Abstract
Purpose: This paper presents a novel conformal thermal monitoring sheet (TMS) sensor array with differential thermal sensitivity for measuring temperature distributions over large surface areas. Performance of the sensor array is evaluated in terms of thermal accuracy, mechanical stability and conformity to contoured surfaces, probe self-heating under irradiation from microwave and ultrasound hyperthermia sources, and electromagnetic field perturbation.
Materials and methods: A prototype with 4 × 4 array of fiber-optic sensors embedded between two flexible and thermally conducting polyimide films was developed as an alternative to the standard 1–2 mm diameter plastic catheter-based probes used in clinical hyperthermia. Computed tomography images and bending tests were performed to evaluate the conformability and mechanical stability respectively. Irradiation and thermal barrier tests were conducted and thermal response of the prototype was compared with round cross-sectional clinical probes.
Results: Bending and conformity tests demonstrated higher flexibility, dimensional stability and close conformity to human torso. Minimal perturbation of microwave fields and low probe self-heating was observed when irradiated with 915 MHz microwave and 3.4 MHz ultrasound sources. The transient and steady state thermal responses of the TMS array were superior compared to the clinical probes.
Conclusions: A conformal TMS sensor array with improved thermal sensitivity and dimensional stability was investigated for real-time skin temperature monitoring. This fixed-geometry, body-conforming array of thermal sensors allows fast and accurate characterization of two-dimensional temperature distributions over large surface areas. The prototype TMS demonstrates significant advantages over clinical probes for characterizing skin temperature distributions during hyperthermia treatments of superficial tissue disease.
Introduction
Superficial tissue disease covers a wide range of cancerous and non-cancerous diseases and abnormal growths that afflict the skin and the underlying subcutaneous tissues. Cancerous superficial tissue diseases include the recurrent and metastatic cancers of the breast, chest wall, and skin, as well as tumor nodules that extend several cm beneath the tissue surface Citation[1]. Numerous clinical studies have shown the efficacy of hyperthermia as an adjuvant to radiotherapy in the treatment of superficial tissue disease Citation[2–4]. Unlike the single aperture heating device developed for small localized tumors, hyperthermia treatment of large area, irregularly shaped superficial tissue disease spread over complex anatomic contours requires multi-element applicators with adjustable heating patterns. Commercially available multi-element array devices in the USA include the Sonotherm 1000 (Labthermics Technologies, Champaign, IL) 4 × 4 array 3.4 MHz ultrasound heating device and the Microtherm 1000 (Labthermics Technologies, Champaign, IL) 4 × 4 planar array 915 MHz microwave heating device for treating superficial tissue regions below the skin Citation[5–8]. Multi-element array devices in clinical use today in Europe include custom sized arrays of 433 MHz current sheet applicators (CSA) Citation[9], lucite cone applicators (LCA) Citation[10–12], and the contact flexible microstrip applicator (CFMA) Citation[13], Citation[14]. Another approach that has been used successfully in the clinic involves computer controlled mechanical rotation of one or two scanning spiral microstrip antennas over surface disease Citation[15]. The largest surface applicators used clinically to date are the 25 aperture 915 MHz spiral microstrip array developed at Stanford University Citation[16] and the conformal microwave array (CMA) applicator Citation[1], Citation[17], Citation[18]. Other multi-element array applicators have been developed that should be available in the hyperthermia clinic soon Citation[19]. A detailed review of the thermal therapy devices and techniques available for the treatment of skin and superficial tissue disease is covered in Citation[1]. Most of these applicators offer the ability to heat larger areas with more uniform heating than the previous single element applicators, given sufficient thermal dosimetry feedback. Current multi-element array applicators lack an appropriate complementary non-invasive thermometry approach to monitor temperatures under each of the ‘independent’ heat sources. Without thermal feedback to control each source independently, the effectiveness of array applicators is reduced to closer to a single aperture heat source of equivalent size. This critical limitation has impeded the entry of recent applicator hardware to the patient clinic.
