the strong correlation between TM-ILP induced deterioration of the tumour vasculature and subsequent necrosis of the tumour is shown in . The effect of TNF-α on limb sarcomas by itself can be summarized as a tumour-specific induction of severe inflammation and subsequent destruction of the vasculature which leads to an immediate and early shut-down of the metabolism within the tumour. These TNF-specific mechanisms were repeatedly demonstrated in clinical use by angiographies as well as in experimental settings using a rat model with intravital microscopy Citation[37], Citation[45–47].
Figure 1. Male, 54 years; repeatedly operated for popliteal entrapment, finally evolving a big lump in the popliteal fossa (high grade leiomyosarcoma). Recommended TM-ILP to achieve limb salvage. Angiogram and dynamic MRI (upper panel). Complete and tumour-specific deterioration of the vasculature eight weeks following ILP with complete corresponding necrosis of the tumour (lower panel). Subsequent marginal resection revealing a complete histopathologic necrosis. Restoration of soft tissue with a microvascular latissimus dorsi flap.
TNF-α: Catalyser for conventional anti-tumour drugs
But direct immune effects, cellular infiltrations or cytokine expressions linked to TNF-α could not be detected which explains why solely use of TNF-α does not improve response rates in ILP Citation[42], Citation[48], Citation[49]. However, de Wilt and co-workers demonstrated that the induction of rapid damage to the tumour vascular endothelial lining by TNF-α leads to an augmented drug accumulation of melphalan into the tumour tissue Citation[50]. The deterioration of the tumour vasculature was shown to induce a four- to six-fold increased uptake of the cytostatic drug into the tumour tissue compared to ILP with melphalan alone. No increased uptake of melphalan could be seen in normal tissues when melphalan-based ILP was supplemented with TNF-α Citation[47], Citation[49], Citation[50] which substantiated the ILP-TNF-α/melphalan setting (TM-ILP) to be a tumour-specific targeted treatment. While response rates are not linked to specific entities of sarcoma, the vessel- and micro-vessel density was shown to influence the drug uptake. The authors found a strong correlation between the degree of tumour vascularization and the synergistic effects of TNF-α and melphalan Citation[51]. Besides this early effect on tumour vasculature and drug uptake, TNF-α seems to provide a late therapeutic effect. Ruegg et al. reported on a reduced activation of the adhesion receptor αVβ3 in human endothelial cells when exposed to TNF-α Citation[52]. These findings indicate that TNF-α might negatively influence tumour-associated neoangiogenesis by inhibition of the αvβ3 integrin signalling Citation[52], Citation[53].
Alternative cytotoxic and vasoactive drugs
Other agents than melphalan were investigated as alternative drugs. Although response rates following TM-ILP with doxorubicin were comparable to those with melphalan it was less effective regarding limb salvage and associated with an increased regional toxicity Citation[33], Citation[54]. Cisplatin had even lower clinical response rates and was also associated with increased regional toxicity Citation[55]. Actinomycin D which has been used in clinical practice for many years was investigated in standardized animal models by the Rotterdam group of Eggermont, ten Hagen and co-workers. They found that the combination of Actinomycin D and TNF-α leads to idiosyncratic toxicity in normal as well as in tumour tissue resulting in severe tissue destruction with loss of the limb Citation[56].
The same group investigated alternative vasoactive agents to diminish potential systemic side effects of TNF-α which is known to be the strongest inductor of inflammation and severe hemodynamic alterations. By replacing TNF-α with histamine, drug induced intra-tumour haemorrhage and increased drug uptake leading to a 66% overall response rate were reported Citation[57]. Likewise Interleukin 2 was investigated and showed a 67% response rate when combined with melphalan Citation[58]. In contrast to the synergistic effects of histamine and interleukin 2 in systemic immunotherapy, the application of both agents had a conflictive and negative effect in the melphalan-based ILP-setting Citation[59]. Although histamine-based ILP provided excellent response rates in these experimental settings which are comparable to those of TNF, no further clinical trial or data exists. Thus, recombinant TNF-α (rh TNF-α, Beromun®) and melphalan (L-phenylalanine-mustard) still represent the standard drugs for hyperthermia isolated limb perfusion in advanced and non-resectable sarcoma of the limbs Citation[53], Citation[60].
Technical prerequisites and dosage
Patient conditions. Sepsis is associated with an increase of TNF-α receptors which could lead to a non-tumour-specific regional inflammation with irreversible tissue damage. Thus, patients scheduled for TM-ILP should not be in septic conditions. Patients with current chemotherapy should have recovered from leucopenia because experimental data demonstrated a complete loss of TNF-α effect as well as of synergy between TNF-α and melphalan in leucopenic rats Citation[61].
