Abstract
Background: After observing rather severe acute neurotoxicity in a few patients following deep hyperthermia treatment for a pelvic tumour, we evaluated the incidence of neurotoxicity in all patients treated with deep hyperthermia of the pelvis between June 1990 and April 2004.
Materials and methods: Hyperthermia treatment registrations and hospital charts of all 736 patients were reviewed. Differences between the incidence of neurotoxicity in subgroups of patients were evaluated by 2 × 2 exact tests.
Results: Grade 2 or 3 acute neurotoxicity occurred in 2.3% of patients, grade 3 in 0.7%. The duration of symptoms was longer than 3 months in 6 patients (0.8%). Neurological examination in 5 patients showed that the most commonly involved structures are the sacral and lower lumbar nerve roots and the sacral plexus. Acute neurotoxicity occurred only after November 1999 and only in patients treated for primary cervical cancer. Comparison of applied powers and achieved temperatures in patients developing neurotoxicity did not show differences between treatment sessions which resulted in neurotoxicity and sessions not resulting in neurotoxicity.
Conclusion: Acute neurotoxicity following hyperthermia for pelvic tumours is a rare complication, but can result in symptoms affecting the activities of daily life. We found no patient, tumour or treatment characteristics predictive for a risk of neurotoxicity.
Introduction
In the period 2002–2004 we observed rather severe acute neurotoxicity (ANT) in a few patients following deep hyperthermia treatment (DHT) of pelvic tumours. For us, this was an uncommon experience. We therefore decided to initiate a retrospective analysis to determine the incidence and severity of symptoms of neurotoxicity in all patients we had treated with DHT.
Patients and methods
We evaluated all patients who had received DHT for a tumour in or near the pelvis between June 1990 and April 2004. All patients were treated with the BSD-2000 system (BSD Medical Corp., Salt Lake City, USA). During all treatments, the pelvic region was within the applicator. The number of DHT sessions varied from 3 to 8, depending on the indication. During each DHT session, and during follow-up, the patients were questioned about symptoms occurring since their previous treatment.
Deep hyperthermia treatments
These were performed by our institutional protocol. Alprazolam 0.5 mg oral was given 30 minutes before treatment preparation. Treatment preparation included placement of thermometry probes in intraluminal, superficial and occasionally interstitial catheters, and positioning of the patient in the applicator. The bladder was kept empty with a Foley catheter. The temperature of the water bolus of the applicator was maintained at 20°C. An additional bolus with circulating water of 13°C was used to cool the perineal skin. The initial power was 400 W, increasing in steps of 100 W every 5 minutes until patients’ discomfort or the achievement of a maximum temperature of 43°C in normal tissue. Thermal mapping was performed every 5 minutes with 1 cm intervals over a catheter length of 14 cm. Additional information concerning temperature distribution came from the patient. Patients were carefully instructed to mention any discomfort occurring during treatment. Any unpleasant feeling disappearing within one minute after switching off the power was considered a hot spot and treatment settings were adjusted accordingly. Phase, amplitude and frequency were adjusted to optimise the temperature distribution in the target volume. The total duration of the treatment was 60 minutes after the intra-tumour temperature reached 42°C, or maximum 90 minutes. During each treatment session, the patient's complaints (character and location of any uncomfortable feeling) indicating hot spots were registered on the treatment chart.
Data retrieval
Hospital and hyperthermia treatment charts of all patients treated with DHT were reviewed. Symptoms indicating neurological involvement were considered ANT caused by hyperthermia when these had developed within 24 hours following treatment. Patients with similar pre-existent, tumour related symptoms of nerve involvement which aggravated following treatment were not considered as having neurotoxicity from hyperthermia. A neurologist examined 5 patients having symptoms of ANT, and for those patients magnetic resonance imaging (MRI), electromyography (EMG) and/or cerebral spinal fluid (CSF) examinations were performed to objectify the complaints and determine the severity and site of the lesion. The grading of ANT was done according to the common terminology criteria for adverse events (CTC) list, version 3.0 Citation[1], in which grade 2 is defined as interfering with function, but not interfering with activities of daily life (ADL) and grade 3 as interfering with ADL, but not disabling. The duration of complaints was measured in days from the treatment session which resulted in neurotoxicity.