Unlike deep-seated tumors, the target tissue in superficial diseases such as chest wall recurrence of breast cancer and plaque psoriasis generally extends only 5–15 mm beneath the tissue surface including the skin. Clinical data from our group have shown skin surface distribution as a good indicator of the underlying tissue temperature distribution within the top 5–10 mm of the surface Citation[8]. Other researchers have observed similar temperatures on the surface and at a depth of 2–3 mm Citation[20]. Theoretical study demonstrates that peak power deposition from 915 MHz microwave hyperthermia occur at the skin surface while peak temperatures are 2–3 mm deep for practical water bolus temperatures around 42°C Citation[21]. Due to practical limitations on the use of interstitial probes for large area surface disease, temperature measurements from probes lying on the skin surface are the common approach used in clinical microwave hyperthermia treatments to monitor thermal dose and control/balance the power radiated by the individual heating elements to deliver a more uniform coverage of the lateral spread of target disease. Present techniques for monitoring two-dimensional temperature distributions of the skin during superficial hyperthermia treatments generally consist of either taping a number of single sensor probes at various irregularly spaced locations on the skin or pulling sensors in 5–10 mm steps through round plastic catheters laying on the skin surface. Unfortunately for the first procedure which is the most common to date, microwave hyperthermia systems generally offer only 8 fiber-optic point sensors – often less than the number of available heat sources. While the second thermal mapping technique provides many more temperature readings along the catheter tracks, the number of catheters is usually small (1–3) and it takes a long time to complete each scan. Thus, the resolution of the temperature measurements is poor both spatially and temporally. Another difficulty with the use of individual sensors involves secure placement of sensors over irregularly shaped anatomy in accurate alignment with all radiating sources in large multi-element array applicators. The round cross section probes taped on to the contoured skin surface often coated with perspiration and natural body oils or protective creams to combat skin breakdown have a tendency to loosen and pull away from the desired positions from patient movement during the 60-minute hyperthermia treatment. In addition to the probe alignment errors, uncertainty of sensor position within the probe is another potential source of error for the soft plastic-sheathed probes which are susceptible to stretching.
Depending on whether heating with microwaves or ultrasound, existing techniques for monitoring surface temperature generally employ a plastic coating for the sensors to make them durable and reusable. The plastic coating provides thermal as well as electrical insulation and thus impacts the temperature measurement. For sensors inside thermal mapping catheters, the second thermal barrier formed by the plastic catheter and air trapped around the sensor add further uncertainty and time delay to the readings obtained by pulling individual sensors through the catheter. The round sensor cross-section and multiple layers of insulating plastic surrounding the sensor further increase measurement uncertainty when located at the interface between a temperature-controlled water bolus and skin at unknown temperature. The temperature gradients across the probe at water bolus-skin interface cause significant differences between measurements recorded by the insulated sensors on the skin surface and the actual temperature of tissue just beneath the skin surface Citation[20].
While still lacking the technology for accurately characterizing surface temperature distributions, several investigators have proceeded with efforts to write automatic feedback control programs assuming they would have at least one feedback temperature for each independent heat source Citation[22–24]. A conformal TMS with appropriately spaced array of non-perturbing stationary fiber-optic sensors is proposed in this paper as an alternative thermometry device to speed up and simplify this critically important thermal monitoring procedure and allow continuous feedback of skin temperature for use in automatic power control of the multi-element heat array applicators. The design and construction details of the prototype TMS array are presented in section 2. Section 3 presents a detailed performance evaluation of the prototype device subjected to mechanical stress, microwave and ultrasound irradiation, thermal loading, and conformity tests on a contoured surface. Results of the evaluation are discussed in section 4 along with plans to further optimize the TMS design for improved directional thermal sensing. Clinical potential of the novel conformal TMS array is summarized in the final section.
Conformal TMS sensor array
The prototype TMS array was constructed in collaboration with IPITEK Inc., (Carlsbad, CA, USA) manufacturer of high precision electromagnetic (EM) field immune fiber-optic thermal sensors with single sensor product lines intended for industrial use as well as high precision sensors intended primarily for medical applications.
Design
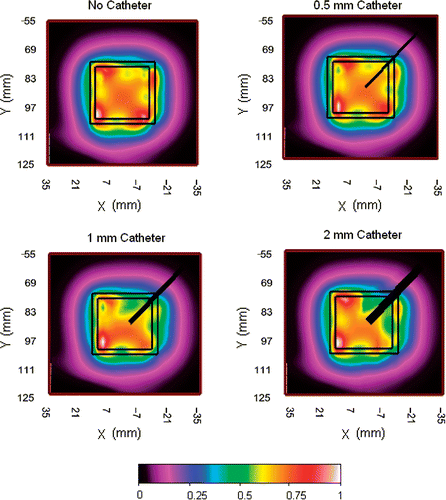
The design work began with the search for optimum materials which included finding small diameter but rugged optical fibers that could be assembled together with soft and flexible plastic materials capable of conforming to complex surface contours of the human torso. The maximum allowable size of plastic fiber-optic sensor material that would avoid perturbation of the heating fields was studied using the 915 MHz dual concentric conductor (DCC) antenna Citation[1], Citation[18], Citation[25], Citation[26]. Plastic catheters with diameter 0.5 mm, 1 mm and 2 mm were placed between the DCC aperture and muscle tissue equivalent liquid phantom. The specific absorption rate (SAR) pattern was measured 5mm deep inside the tissue phantom using a 3 mm diameter electric field probe (ALS-E020 Aprel Laboratories, Ottowa, Ontario, Canada). shows the normalized SAR measured under a 3 cm square DCC slot antenna driven at 30 W (maximal clinical power level) with the plastic catheter crossing the corner of the radiating slot. Comparison of the SAR measurements in shows large field perturbation for catheters with diameter >0.5 mm and no perceptible change in the SAR pattern for catheter with diameter ≤0.5 mm.