Hyperthermia and oxygenation
To some extent hyperthermia increases the permeability of the endothelial layer and induces recruitment of poorly perfused areas of the vastly pleomorphous sarcomas by arteriovenous shunting. While temperatures below 38°C are associated with a complete loss of all anti-tumour effects, mild hyperthermia (38°–39.0°C) is proven to be most effective Citation[62]. Hyperthermia exceeding 39°C is indeed associated with increasing rates of complete remissions but is also strictly associated with severe damage to normal tissues Citation[42], Citation[62]. Despite the fact that hypoxemia can improve the anti-tumour effects of ILP with either TNF-α or melphalan it does not reach the efficacy of ILP in conditions of normoxaemia or hyperoxaemia with the combined use of TNF-α and melphalan Citation[62].
Isolation and leakage monitoring
TNF-α release in the systemic circuit can induce dose dependent hemodynamic alterations up to shock-like syndromes, therefore a reliable isolation must be warranted throughout the perfusion Citation[35]. This is easily feasible in distal ILPs of the arm and leg where an inflatable tourniquet can be applied proximal to the cannulas and a complete disruption of all collaterals can be achieved by pneumatic pressure. To achieve sufficient isolation at the iliac or axillary level is much more sophisticated where only a tightened Esmarch bandage can be applied in the groin or in the axilla. Numerous collaterals in the pelvic region require a smart balancing between the extracorporal circuit (pressure, flow rate) and systemic hemodynamic (fluid administration, central venous pressure, middle arterial pressure, inotropes, catecholamines, etc.) to obtain sufficient isolation Citation[63]. Although continuous leakage monitoring by radio-labelled serum (99mTc, Indium, Iodine) is in general mandatory for TM-ILP, it is of utmost importance in those proximal perfusions where the balance between the extracorpral and systemic circuit is particularly fragile. Leakage monitoring has definitively proven over the years to enable secure performance of TM-ILP.
Dosage
Dosages of 3-4 mg were established in the initial trials which have led to the approval of TNF-α for sarcoma treatment. Based on a very early single institutional experience (complete local response in all of eight patients with sarcoma of the limb) with TM-ILP and only 1 mg TNF-α Citation[65], it remained unclear whether lower dosages of TNF-α could be as sufficient as the initial dosages which have led to approval. Confirmation of this early report came from an Italian study which consisted of a few patients where ILP using 1 mg TNF-α and doxorubicin (TD-ILP) also had an overall response rate of 90% Citation[66], Citation[67]. Data from further experimental models also demonstrated that dose reduction from the original 50 µg down to 10 µg did not negatively affect clinical response rates. However, the synergistic effect of TNF-α and melphalan was completely lost when further dose reduction beyond this 10 µg threshold was executed Citation[62]. Data from the recent French randomized trial on 100 patients with four assigned dosages (0.5, 1.0, 2, 3–4 mg) did not reveal any dose dependent difference in clinical response rates Citation[66]. Additionally, the Rotterdam experience which is based on the retrospective analysis of more than 200 patients indicated that reduced TNF-α dosages (1–2 mg) resulted in comparable response rates to those where high dose TNF-α was administered Citation[68].
Complications, clinical response rates and clinical outcome
Complications
Complications in melphalan-based ILP with TNF-α derive either from hemodynamic alterations caused by leakage of TNF-α from the systemic circuit or from regional toxicity in the perfused limb. There are no current reports on leakage related complications, which indicates that leakage monitoring seems to be very efficient and thus leakage was not a substantial problem in TM-ILP. Earlier and sparse reports estimated leakage to be a minor problem with 2% leakage on average causing only mild and reversible hemodynamic effects as hypotension or tachycardia Citation[35], Citation[63], Citation[64], Citation[69]. All symptoms related to regional toxicity are classified according to the five grade scale implemented by Wieberdink Citation[70]. This scale is displayed in and shows that grade II reactions consisting of mild hyperaemia, erythema or mild oedema are most frequent following TM-ILP. In contrast to a very mild regional toxicity in most of the patients, the incidence of severe regional toxicity with epidermolysis, compartment syndrome (grade IV) or even tissue damage with loss of the limb (grade V) remains very low Citation[38–40], Citation[71]. Interestingly, the results of the Italian trials with doxorubicin-based ILP showed higher incidences of grade III and IV toxicities whereas melphalan is recommended as the standard cytotoxic agent Citation[66], Citation[67]. However, whenever performing ILP with TNF-α caution must be taken with the limb after revascularization to accurately judge the swelling of the muscle and to execute immediate fasciotomy in case the clinical situation is doubtful Citation[72]. Other complications related to TM-ILP are rare and consist mainly of thromboses at the site of the arteriotomy with an incidence below 2% Citation[73].