Treatment temperature and power data were retrieved as described by Fatehi et al. Citation[2]. Differences in the incidence of ANT between subgroups of patients were evaluated by Fisher's 2 × 2 exact tests. Reported p values are two-sided, and a p value <0.05 was considered significant.
Results
In the period June 1990 to April 2004, 736 patients (585 female, 151 male) received 750 treatment series to a total of 3180 DHT sessions. The Sigma-Eye applicator was used for 231 treatment sessions. Six hundred seventy-six treatment series were given combined with radiotherapy, 38 with chemotherapy (36 cisplatin and 2 capecitabine) and 36 combined with both radiotherapy and cisplatin. Four hundred sixty-four patients were treated for primary or recurrent cervical cancer, 201 for rectal cancer, 33 for bladder cancer and 38 for various other intra-pelvic tumours.
Seventeen patients, who received a total of 80 hyperthermia treatment sessions, reported symptoms of ANT, or aggravation of previously developed hyperthermia-induced ANT, following 32 treatment sessions (). The first symptoms of ANT occurred after the first treatment session in 6 patients, after the second in 7, after the third in 2, and after the fourth session in 2 patients. ANT occurred in 2.3% of the patients and following 1% of the treatment sessions. Twelve patients reported grade 2 ANT and 5 grade 3 (0.7%). The grade 3 ANT included sensory deficits leading to urinary incontinence (2 patients) and combined urinary and faecal incontinence (3 patients). All 17 patients with ANT were treated for cervical cancer in combination with radiotherapy, two of them also with concomitant cisplatin. One of these patients was treated in the Sigma-Eye applicator. None of the patients with ANT had been treated previously with radiotherapy or chemotherapy.
Table I. Patients with acute neurotoxicity: reported symptoms.
All 17 patients had sensory symptoms (numbness, paresthesias, or radiating pain), while 4 complained of additional leg weakness. Five patients suffered sphincter function loss. The reported symptoms were symmetrical in 13 patients, left-sided in 1 patient and right-sided in 3 patients. In 10 patients the symptoms lasted less than 1 month, in 5 less than 1 week. One patient had fully recovered 19 months after treatment. In 6 patients the symptoms persisted until last follow-up date or death, 1–23 months (median 7 months) after treatment, with only partial recovery in 3 patients. In 7 patients the occurrence of neurotoxicity was incidental: symptoms did not aggravate or reoccur after a subsequent treatment session. In 10 patients subsequent treatment sessions, applied with a one-week time interval, did aggravate the symptoms, and for 2 patients this was reason to discontinue DHT. One of these patients (HTNR 1585), initially treated with radiotherapy and DHT, was retreated 6 months later with cisplatin and DHT for a local recurrence while still experiencing symptoms of neurotoxicity. During the second DHT series there was no aggravation of the symptoms.
Five patients (4 with grade 3 toxicity) were seen by a neurologist and underwent further examination. The most commonly involved structures in hyperthermia-induced neurotoxicity were the sacral and lower lumbar nerve roots and the sacral plexus (). In patients with confirmed neurological signs, examination revealed hypesthesia and hypalgesia in the sacral (S1-S5) dermatomes. Motor exam showed weakness predominantly of the hamstrings (S1-S2 innervated) and gastrocnemius (also S1-S2) muscles. In one patient the anterior tibial and extensor hallucis longus muscles (L5) were also affected. MRI and CSF examinations were performed to exclude leptomeningeal metastases or tumour infiltration of the lumbosacral plexus. Two of four MRI scans showed enhancement of the lumbosacral meninges or cauda equina after gadolinium administration consistent with inflammation (). CSF examination was abnormal in all four patients tested with elevated total protein concentration. Increased white cell count was present in the CSF of two patients (). To locate the lesion in the nervous system, electrophysiological studies were performed. EMG demonstrated denervation in S1-S2 innervated muscles in all five patients, indicating axonal damage. In two patients the site of the lesion was both radicular and plexus, in one patient radicular only and in one patient undetermined.
Figure 1. MRI of the lumbosacral spine of patient with HTNR 1497. T1-weighted images before, (a), and after, (b), the administration of gadolinium show enhancement of the conus and cauda equine. In addition, degenerative changes including listhesis are present at the L5-S1 level.