Figure 1. Measured SAR patterns 5 mm deep inside liquid muscle phantom under 3 cm square DCC antenna driven with a clinically relevant power of 30 W at 915 MHz, with 0, 0.5, 1, and 2 mm diameter plastic catheters across one corner of the radiating slot.
This study investigated the use of standard multi-layer Kapton® polyimide film (DuPont, Circleville, OH, USA) commonly used in flexible printed circuit board (PCB) fabrication Citation[27]. The Kapton® film possesses stable mechanical, physical and thermal properties over a wide range of temperatures. The low dielectric constant (3.4–3.5) and dissipation factor (0.0018–0.0026) of the Kapton film makes it compatible with PCB-based conformal array heating devices. The high tensile strength (1.14–1.59 mpa) and folding endurance (285 k cycles) of the Kapton film provides dimensional stability to the TMS array when wrapped around a contoured human torso. The field perturbation experiments and the material properties of Kapton polyimide films led to the design of a TMS array with 250 µm diameter IPITEK fiber-optic sensors embedded between two layers of Kapton film. Kapton layers with differential thickness were used to provide directional thermal sensitivity to the buried fiber-optic sensors towards one side of the array. The flexible TMS assembly provides secure sensor placement with known position and spacing over highly contoured human anatomy, minimizing alignment errors so common with current single sensor placement techniques.
Construction
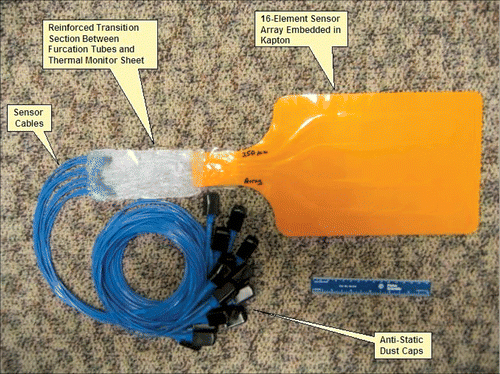
IPITEK fiber-optic sensors were adapted to conform to a two-dimensional array format in order to achieve compatibility with the multi-element array heat applicators. Plastic fiber used in standard IPITEK sensors was cut to form array segments with appropriate lengths. A temperature-sensitive phosphor was then added at the sensing end of each segment, and the segments were arranged in a 4 × 4 array. For prototype 1, the spacing between sensors was chosen to match the center-to-center spacing of 16 heat sources in the Microtherm and Sonotherm (Labthermics Technologies Inc., Champaign, IL) planar array applicators. The fiber-optic sensor array with exposed plastic fibers was sandwiched between two layers of Kapton film; one layer was 100 µm thick while the other was 175 µm to provide increased thermal resistance in one direction. shows the rectangular shaped 4×4 prototype Kapton TMS array with sixteen 250 µm IPITEK fiber-optic sensors. Each of the sensors is terminated with proprietary IPITEK fiber-optic connectors and electronics modules for real time data acquisition and calibration. The entire TMS-250 conformal array prototype investigated in this work is shown in .
Prototype testing
A series of tests were performed to evaluate the performance of the TMS-250 array in terms of thermal accuracy, conformity to contoured human anatomy, probe self-heating and field perturbation during irradiation by electromagnetic and ultrasound applicators used in superficial hyperthermia treatments.
Thermal accuracy
The accuracy discussed in this paper is the deviation of the average sensor temperature measurement from the true known value (Tavg−Tstd) established by repeatedly measuring using a temperature standard that has been calibrated against standards maintained by the National Institute of Standards and Technology (NIST). This type of measurement requires a stable temperature environment and was conducted at the manufacturer site in a stirred oil bath. The thermal monitoring sheet was placed inside an oil bath held at a constant temperature of 38°C. Each sensor in the TMS-250 array was calibrated separately and sensor responses were measured against a calibrated resistance temperature detector traceable to NIST. After calibration, accuracy tests were performed at 35°C, 38°C, and 45°C and all sensor readings were compared against the temperature standard. The accuracy of the 250 µm sensor measurements compared to the temperature standard was 0.01°C at 38°C, 0.06°C at 35°C and 0.10°C at 45°C. The TMS sensor measurements logged for 2 h after calibration were measured to be stable.