Table I. Classification of the regional toxicity in TM-ILP according to Wieberdink Citation[70] (*summarizing results from studies Citation[36], Citation[38], Citation[53], Citation[54], Citation[66], Citation[67], Citation[71], Citation[74]).
Clinical response rates
Lejeune and colleagues pioneered the use of TNF-α in the ILP-setting and were among the first to describe enormous local response rates with this treatment in advanced melanoma and sarcoma Citation[36]. Meanwhile, melphalan-based ILP with TNF-α is successfully established in currently more than 40 sarcoma centres in Europe. Based on patent and licensing rates, the registration file of TM-ILP is yet not presented to the FDA, which is why TM-ILP unfortunately cannot be offered to suitable patients in North America. In Europe TM-ILP has been an approved and registered treatment for advanced soft tissue sarcoma since 1988 Citation[74].
The results of the multicentre trial which has led to approval of TNF-α, revealed complete and partial local responses in amounts which were not previously known in local treatment of advanced soft tissue sarcomas of the limbs Citation[39], Citation[74]. Furthermore, local complication rates were dramatically low compared to what was known from alternative multimodality treatment options Citation[14], Citation[39]. It is quite important to mention that local tumours in all 246 patients of this trial were either judged to be non-resectable (87%) or only resectable if mutilating surgery plus radiotherapy would have been performed (13%); the judgement was performed by an independent surgical review committee. Another aspect which strengthens the superiority of the results of TM-ILP is the selection bias of patients included in the multicentre trial. Only 55% of patients had primary sarcomas, while 45% had local multifocal primary or even multiple recurrences Citation[39]. The 95% rate of patients suffering from high grade sarcomas with a considerable number of tumours exceeding 10 cm and 15% patients with overt distant metastases underline that this collective represents the most problematic subgroup of patients with soft tissue sarcoma. The five year overall survival rate was 50% and did not differ from the survival rate of patients from the Scandinavian sarcoma register (matched control study), thus indicating that TM-ILP does not negatively influence survival Citation[39].
Over the past years reports from different institutions strengthened the results of the initial multicentre study. Focusing on those papers with patient numbers greater than 20, local response rates ranged from 63% to 91% (). Those studies have in common that beyond excellent response rates regional toxicities were reported to be very low, thus resulting in an extraordinarily high limb salvage rate ranging from 58% to 87% Citation[38], Citation[39], Citation[66–68],Citation[71], Citation[75–81].
Table II. Local response rates and limb salvage rates for non-resectable limb sarcoma (TM-ILP).
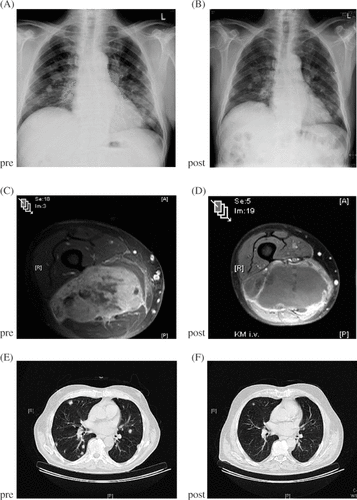
Several studies even demonstrated that TM-ILP can be successfully applied in local recurrent sarcomas with previous surgery and/or radiotherapy Citation[74], Citation[76], Citation[78], Citation[79]. Because of its low toxicity profile and low overall complication rate TM-ILP may serve as the most useful neoadjuvant induction therapy in multimodality treatment concepts. Patients with overt metastatic disease and a limb threatening tumour seem to represent a patient category with a special need for TM-ILP. Extensive surgical interventions to control local tumour and relief symptoms are not indicated prior to systemic chemotherapy because of the delay in systemic chemotherapy Citation[20]. TM-ILP does not hamper the onset of systemic chemotherapy because the local tumour does not get touched surgically thus not resulting in a delay of systemic treatment Citation[20], Citation[45], Citation[60], Citation[82]). demonstrates the typical clinical course of such patients where chemotherapy was almost simultaneously conducted with TM-ILP thus resulting in sufficient local control by complete devitalization and in stabilization of systemic disease by chemotherapy. The necrotic tumours can then be easily resected when systemic treatment is finished or the treatment schedule allows a limited surgical intervention Citation[82]. Analogous to the utilization of chemotherapy, radiotherapy may be applied subsequent to TM-ILP and resection if the margins are not secure. Thereto several authors reported the feasibility of external beam radiotherapy after ILP and its potential to increase local tumour control Citation[81], Citation[83], Citation[84].
Figure 2. Overt metastatic disease (A) of a pleomorphous fibrosarcoma in the thigh in a 72-year-old male with progressive palsy of the sciatic nerve (MRI, C). Onset of chemotherapy 10 days after iliac TM-ILP. Complete response int the thigh within six weeks (MRI, D) with impetuous recovery of the nerve. Partial response of the pulmonary metastases (A, B, E, F) following four cycles of chemotherapy (adriamycin/ifosfamide). Histology of the resection subsequent to completion of chemotherapy showed a 99% necrosis.