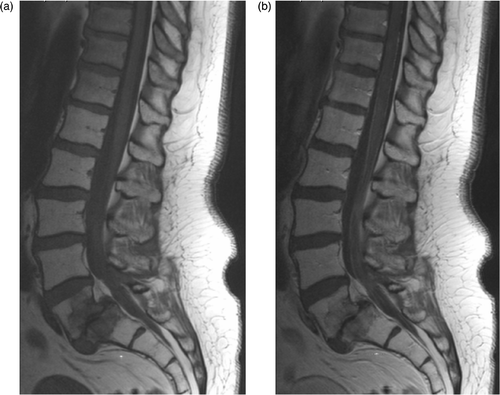
Table II. Results of neurological examination in patients with acute neurotoxicity.
We compared the power levels applied and the temperatures achieved in the patients developing ANT to those in all patients with cervical cancer, treated between 1990 and 2005. In the patients with ANT, temperatures were slightly, but not significantly, higher: the T50 of all intraluminal measurements was 40.6°C for the sessions resulting in ANT, while this was 40.5°C for all patients Citation[3]. The average net mean power applied during sessions after which ANT occurred was 699 W, which is in the higher range of the power used in all patients with cervical cancer (yearly average 412–731 W) Citation[3].
Within the subgroup of patients developing ANT, we compared the applied power and the temperatures achieved during treatment sessions with and without (aggravation of) ANT (). On the average, power levels of treatment sessions resulting in ANT were slightly, but not significantly, higher, and the temperatures not higher than those of treatment sessions not resulting in ANT.
Table III. Hyperthermia treatment characteristics (average, range) for patients with acute neurotoxicity (ANT).
The next evaluation was a comparison of applied power and achieved temperatures during sessions in the same patient. The power levels and temperatures achieved during the majority of treatment sessions causing ANT were equal to or lower than those during treatment sessions not causing ANT (). During treatments causing ANT, the rectal temperature was usually lower than the bladder temperature.
Table IV. Intra-patient comparison of treatment data of sessions resulting in acute neurotoxicity (ANT) with those of treatments not resulting in ANT, for the subgroup of patients developing ANT.
Remarkably, no ANT was observed before 1999 and all grade 3 toxicity after January 2002. In the period January 1999 - April 2004 ANT developed in 4.5% of the patients and following 2% of the sessions. The incidence of ANT during the period 1990–2004 was different between male and female patients (0/151 versus 17/585; p = 0.02). However, when comparing the incidence between male and female patients for the period January 1999 – April 2004, the difference was not significant (). Further comparisons were restricted to the patients treated after December 1998. We found no differences between treatments in the Sigma-Eye or Sigma-60, between treatments with or without chemotherapy, or between female patients with cervical cancer or another tumour type ().
Table V. Incidence of acute neurotoxicity, comparison between subgroups.
Evaluation of the notes on the treatment charts of patients who developed ANT did not reveal specific symptoms that indicated hot spots near the lumbosacral plexus. Complaints during the DHT sessions we heard of patients developing ANT were not different from those we heard of other patients. Neither did we find obvious specific characteristics which were common for the patients who developed ANT. For example, their ages (35–78, mean 59 year), height (1.58–1.76 m, mean 1.64 m), weight (42–92 kg, mean 63.5 kg), and body mass index (16.8–35.9 kg/m2, mean 23.6 kg/m2) were in the range of those in other patients. One patient with ANT was diabetic, while many more patients with diabetes had been treated. At the time that ANT developed, patients had received a total radiotherapy dose of 6 to 40 Gy (median 18 Gy).
We compared treatment characteristics of the one patient who received a second treatment series with DHT, after developing ANT during the first series (). We used higher power levels and achieved higher temperatures during the first series, but some treatment sessions of the second series had higher power and temperature levels than sessions in the first series, resulting in ANT.
Table VI. Comparison of treatment settings and achieved temperatures in one patient (#1585) who received two series of hyperthermia.