Bending tests were performed to study the fluctuation in sensor measurements for curved TMS geometry. The TMS-250 array was suspended flat inside a temperature controlled empty chamber with the thinner Kapton layer facing upwards. The sensor was left undisturbed and temperature was logged for all 16 sensors for 60 s. The array was later rolled around 10.16 and 15.24 cm diameter cylindrical rings respectively and suspended inside the chamber while temperature data was again logged for all 16 sensors for 60 s each. This procedure was repeated twice for the 10.16 and 15.24 cm diameter curvatures. The steady state temperatures logged during flat and rolled conditions were used to quantify fluctuation in sensor measurements for curved geometry. The average steady state accuracy of the sensors at flat and rolled conditions for the 15.24 cm diameter roll was −0.06°C and 0.21°C respectively. The sensor measurement accuracy at flat and rolled conditions was measured to be 0.05°C and 0.23°C respectively for the 10.16 cm diameter curvature.
Conformity to contoured anatomy
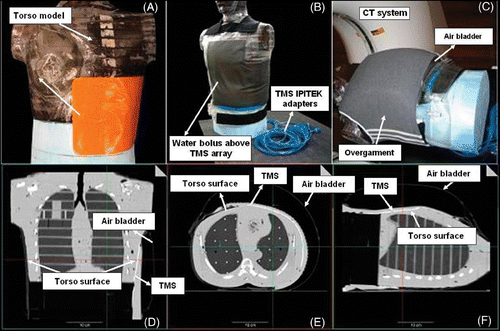
Conformity tests were performed using a Computer Tomography (CT) scanner to quantify the contact between the prototype TMS sensor array and the surface of a human torso phantom under a CMA type water bolus Citation[28]. shows the placement of the prototype TMS-250 array on the human torso phantom extending across the left chest wall and around the side under the arm. show the vest-shaped CMA applicator, and the 6 mm thick water bolus layer with overlying air bladder and elastic overgarment in place over the TMS, in an appropriate clinical heating configuration. The three orthogonal CT views of the torso phantom in to F indicate the TMS array as a thin white line (arrows) conforming closely to the contoured phantom surface (gray). The conformity (and thermal contact) to the realistically shaped human torso model in is excellent with the TMS bending smoothly both around the side, under the arm, and simultaneously twisting in the axial direction to fit the complex surface contour. When integrated into the multi-layered CMA applicator Citation[17], Citation[27], the conformal TMS array can maintain fixed sensor placement relative to the individual heating elements regardless of patient movements during a typical hour long hyperthermia treatment.
Figure 3. Conformity test showing (A) TMS position on torso phantom with (B) overlying water bolus, (C) air bladder vest and elastic overgarment as in typical CMA clinical setup; (D)–(E) Orthogonal views of the CT scans demonstrate excellent conformity and thermal contact to realistic curving and twisting body contour.
Evaluation of TMS with clinical hyperthermia devices
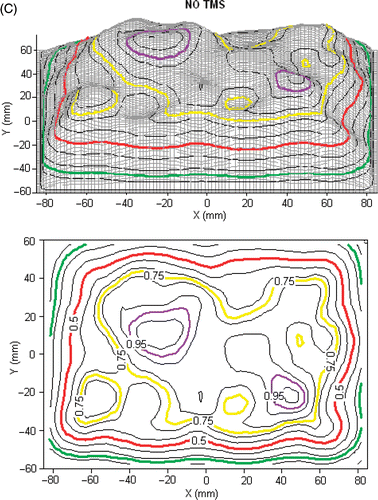
Because these TMS arrays are intended for thermometry in microwave and ultrasound fields, perturbation of the heating patterns due to energy absorption in the TMS array was investigated in two series of tests. In the first test, the TMS was securely taped to a 16-element CMA applicator that was placed flat above a rectangular tank filled with liquid muscle tissue equivalent phantom as shown in . The TMS lying on the liquid tissue phantom was irradiated by a 6 element sub-array of the 16-element 915 MHz CMA applicator at 30 W per antenna to measure perturbation in the radiated field. The relative power density was measured with a scanning electric field probe (ALS-E020 Aprel Laboratories, Ottowa, Ontario, Canada) in the XY plane 5 mm deep (z = 5 mm) inside the muscle phantom under the 6-element sub array using the procedure described previously Citation[5]. and C show the relative SAR plots measured with and without the TMS array between the applicator and the tissue phantom. As seen in , insertion of the 0.275 mm thick TMS with 250 µm fibers had essentially no effect on the field radiated from the 915 MHz microwave array other than to reduce the peak SAR by about 5%. These measurements confirmed our expectations since both Kapton and the adhesive used in TMS construction are extremely low loss materials.