Conclusion and key points
Neoadjuvant treatment options are required to achieve sufficient local tumour control in advanced soft tissue sarcomas of the limbs. Preoperative radiotherapy and chemotherapy are well established and accessible at any oncology centre. However, experience from the past decade reveals that these treatment modalities are associated with a significant number of substantial complications as the response rate is limited to some extent Citation[20]. TM-ILP offers an excellent treatment option to efficiently achieve local tumour control even in catastrophic situations, thus avoiding mutilation and amputation in most of these patients. Far more, TM-ILP is associated with a low rate and severity of complications. Although the logistic efforts in executing this treatment are more demanding it is thus far the most effective concept to control advanced local tumour and does not interfere with the necessity and time schedule of inevitable systemic treatment. TM-ILP is now established in more than 50 centres in Europe with growing demand for this treatment option. Besides the challenge to resolve the patent and licensing issue in North America in order to implement and offer this effective treatment, a prospective randomized protocol would be mandatory to gain even more scientific evidence.
References
- Dickinson IC, Whitwell DJ, Battistuta D, Thompson B, Strobel N, Duggal A, Steadman P. Surgical margin and its influence on survival in soft tissue sarcoma. ANZ J Surg 2006; 76: 104–109
- McKee MD, Liu DF, Brooks JJ, Gibbs JF, Driscoll DL, Kraybill WG. The prognostic significance of margin width for extremity and trunk sarcoma. J Surg Oncol 2004; 85: 68–76
- Stojadinovic A, Leung DH, Hoos A, Jaques DP, Lewis JJ, Brennan MF. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg 2002; 235: 424–434
- Trovik CS, Bauer HC, Alvegard TA, Anderson H, Blomqvist C, Berlin O, Gustafson P, Saeter G, Wallöe A. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian Sarcoma Group Register. Eur J Cancer 2000; 36: 710–716
- Rosenberg SA, Tepper J, Glatstein E, Costa J, Baker A, Brennan M, DeMoss EV, Seipp C, Sindelar WF, Sugarbaker P, et al. The treatment of soft-tissue sarcomas of the extremities: Prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982; 196: 305–315
- Stojadinovic A, Jaques DP, Leung DH, Healey JH, Brennan MF. Amputation for recurrent soft tissue sarcoma of the extremity: Indications and outcome. Ann Surg Oncol 2001; 8: 509–518
- Williard WC, Hajdu SI, Casper ES, Brennan MF. Comparison of amputation with limb-sparing operations for adult soft tissue sarcoma of the extremity. Ann Surg 1992; 215: 269–275
- Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin 2004; 54: 94–109
- Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS. Surgical margins and reresection in the management of patients with soft tissue sarcoma using conservative surgery and radiation therapy. Cancer 2003; 97: 2544–2553
- Delaney TF, Kepka L, Goldberg SI, Hornicek FJ, Gebhardt MC, Yoon SS, Springfield DS, Raskin KA, Harmon DC, Kirsch DG, et al. Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys 2007; 67: 1460–1469
- Kepka L, Suit HD, Goldberg SI, Rosenberg AE, Gebhardt MC, Hornicek FJ, Delaney TF. Results of radiation therapy performed after unplanned surgery (without re-excision) for soft tissue sarcomas. J Surg Oncol 2005; 92: 39–45
- Suit HD, Mankin HJ, Wood WC, Proppe KH. Preoperative, intraoperative, and postoperative radiation in the treatment of primary soft tissue sarcoma. Cancer 1985; 55: 2659–2667
- Trovik CS, Bauer HC, Berlin O, Tukiainen E, Erlanson M, Gustafson P, Klepp R, Saeter G, Wahlström O. Local recurrence of deep-seated, high-grade, soft tissue sarcoma: 459 patients from the Scandinavian Sarcoma Group Register. Acta Orthop Scand 2001; 72: 160–166
- Pisters PW, Harrison LB, Woodruff JM, Gaynor JJ, Brennan MF. A prospective randomized trial of adjuvant brachytherapy in the management of low-grade soft tissue sarcomas of the extremity and superficial trunk. J Clin Oncol 1994; 12: 1150–1155
- Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, DeLaney T, Glatstein E, Steinberg SM, Merino MJ, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 1998; 16: 197–203
- Davis AM, O'Sullivan B, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Hammond A, Benk V, Kandel R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 2005; 75: 48–53
- O'Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Kandel R, Goddard K, Sadura A, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002; 359: 2235–2241
- Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS. Preoperative versus postoperative radiation therapy for soft tissue sarcoma: A retrospective comparative evaluation of disease outcome. Int J Radiat Oncol Biol Phys 2003; 56: 482–488
- Cannon CP, Ballo MT, Zagars GK, Mirza AN, Lin PP, Lewis VO, Yasko AW, Benjamin RS, Pisters PW. Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer 2006; 107: 2455–2461
- Pisters PW, O'Sullivan B, Maki RG. Evidence-based recommendations for local therapy for soft tissue sarcomas. J Clin Oncol 2007; 25: 1003–1008
- Tseng JF, Ballo MT, Langstein HN, Wayne JD, Cormier JN, Hunt KK, Feig BW, Yasko AW, Lewis VO, Lin PP, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol 2006; 13: 1209–1215
- Meric F, Milas M, Hunt KK, Hess KR, Pisters PW, Hildebrandt G, Patel SR, Benjamin RS, Plager C, Papadopolous NE, et al. Impact of neoadjuvant chemotherapy on postoperative morbidity in soft tissue sarcomas. J Clin Oncol 2000; 18: 3378–3383
- Pisters PW. Preoperative chemotherapy and split-course radiation therapy for patients with localized soft tissue sarcomas: Home run, base hit, or strike out?. J Clin Oncol 2006; 24: 549–551
- Meric F, Hess KR, Varma DG, Hunt KK, Pisters PW, Milas KM, Patel SR, Benjamin RS, Plager C, Papadopoulos NE, et al. Radiographic response to neoadjuvant chemotherapy is a predictor of local control and survival in soft tissue sarcomas. Cancer 2002; 95: 1120–1126
- Pisters PW, Patel SR, Prieto VG, Thall PF, Lewis VO, Feig BW, Hunt KK, Yasko AW, Lin PP, Jacobson MG, et al. Phase I trial of preoperative doxorubicin-based concurrent chemoradiation and surgical resection for localized extremity and body wall soft tissue sarcomas. J Clin Oncol 2004; 22: 3375–3380
- DeLaney TF, Spiro IJ, Suit HD, Gebhardt MC, Hornicek FJ, Mankin HJ, Rosenberg AL, Rosenthal DI, Miryousefi F, Ancukiewicz M, et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys 2003; 56: 1117–1127
- Kraybill WG, Harris J, Spiro IJ, Ettinger DS, DeLaney TF, Blum RH, Lucas DR, Harmon DC, Letson GD, Eisenberg B, et al. Phase II study of neoadjuvant chemotherapy and radiation therapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. J Clin Oncol 2006; 24: 619–625
- Issels RD. High-risk soft tissue sarcoma: Clinical trial and hyperthermia combined chemotherapy. Int J Hyperthermia 2006; 22: 235–239
- Krementz ET, Carter RD, Sutherland CM, Hutton I. Chemotherapy of sarcomas of the limbs by regional perfusion. Ann Surg 1977; 185: 555–564
- van Ginkel RJ, Schraffordt KH, de Vries EG, Molenaar WM, Uges DR, Hoekstra HJ. Hyperthermic isolated limb perfusion with cisplatin in four patients with sarcomas of soft tissue and bone. Eur J Surg Oncol 1996; 22: 528–531
- Fletcher WS, Pommier RF, Woltering EA, Mueller CR, Ash KO, Small KA. Pharmacokinetics and results of dose escalation in cis-platin hyperthermic isolation limb perfusion. Ann Surg Oncol 1994; 1: 236–243
- Pommier RF, Moseley HS, Cohen J, Huang CS, Townsend R, Fletcher WS. Pharmacokinetics, toxicity, and short-term results of cisplatin hyperthermic isolated limb perfusion for soft-tissue sarcoma and melanoma of the extremities. Am J Surg 1988; 155: 667–671
- Rossi CR, Vecchiato A, Foletto M, Nitti D, Ninfo V, Fornasiero A, Sotti G, Tregnaghi A, Melanotte P, Lise M. Phase II study on neoadjuvant hyperthermic-antiblastic perfusion with doxorubicin in patients with intermediate or high grade limb sarcomas. Cancer 1994; 73: 2140–2146