Discussion
Overall, the incidence of ANT we observed following hyperthermia treatment of the pelvis is low: 2.3% in all patients treated with DHT until April 2004. However, five patients (0.7%) developed toxicity with a large impact on quality of life: faecal and/or urinary incontinence lasting more than 1 month and only partly recovering during follow-up. In this retrospective evaluation, only symptomatic toxicity was scored, which means that we have no information about grade 1 toxicity (abnormalities in motor or sensory function found on examination or testing only). We think that the ANT in our patients can only be explained as hyperthermia-induced toxicity. The symptoms developed within 24 hours following treatment in patients who had no prior signs of nerve involvement. None of the patients had tumour progression; two months after treatment 4 showed partial response and 13 complete response. The radiation dose at the time that ANT developed was relatively low (6–40, median 18 Gy) and the symptoms were different from those of cisplatin neurotoxicity. Neurotoxicity is relatively rare after treatment of intra-pelvic malignant tumours (). Following radiotherapy of cervical cancer, neurotoxicity is not mentioned as one of the side effects Citation[4–5] or with an incidence of ≥grade 3 neurotoxicity between 0% and 0.5% Citation[6–8]. With the addition of cisplatin to radiotherapy, the incidence of neurotoxicity appears to increase: ≥grade 3 was observed in 1.1% to 1.6% Citation[7–8].
Table VII. Neurotoxicity following treatment of cervical cancer.
The five patients with objective neurological signs demonstrate that ANT following deep pelvic hyperthermia mainly affects the sacral nerve roots and/or sacral plexus. The increased protein concentration in the CSF and the pleiocytosis in two of four patients points to an inflammatory reaction causing a polyradiculitis. Also, the meningeal enhancement on MRI, following gadolinium administration in two patients is compatible with aseptic meningitis. When a lesion is restricted to the nerve root(s), the sensory nerve action potential (SNAP) following stimulation of the sural nerve should be present. In two of our patients, the SNAP could not be induced, indicating additional pathology at the level of the sacral plexus, at least in these patients. The other 12 patients without objective neurological signs had symptoms compatible with a sacral root or plexus distribution: numbness or paresthesias in the perianal and vulva area with or without irradiation into the legs. Also, numbness while voiding was common.
Hyperthermia can severely and irreversibly damage the peripheral nerves when the heat dose is in excess of 30 minutes at 44°C or equivalent, and has been observed occasionally in human patients. The review by Haveman et al. Citation[9] summarizes the history of 6 patients developing ANT after hyperthermia of the pelvic region. The incidence of neuropathy following hyperthermic isolated limb perfusion appears more frequent than following other hyperthermia techniques, which may be explained by the use of a tight tourniquet to isolate the limb circulation, and/or to the simultaneous application of platin containing drugs Citation[10–12]. In our patients, the indidence of ANT was not higher in patients treated with cisplatin simultaneously. Hyperthermia can also enhance radiation effects Citation[13–14], but radiation neurotoxicity is a late effect and is unlikely to develop after a dose of 40 Gy or less. In one experimental study, diabetes was found to be a predisposing risk factor Citation[15]. In our patients, only one patient with diabetes developed ANT, while many other diabetic patients did not. The temperatures that we have measured in our patients are lower than those reported to result in neurotoxicity in preclinical studies Citation[9], but with the available limited thermometry, we are not well informed about the temperatures at the site of the lesion. Computer simulations of power distributions achieved with the BSD-2000 system have shown that in some patients a high power level is deposited in the presacral region, the site where the lesions have been located by neurological examination Citation[16].
ANT was not observed before 1999, and grade 3 ANT only after 2001. We have evaluated whether there had been changes in equipment which might explain this finding. We started performing DHTs with a BSD-2000 system with Quad amplifiers. In June 1998, a new treatment base and Sigma-60 applicator was installed together with the BSD-2000-3D Quad amplifiers. In July 1999 a 3D drawer was installed, dividing the 4 power channels into 12 power channels. In August 2000 the Quad amplifiers were replaced by Dodek solid state amplifiers. Phantom measurements have shown that the performance of the new applicator with the Dodek amplifiers was not different from that of the previous applicator with the solid state amplifiers. The treatment base, installed in 1998, has a somewhat tighter sling and a wider waterbolus than the previous one. We think that it is unlikely that the wider waterbolus of the new applicator has made a difference, since we always have placed extra waterboluses on the legs as an extension of the applicator waterbolus. It is possible that with the tighter sling, the position of the patient is somewhat different compared to the wider sling, which can influence the intrapelvic power distribution.