Figure 4. (A) Schematic diagram of the experimental setup for measuring SAR pattern at 5 mm depth inside muscle tissue equivalent liquid phantom irradiated with 915 MHz from a 6-element sub array of a CMA applicator; (B) SAR pattern with intervening 0.275mm thick TMS-250 sheet, superimposed above the outline of the 6 DCC antennas; (C) SAR pattern without TMS sheet.
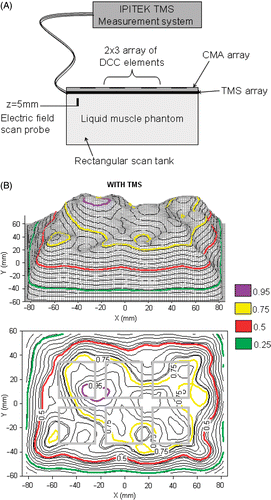
Although the TMS concept was originally intended for microwave field dosimetry, a second test setup was constructed to characterize the absorption/self-heating of the TMS exposed to an ultrasound field that is also commonly used in superficial hyperthermia treatments. The self-heating and thermal barrier characteristics of the TMS array were measured under a 3.4 MHz ultrasound (US) planar array applicator (Sonotherm 1000, Labthermics Technologies Inc., Champaign, IL). In this test setup, the TMS array was placed on a solid phantom load and a 40.5°C water bolus coupled 16-transducer US array was pressed down over the top. Temperatures were recorded in two TMS sensors near the distal edge of the TMS array and compared to the measurements from two standard clinical thermocouple probes. Using a clinical level of ultrasound power (35W per transducer), the interface temperature was measured with two fiber-optic sensors of the TMS, two M-CC-U-0-4/7 polyethylene encased thermocouples (Ella-CS, Czech Republic) on the phantom surface just under the TMS array, and two M-CC-U-0-4/7 thermocouples in direct contact with the ultrasound bolus and muscle load just off the distal end of TMS as shown in . shows the average temperature of the thermocouple and TMS sensors. In the absence of ultrasound field, the thermocouple sensors at load-bolus interface read approximately 1°C higher than those shielded by TMS from the warm water bolus. With the ultrasound energy on, the situation reverses and the thermocouples below the TMS array read approximately 1°C warmer than those at the bolus-load interface due to self-heating in the Kapton. Temperatures recorded by the fiber-optic sensors inside the double layer TMS were about 0.5°C lower than the true interface temperature during the transient thermal conduction heating before ultrasound power was applied, due to the time delay of phantom heating through a double Kapton layer TMS. With a clinical level of ultrasound power, the TMS sensor read 2.0°C higher than the interface due to power absorption in the Kapton.
Figure 5. TMS probe self-heating from irradiation by Sonotherm 3.4 MHz ultrasound array hyperthermia applicator. (A) Experimental setup. (B) Comparison of standard thermocouple (TC) probes with TMS sensors reading tissue phantom temperature before and after application of maximal clinical power level. Note close correspondence of all probe temperatures at the interface after contact with the water bolus at 30 sec, and consistent 1–2°C self-heating of the Kapton sheet fiber-optic sensors during application of a high clinical power level of ultrasound at time 10–20 min.
Comparison with standard clinical hyperthermia monitoring techniques
Finally, the performance of the prototype TMS array was compared with other temperature probes that are commonly used for clinical microwave hyperthermia treatments. shows the experimental set-up with various thermometry sensors including the TMS-250 array, bare Teflon encased fluoro-optic probe (Luxtron Corp, Santa Clara, CA) underneath the TMS array, Teflon encased fluoro-optic probe both bare and inside 19 gauge (0.91 mm dia) PVC and Teflon thermal mapping catheters was securely taped in close proximity on a PVC bolus bag. During the experiment, all temperature sensors were sandwiched between the PVC bolus bag and a high density polystyrene sheet of 1 cm thickness. The polystyrene sheet insulated the sensors from fluctuations in room temperature and enabled characterization of the thermal response of individual sensors. To demonstrate differences in the transient as well as steady state response of the various probes, the probes were placed close together on a large uniform temperature water bolus that was subjected to a step change in temperature of 18°C while recording thermal response of all sensors. To accomplish this step change, 23°C water was uniformly and rapidly circulated at 2 L/min flow rate through a large 6 mm thick water bolus till steady state was reached. After recording temperatures from all sensors for approximately 4 minutes, the heat exchanger in the water circuit was moved from the 23°C bath and plunged into a heated bath to circulate 41.5°C water through the bolus.