- Chapman PB, Lester TJ, Casper ES, Gabrilove JL, Wong GY, Kempin SJ, Gold PJ, Welt S, Warren RS, Starnes HF. Clinical pharmacology of recombinant human tumor necrosis factor in patients with advanced cancer. J Clin Oncol 1987; 5: 1942–1951
- Eggimann P, Chiolero R, Chassot PG, Lienard D, Gerain J, Lejeune F. Systemic and hemodynamic effects of recombinant tumor necrosis factor alpha in isolation perfusion of the limbs. Chest 1995; 107: 1074–1082
- Lienard D, Ewalenko P, Delmotte JJ, Renard N, Lejeune FJ. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol 1992; 10: 52–60
- Renard N, Lienard D, Lespagnard L, Eggermont A, Heimann R, Lejeune F. Early endothelium activation and polymorphonuclear cell invasion precede specific necrosis of human melanoma and sarcoma treated by intravascular high-dose tumour necrosis factor alpha (rTNF alpha). Int J Cancer 1994; 57: 656–663
- Eggermont AM, Schraffordt KH, Klausner JM, Kroon BB, Schlag PM, Liénard D, van Geel AN, Hoekstra HJ, Meller I, Nieweg OE, et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter European experience. Ann Surg 1996; 224: 756–764
- Eggermont AM, Schraffordt KH, Liénard D, Kroon BB, van Geel AN, Hoekstra HJ, Lejeune FJ. Isolated limb perfusion with high-dose tumor necrosis factor-alpha in combination with interferon-gamma and melphalan for nonresectable extremity soft tissue sarcomas: A multicenter trial. J Clin Oncol 1996; 14: 2653–2665
- Lejeune F, Liénard D, Eggermont A, Schraffordt KH, Rosenkaimer F, Gérain J, Klaase J, Kroon B, Vanderveken J, Schmitz P. Administration of high-dose tumor necrosis factor alpha by isolation perfusion of the limbs. Rationale and results. J Infus Chemother 1995; 5: 73–81
- Lejeune F, Liénard D, Eggermont A, Schraffordt KH, Rosenkaimer F, Gérain J, Klaase J, Kroon B, Schmitz P. [Efficacy of the tumor necrosis factor-alpha (rTNF-alpha) associated with interferon-gamma and chemotherapy in extracorporeal circulation in the limb in inoperable malignant melanoma, soft tissue sarcoma and epidermoid carcinoma. A 4-year experience]. Bull Cancer 1995; 82: 561–567
- Posner MC, Lienard D, Lejeune FJ, Rosenfelder D, Kirkwood J. Hyperthermic isolated limb perfusion with tumor necrosis factor alone for melanoma. Cancer J Sci Am 1995; 1: 274–280
- Palladino MA, Jr, Shalaby MR, Kramer SM, Ferraiolo BL, Baughman RA, Deleo AB, Crase D, Marafino B, Aggarwal BB, Figari IS. Characterization of the antitumor activities of human tumor necrosis factor-alpha and the comparison with other cytokines: Induction of tumor-specific immunity. J Immunol 1987; 138: 4023–4032
- Hoving S, Seynhaeve AL, van Tiel ST, aan de Wiel-Ambagtsheer G, de Bruijn EA, Eggermont AM, ten Hagen TL. Early destruction of tumor vasculature in tumor necrosis factor-alpha-based isolated limb perfusion is responsible for tumor response. Anticancer Drugs 2006; 17: 949–959
- Eggermont AM, de Wilt JH, ten Hagen TL. Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol 2003; 4: 429–437
- Sijens PE, Eggermont AM, van Dijk PV, Oudkerk M. 31P-magnetic resonance spectroscopy as predictor of clinical response in human extremity sarcomas treated by single dose TNF-alpha + melphalan isolated limb perfusion. NMR Biomed 1995; 8: 215–224
- van Der V, de Wilt JH, Eggermont AM, van Tiel ST, Seynhaeve AL, ten Hagen TL. TNF-alpha augments intratumoural concentrations of doxorubicin in TNF-alpha-based isolated limb perfusion in rat sarcoma models and enhances anti-tumour effects. Br J Cancer 2000; 82: 973–980
- De Blaauw I, Eggermont AM, Deutz NE, de Vries M, Buurman WA, Von Meyenfeldt MF. TNF-alpha has no direct in vivo metabolic effect on human muscle. Int J Cancer 1997; 71: 148–154
- Manusama ER, Stavast J, Durante NM, Marquet RL, Eggermont AM. Isolated limb perfusion with TNF alpha and melphalan in a rat osteosarcoma model: A new anti-tumour approach. Eur J Surg Oncol 1996; 22: 152–157
- de Wilt JH, ten Hagen TL, de Boeck G, van Tiel ST, de Bruijn EA, Eggermont AM. Tumour necrosis factor alpha increases melphalan concentration in tumour tissue after isolated limb perfusion. Br J Cancer 2000; 82: 1000–1003