The occurrence of ANT only after January 1999 made us wonder whether this could be explained by a more aggressive treatment approach. An analysis of the net power delivered per treatment shows that the mean value over the year gradually increases between 1990 and 1997, but thereafter remained at the same level of approximately 600 W Citation[3]. The net power levels of patients developing ANT were, on average, relatively high (∼700 W), but not higher during sessions resulting in ANT than in sessions not resulting in ANT. Also the intraluminal temperatures (T50 in bladder, vagina and rectum, and rectum Tmax) were not higher in treatments resulting in ANT than in treatments not resulting in ANT. The temperatures measured in the rectum, closest to the site of the lesion, was usually not higher than the bladder temperature.
Unfortunately, we have not found a way to prevent the occurrence of ANT in future patients. Our treatment protocol prescribes that the power input is increased to levels as high as possible, to a temperature distribution as homogeneous as possible in the pelvis, while avoiding temperatures >43°C in normal tissues and uncomfortable hot spots mentioned by the patient. Complaints which we relate to too high temperatures in the nervous tissue are tingling sensations, a tired feeling or a feeling of pressure in the lower extremities. In response to such sensations, the power is turned off until the symptoms have disappeared, and thereafter the treatment is continued after changing the treatment settings. Such complaints have been expressed by many patients not developing neurotoxicity, and by only a few patients who have developed neurotoxicity. Thermometry near the nerve roots or sacral plexus is, in our view, not a realistic option. It is likely that the patient-positioning in the applicator is important, since small changes in position can alter the intra-pelvic power distribution, as demonstrated by hyperthermia treatment planning Citation[17]. This may explain the different outcome of the two treatment series in our patient with HTNR 1585 (). The relatively small change of position in this patient, with the centre of the target volume 5 cm instead of 3 cm cranial of the pubic bone, led us to select a phase steering 2 cm more dorsally in order to get the optimum achievable temperature distribution.
The observation that ANT only occurred in patients with cervical cancer may also be explained by the positioning of these patients within the applicator, with the centre of the target volume usually 2–3 cm cranial of the pubic bone.
We expect that we will improve the ability to prevent the occurrence of ANT when reliable hyperthermia treatment planning systems Citation[18–19] and/or 3-D non-invasive thermometry systems Citation[20–21] will become part of routine treatment procedures. At present, we are testing whether the Hyperplan modelling system Citation[16], Citation[18] is helpful in optimizing treatments. In the Dutch Deep Hyperthermia Trial, the addition of hyperthermia to radiotherapy in patients with advanced cervical cancer was shown to result in an increase of 3-year local tumour control from 41% to 61%, and of 3-year overall survival from 27% to 51% Citation[22]. In view of this large improvement, the low incidence of ANT appears acceptable.
Conclusion
Acute neurotoxicity following deep hyperthermia treatment of the pelvis is rare, but can lead to deficiencies which have a negative impact on the quality of life. For prevention of ANT in future patients, continuation of developing hyperthermia planning systems and non-invasive thermometry is essential.