Figure 6. (A) Experimental set-up showing the placement of the TMS array and standard clinical temperature probes close together on a large equi-temperature water bolus sheet with rapidly circulating water (4 × 4 array of black dots indicate sensor location). (B) Comparison of the thermal response of TMS sensor array with standard clinical probes for a rapid change in circulating water temperature.
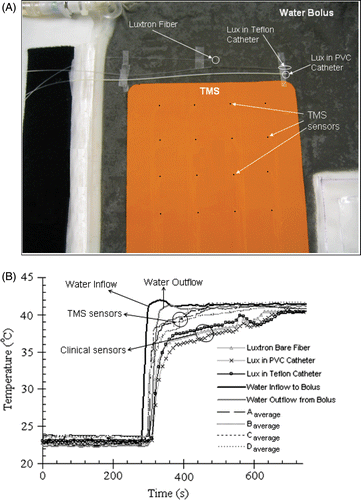
shows the thermal response curves of the TMS-250 sensor array and standard clinical probes in contact with the PVC water bolus bag during this 18°C step change. Note that all TMS sensors respond more rapidly to the steep transition and read closer to the actual bolus temperature than the current clinical monitoring techniques. The spread in readings of temperature among the multiple sensors of each type is due to minor temperature differences across the large water bolus surface. The average temperature of water flowing in and out of the entire water bolus compartment is seen to vary rapidly from about 23°C to 41°C, with a time delay of approximately 25 s for the transit of water across the large bolus surface. Twenty seconds after the step change, the TMS and Luxtron sensors read 2°C and 4–5°C respectively cooler than the average water outflow temperature. By 180 sec, all TMS sensors reached steady state and remained within 0.5°C of the bolus outflow temperature while the standard fiber-optic sensors were still lagging by 2.5–3°C. At steady state, the average of the temperatures read by the TMS sensor array was 41.08°C compared to the water bolus outflow temperature of 41.1°C while the round cross section probes read approximately 1°C below the water bolus outflow temperature.
Discussion
This work describes a detailed investigation carried out during the early testing phase of a novel prototype device for monitoring temperature distributions over the skin surface. The mechanical stability, microwave and ultrasound absorption loss, and field perturbation properties of the materials were studied carefully before determining optimum construction of the TMS array. SAR measurements in liquid muscle phantom established that use of sensors with ≤0.5 mm diameter should avoid significant microwave field perturbation. The design work enabled fabrication of a thin and flexible thermal monitoring sheet using readily available Kapton polyimide film and 250 µm diameter IPITEK plastic fibers with overall thickness smaller than the standard Teflon encased Luxtron fluor-optic probes. Additional plastic coating typically used with existing single or multi-sensor plastic fiber-optic probes serve as an insulating layer that introduces further time delay and impacts temperature measurement. The standard Teflon or PVC encased round fiber-optic sensors are useful for interstitial tissue temperature monitoring during deep hyperthermia or thermal ablation procedures, where the depth profile of tissue temperature can be obtained by pulling the sensor to various positions inside the catheter. However, for the skin surface, temperature measured by the round plastic sensors with omni-directional thermal sensitivity is significantly influenced by the bolus pressure and toughness of the skin which affect how deeply the round probe sinks into either the skin or water bolus surface, and by limitations in spatial sampling of large surface areas. On the other hand, a conformal TMS probe configuration with stationary regularly spaced grid array of fiber-optic temperature sensors embedded between differential thickness Kapton layers can gather sufficient measurements to characterize the entire surface temperature distribution of the skin over large contoured anatomy without uncertainties in sensor position.