- van Etten B, de Vries MR, van Ijken MG, Lans TE, Guetens G, Ambagtsheer G, van Tiel ST, de Boeck G, de Bruijn EA, Eggermont AM, et al. Degree of tumour vascularity correlates with drug accumulation and tumour response upon TNF-alpha-based isolated hepatic perfusion. Br J Cancer 2003; 88: 314–319
- Ruegg C, Yilmaz A, Bieler G, Bamat J, Chaubert P, Lejeune FJ. Evidence for the involvement of endothelial cell integrin alphaVbeta3 in the disruption of the tumor vasculature induced by TNF and IFN-gamma. Nat Med 1998; 4: 408–414
- Lejeune FJ, Lienard D, Matter M, Ruegg C. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun 2006; 6: 6
- Di Filippo F, Garinei R, Anzà M, Cavaliere F, Botti C, Perri P, Di Filippo S. Doxorubicin in isolation limb perfusion in the treatment of advanced limb soft tissue sarcoma. J Exp Clin Cancer Res 2003; 22(Suppl.4)81–817
- Thompson JF, Gianoutsos MP. Isolated limb perfusion for melanoma: Effectiveness and toxicity of cisplatin compared with that of melphalan and other drugs. World J Surg 1992; 16: 227–233
- Seynhaeve AL, de Wilt JH, van Tiel ST, Eggermont AM, ten Hagen TL. Isolated limb perfusion with actinomycin D and TNF-alpha results in improved tumour response in soft-tissue sarcoma-bearing rats but is accompanied by severe local toxicity. Br J Cancer 2002; 86: 1174–1179
- Brunstein F, Hoving S, Seynhaeve AL, van Tiel ST, Guetens G, de Bruijn EA, Eggermont AM, ten Hagen TL. Synergistic antitumor activity of histamine plus melphalan in isolated limb perfusion: preclinical studies. J Natl Cancer Inst 2004; 96: 1603–1610
- Hoving S, Brunstein F, aan de Wiel-Ambagtsheer G, van Tiel ST, de Boeck G, de Bruijn EA, Eggermont AM, ten Hagen TL. Synergistic antitumor response of interleukin 2 with melphalan in isolated limb perfusion in soft tissue sarcoma-bearing rats. Cancer Res 2005; 65: 4300–4308
- Brunstein F, Hoving S, de Wiel-Ambagtsheer G, de Bruijn EA, Guetens G, Eggermont AM, ten Hagen TL. Decreased response rates by the combination of histamine and IL-2 in melphalan-based isolated limb perfusion. Cancer Immunol Immunother 2007; 56: 573–580
- Grunhagen DJ, de Wilt JH, ten Hagen TL, Eggermont AM. Technology insight: Utility of TNF-alpha-based isolated limb perfusion to avoid amputation of irresectable tumors of the extremities. Nat Clin Pract Oncol 2006; 3: 94–103
- Manusama ER, Nooijen PT, Stavast J, de Wilt JH, Marquet RL, Eggermont AM. Assessment of the role of neutrophils on the antitumor effect of TNF-alpha in an in vivo isolated limb perfusion model in sarcoma-bearing brown Norway rats. J Surg Res 1998; 78: 169–175
- de Wilt JH, Manusama ER, van Tiel ST, van Ijken MG, ten Hagen TL, Eggermont AM. Prerequisites for effective isolated limb perfusion using tumour necrosis factor alpha and melphalan in rats. Br J Cancer 1999; 80: 161–166
- van Ginkel RJ, Limburg PC, Piers DA, Koops HS, Hoekstra HJ. Value of continuous leakage monitoring with radioactive iodine-131-labeled human serum albumin during hyperthermic isolated limb perfusion with tumor necrosis factor-alpha and melphalan. Ann Surg Oncol 2002; 9: 355–363
- Thom AK, Alexander HR, Andrich MP, Barker WC, Rosenberg SA, Fraker DL. Cytokine levels and systemic toxicity in patients undergoing isolated limb perfusion with high-dose tumor necrosis factor, interferon gamma, and melphalan. J Clin Oncol 1995; 13: 264–273
- Hill S, Fawcett WJ, Sheldon J, Soni N, Williams T, Thomas JM. Low-dose tumour necrosis factor alpha and melphalan in hyperthermic isolated limb perfusion. Br J Surg 1993; 80: 995–997
- Rossi CR, Foletto M, Di Filippo F, Vaglini M, Anza' M, Azzarelli A, Pilati P, Mocellin S, Lise M. Soft tissue limb sarcomas: Italian clinical trials with hyperthermic antiblastic perfusion. Cancer 1999; 86: 1742–1749
- Rossi CR, Mocellin S, Pilati P, Foletto M, Campana L, Quintieri L, De Salvo GL, Lise M. Hyperthermic isolated perfusion with low-dose tumor necrosis factor alpha and doxorubicin for the treatment of limb-threatening soft tissue sarcomas. Ann Surg Oncol 2005; 12: 398–405
- Grunhagen DJ, de Wilt JH, van Geel AN, Graveland WJ, Verhoef C, Eggermont AM. TNF dose reduction in isolated limb perfusion. Eur J Surg Oncol 2005; 31: 1011–1019