References
- Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0. 2003, http://ctep.cancer.gov
- Fatehi D, De Bruijne M, Van der Zee J, Van Rhoon GC. RHyThM, a tool for analysis of PDOS formatted hyperthermia treatment data generated by the BSD2000/3D system. Int J Hyperthermia 2006; 22: 173–184
- Fatehi D, Van der Zee J, De Bruijne M, Franckena M, Van Rhoon GC. RF-power and temperature data analysis of 444 patients with primary cervical cancer: Deep hyperthermia using the Sigma-60 applicator is reproducible. Int J Hyperthermia 2007; 23: 623–643
- Denton AS, Bond SJ, Matthews S, Bentzen SM, Maher EJ. and the UK Link Gynaecology-Oncology Group. National Audit of the management and outcome of carcinoma of the cervix treated with radiotherapy in 1993. Clin Oncol 2000; 12: 347–353
- Eifel PJ, Levenback C, Wharton T, Oswald MJ. Time course and incidence of late complications in patients treated with radiation therapy for FIGO stage IB carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 1995; 32: 1289–1300
- Perez CA, Grigsby PW, Lockett MA, Chao KSC. Radiation therapy morbidity in carcinoma of the uterine cervix: Dosimetric and clinical correlation. Int J Radiat Oncol Biol Phys 1999; 44: 855–866
- Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, Walker JL, Gersell D. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 1999; 340: 1154–1161
- Pearcey R, Brundage M, Drouin P, Jeffrey J, Johnston D, Lukka H, MacLean G, Souhami L, Stuart G, Tu D. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell carcinoma of the cervix. J Clin Oncol 2002; 20: 966–972
- Haveman J, Van der Zee J, Wondergem J, Hoogeveen JF, Hulshof MCCM. Effects of hyperthermia on the peripheral nervous system: A review. Int J Hyperthermia 2004; 20: 371–391
- Busse O, Aigner K, Wilimzig H. Peripheral nerve damage follosing isolated extremity perfusion with cis-platinum. Recent Results Cancer Res 1983; 86: 264–267
- Kettelhack C, Kraus T, Hupp T, Manner M, Schlag P. Hyperthermia limb perfusion for malignant melanoma and soft tissue sarcoma. Eur J Surg Oncol 1990; 16: 370–375
- Hoogeveen JF, Van der Kracht AHW, Wondergem J, Gonzalez Gonzalez D, Haveman J. Influence of cisplatin on the sensitivity of the rat sciatic nerve to local hyperthermia. Neurotoxicology 1993; 14: 1–7
- Haveman J, Wondergem J, Van Dam WM, Van der Kracht AHW. Irradiation of the rat sciatic nerve leads to delayed recovery from function loss after heat treatment or mechanical damage. Neurosci Res Comm 1994; 14: 1–7
- Vujaskovic Z, Gillette SM, Powers BE, Stukel TA, Larue SM, Gillette EL, Borak TB, Scott RJ, Weiss J, Colacchio TA. Effects of intraoperative irradiation and intraoperative hyperthermia on canine sciatic nerve: Neurologic and electrophysiologic study. Int J Radiat Oncol Biol Phys 1996; 34: 125–131
- Hoogeveen JF, Van der Kracht AHW, Wondergem J, Haveman J. Effects of local hyperthermia on the sensitivity of rat sciatic nerve from diabetic rats. Neurosci Res Comm 1993; 12: 63–70
- Sreenivasa G, Gellermann J, Rau B, Nadobny J, Schlag P, Dueflhard P, Felix R, Wust P. Clinical use of the hyperthermia treatment planning system Hyperplan to predict effectiveness and toxicity. Int J Radiat Oncol Biol Phys 2003; 55: 407–419
- Gellermann J, Göke J, Figiel R, Weihrauch M, Cho CH, Budach V, Felix R, Wust P. Simulation of different applicator positions for treatment of a presacral tumour. Int J Hyperthermia 2007; 23: 37–47
- Gellermann J, Wust P, Stalling D, Seebass M, Nadobny J, Beck R, Hege H-C, Deuflhard P, Felix R. Clinical evaluation and verification of the hyperthermia treatment planning system Hyperplan. Int J Radiat Oncol Biol Phys 2000; 47: 1145–1156
- Van Haaren PMA, Kok HP, Van den Berg CAT, Zum Vorde sive Vörding PJ, Oldenburg S, Stalpers LJA, Schilthuis MS, De Leeuw AC, Crezee J. On verification of hyperthermia treatment planning for cervical cancer patients. Int J Hyperthermia 2007; 23: 303–314
- Gellermann J, Wlodarczyk W, Feussner A, Fähling H, Nadobny J, Hildebrandt B, Felix R, Wust P. Methods and potentials of magnetic resonance imaging for monitoring radiofrequency hyperthermia in a hybrid system. Int J Hyperthermia 2005; 21: 497–513
- Wust P, Cho CH, Hildebrandt B, Gellermann J. Thermal monitoring: Invasive, minimal-invasive and non-invasive approaches. Int J Hyperthermia 2006; 22: 255–262
- Van der Zee J, González González D, Van Rhoon GC, Van Dijk JDP, Van Putten WLJ, Hart AAM. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Lancet 2000; 355: 1119–1125