The thermal accuracy of the prototype 16 sensor TMS array was measured to be at least 0.1°C at three clinically relevant test temperatures. The calibration bath used in thermal accuracy tests was marginal in size to accommodate the TMS. Warping the sheet to fit inside the bath produced small temperature gradients that degraded the thermal accuracy of the array during calibration. The calibrated TMS sensor measurements logged for a duration of 2 h inside a temperature controlled water-bath were stable and matched the temperature standard with 0.1°C accuracy. The stability and accuracy of the prototype TMS sensor array are reproducible even after more than a year since the prototype fabrication. The thermal accuracy and readout stability of the TMS array in flat and tightly curved conditions tested over the range of 35–45°C indicated a larger but still acceptable 0.23°C temperature deviation for a 5.08 cm radius of curvature. But a 10.16 diameter curvature is tighter than most surfaces encountered in clinical measurements of the human torso. The thermal gradient established by convection air currents around the curved TMS array adversely degraded the sensor accuracy in the bend tests. Performing the bend tests inside a well stirred oil bath would minimize the thermal gradients across the TMS surface and enable a better characterization of the device. The close conformity of the TMS array both under the arm and around the body seen in indicates that excellent body contact can be maintained over the highly contoured human torso.
As shown in , irradiation of the TMS array exposed to 915 MHz microwave fields at power levels in excess of the clinical requirements produced no perceptible perturbation of the radiated field pattern and no perceptible self-heating of the Kapton TMS array. A uniform approximately 5% decrease in SAR observed across the entire pattern could easily be accommodated in clinical treatments by increasing the applied power as necessary to achieve the desired skin surface temperature which is accurately characterized by the TMS sensor array. The 1–2°C error in temperature measurement seen in for the 4×4 array of 3.4 MHz ultrasound applicator with 38 W per transducer clearly indicates probe self-heating due to absorption of ultrasound energy within the soft plastic probe and Kapton layers. As with other probes used to monitor ultrasound therapy, this probe self-heating must be accommodated with appropriate temperature correction procedures Citation[29], Citation[30].
Comparison of the thermal response curves in clearly demonstrates the superior ability of the TMS array to respond rapidly to a steep rise in temperature (18°C). Even though the four Luxtron sensors (current gold standard for clinical microwave hyperthermia treatments) were taped directly to the water bolus (heat source) surface, the Luxtron sensors were slower to respond and by 35 s read temperatures 2–3°C cooler than the TMS array measurements and 3–4°C cooler than the bolus outflow temperature. The thin Kapton layer overlying the IPITEK 250 µm sensor on the bolus side introduced less insulation and time delay in reading the bolus temperature than the bare Luxtron probe and especially the catheter encased Luxtron sensors which have a polyurethane insulated round cross section that is less suited for accurate temperature measurement at an interface between two planar surfaces. This is a significant finding that supports TMS sensor array thermometry as superior to the current best clinical approaches used for thermal characterization of large surface tissue regions Citation[1].
The primary motivation to develop the TMS array is thermal dosimetry of temperature distributions across large surfaces of skin, a thermometry application that is currently unmet with current systems Citation[8]. It should be noted that this paper does not address the ability of hyperthermia applicators to treat to any specific depth, but rather this thermometry approach will provide the critical information necessary to change power levels of multi-element applicators to deliver most uniform lateral distribution of heating across large superficial tissue disease Citation[31]. Efforts thus far have been aimed at evaluating the prototype TMS for use in monitoring and control of surface temperature distributions under multi-element array microwave hyperthermia applicators like the 16-element Microtherm and 32-element CMA applicator developed for treatments of chest wall recurrence of breast cancer Citation[1], Citation[5], Citation[32], Citation[33]. This thermometry approach should be equally useful for superficial hyperthermia induced with numerous other multi-element array microwave applicators Citation[9], Citation[13], Citation[14], Citation[16], Citation[34], Citation[35]. Clinical evaluations of these multi-element applicators have demonstrated the ability to deliver adjustable heating patterns to large surface areas over highly contoured portions of the anatomy if sufficient feedback information is available to guide power adjustments Citation[1], Citation[5]. Unfortunately, existing thermometry techniques rely on a small number of sensors taped to the skin or thermal mapping of a single fiber-optic sensor through long plastic catheters on the skin which requires long times, up to half the available treatment time, before adjustments can be made to the applicator power settings to improve heating distribution for the second half of therapy. In contrast, the TMS array investigated in this work can provide continuous readings of 16 surface measurements in a regular grid that matches the center-to-center spacing of the heat sources. The multilayered conformal design with stationary arrangement of embedded fiber-optic sensors eliminates uncertainties in sensor position over the contoured human anatomy. The differential thickness TMS with the thinner Kapton film on the tissue side lowered the thermal barrier between buried fiber-optic sensors and skin while at the same time increased the thermal isolation of the water bolus. As seen in , the differential thickness enabled the prototype TMS sensor array to read surface temperatures on the thinner Kapton film side with an accuracy of 0.1°C compared to the standard round cross section clinical probes that exhibited steady state temperature differences of ≥1°C from the true surface temperature. The thin (<0.3 mm) TMS sensor array with thermal resistance equal to 0.0023 m2K/W results in an incremental change in the thermal impedance of the coupling water bolus towards the tissue surface. A number of new TMS probe configurations with improved directional thermal sensitivity and low overall thermal barrier characteristics for good regulation of spatial inhomogeneities in skin temperature are investigated in a related study Citation[36].