- Sleijfer S, van Ginkel RJ, van der Mark TW, Hoekstra HJ, Zwaveling JH, Schraffordt KH, Mulder NH. Effects of hyperthermic isolated limb perfusion with tumor necrosis factor-alpha and melphalan on pulmonary function assessments. J Immunother 1997; 20: 202–207
- Wieberdink J, Benckhuysen C, Braat RP, van Slooten EA, Olthuis GA. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol 1982; 18: 905–910
- Noorda EM, Vrouenraets BC, Nieweg OE, Coeforden van C, van Slooten GW, Kroon BB. Isolated limb perfusion with tumor necrosis factor-alpha and melphalan for patients with unresectable soft tissue sarcoma of the extremities. Cancer 20031; 98: 1483–1490
- Hohenberger P, Finke LH, Schlag PM. Intracompartmental pressure during hyperthermic isolated limb perfusion for melanoma and sarcoma. Eur J Surg Oncol 1996; 22: 147–151
- Klicks RJ, Vrouenraets BC, Nieweg OE, Kroon BB. Vascular complications of isolated limb perfusion. Eur J Surg Oncol 1998; 24: 288–291
- Eggermont AM, Schraffordt KH, Klausner JM, Schlag PM, Kroon BB, Ben-Ari G, van Geel AN, ten Hagen T. Limb salvage by isolated limb perfusion with tumor necrosis factor alpha and melphalan for locally advanced extremity soft tissue sarcomas: Results of 270 perfusions in 246 patients. Proc Am Soc Clin Oncol USA 1999; 11: 497
- Bonvalot S, Laplanche A, Lejeune F, Stoeckle E, Le Péchoux C, Vanel D, Terrier P, Lumbroso J, Ricard M, Antoni G, et al. Limb salvage with isolated perfusion for soft tissue sarcoma: Could less TNF-alpha be better?. Ann Oncol 2005; 16: 1061–1068
- Grunhagen DJ, de Wilt JH, Graveland WJ, Verhoef C, van Geel AN, Eggermont AM. Outcome and prognostic factor analysis of 217 consecutive isolated limb perfusions with tumor necrosis factor-alpha and melphalan for limb-threatening soft tissue sarcoma. Cancer 2006; 106: 1776–1784
- Gutman M, Inbar M, Lev-Shlush D, Abu-Abid S, Mozes M, Chaitchik S, Meller I, Klausner JM. High dose tumor necrosis factor-alpha and melphalan administered via isolated limb perfusion for advanced limb soft tissue sarcoma results in a >90% response rate and limb preservation. Cancer 1997; 79: 1129–1137
- Lans TE, Grunhagen DJ, de Wilt JH, van Geel AN, Eggermont AM. Isolated limb perfusions with tumor necrosis factor and melphalan for locally recurrent soft tissue sarcoma in previously irradiated limbs. Ann Surg Oncol 2005; 12: 406–411
- Lejeune FJ, Pujol N, Liénard D, Mosimann F, Raffoul W, Genton A, Guillou L, Landry M, Chassot PG, Chiolero R, et al. Limb salvage by neoadjuvant isolated perfusion with TNFalpha and melphalan for non-resectable soft tissue sarcoma of the extremities. Eur J Surg Oncol 2000; 26: 669–678
- Lev-Chelouche D, Abu-Abeid S, Kollander Y, Meller I, Isakov J, Merimsky O, Klausner JM, Gutman M. Multifocal soft tissue sarcoma:Limb salvage following hyperthermic isolated limb perfusion with high-dose tumor necrosis factor and melphalan. J Surg Oncol 1999; 70: 185–189
- Olieman AF, Pras E, van Ginkel RJ, Molenaar WM, Schraffordt KH, Hoekstra HJ. Feasibility and efficacy of external beam radiotherapy after hyperthermic isolated limb perfusion with TNF-alpha and melphalan for limb-saving treatment in locally advanced extremity soft-tissue sarcoma. Int J Radiat Oncol Biol Phys 1998; 40: 807–814
- Grunhagen DJ, de Wilt JH, Graveland WJ, van Geel AN, Eggermont AM. The palliative value of tumor necrosis factor alpha-based isolated limb perfusion in patients with metastatic sarcoma and melanoma. Cancer 2006; 106: 156–162
- Hoven-Gondrie ML, Thijssens KM, Van den Dungen JJ, Loonstra J, van Ginkel RJ, Hoekstra HJ. Long-term locoregional vascular morbidity after isolated limb perfusion and external-beam radiotherapy for soft tissue sarcoma of the extremity. Ann Surg Oncol 2007; 14: 2105–2112
- Thijssens KM, van Ginkel RJ, Pras E, Suurmeijer AJ, Hoekstra HJ. Isolated limb perfusion with tumor necrosis factor alpha and melphalan for locally advanced soft tissue sarcoma: The value of adjuvant radiotherapy. Ann Surg Oncol 2006; 13: 518–524