Conclusions
This work describes the design and performance of a prototype thermal monitoring sheet (TMS) conformal array of miniature fiber-optic sensors intended for characterization of surface temperature distributions across large areas of skin during superficial clinical hyperthermia treatments. TMS sensor arrays fabricated from fiber-optic sensors embedded in <0.3 mm thick Kapton sheets exhibit very small temperature offset and time delay in reading true skin surface temperatures. Compared to the current clinical probes with omni-directional thermal sensitivity, TMS sensor arrays with differential thickness lowered the thermal resistance and capacitance to the side with thinner Kapton, and significantly improved the speed and accuracy of surface temperature measurements. The fast thermal response time, low probe self-heating under EM field and low EM field perturbation of the TMS sensor array, dimensional stability of sensor locations over contoured anatomy, and measurement accuracy of 0.1°C even when adjacent to another (water bolus) surface at a different temperature appears very promising for use in surface thermometry of the skin. The TMS array investigated in this paper provides a novel thermometry approach capable of yielding high density and/or large area two-dimensional thermal distributions with improved sensitivity and accuracy. Successful completion of this fixed geometry, body conforming array of thermal sensors should produce a non-invasive thermal dosimetry device that can significantly improve monitoring and real time control of superficial hyperthermia treatments with multi-element array heat applicators.
Acknowledgements
This work was supported by NIH grants R43-AR51278, R43-CA104061 and RO1-CA70761. The authors would like to express their appreciation of efforts by collaborators at UCSF Medical Center (Daniel Neuman) and IPITEK Corp. (Dave Schafsma, John Rozanski, Doug Bonnell and Rick Forber).
References
- Stauffer PR. Thermal therapy techniques for skin and superficial tissue disease. A critical review, matching the energy source to the clinical need, TP Ryan. SPIE Optical Engineering Press, Bellingham, WA 2000; 327–367
- Overgaard J, Gonzales Gonzales D, Hulshof MCCH, Arcangeli G, Dahl O, Mella O, Van der Zee J. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European Society for Hyperthermia Oncology. Int J Hyperthermia 1996; 12: 3–20
- Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, Van Putten WLJ, Van Rhoon GC, Van Dijk JDP, Gonzalez DG, Fei-fei L, Goodman P, Sherar M. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. Int J Radiat Oncol Biol Physics 1996; 35: 731–744
- Jones E, Oleson J, Prosnitz L, Samulski T, Vujaskovic Z, Yu D, Sanders L, Dewhirst M. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 2005; 23: 3079–3085
- Diederich CJ, Stauffer PR. Pre-clinical evaluation of a microwave planar array applicator for superficial hyperthermia. Int J Hyperthermia 1993; 9: 227–246
- Diederich CJ, Stauffer PR, Bozzo D. An improved bolus configuration for commercial multielement ultrasound and microwave hyperthermia systems. Med Phys 1994; 21: 1401–1403
- Samulski TV, Grant WJ, Oleson JR, Leopold KA, Dewhirst MW, Vallario P, Blivin J. Clinical experience with a multi-element ultrasonic hyperthermia system: Analysis of treatment temperatures. Int J Hyperthermia 1990; 6: 909–922
- Stauffer PR, Diederich CJ, Sneed PK, Fidel JA, Phillips TL. Preliminary clinical experience with planar and conformal microwave array applicators for hyperthermia. 14th Annual Meeting of the North American Hyperthermia Society Meeting. Nashville, TN, USA 1994; 113, 1994
- Gopal MK, Hand JW, Lumori MLD, Alkhairi S, Paulsen KD, Cetas TC. Current sheet applicator arrays for superficial hyperthermia of chestwall lesions. Int J Hyperthermia 1992; 8: 227–240
- Rietveld PJM, Van Putten WLJ, Van Der Zee J, Van Rhoon GC. Comparison of the clinical effectiveness of the 433 MHz Lucite cone applicator with that of a conventional waveguide applicator in applications of superficial hyperthermia. Int J Radiat Oncol Biol Phys 1999; 43: 681–687
- Van Der Gaag G, De Bruijne M, Samaras T, Van Der Zee J, Van Rhoon GC. Development of a guideline for the water bolus temperature in superficial hyperthermia. Int J Hyperthermia 2006; 22: 637–656
- Van Rhoon GC, Rietveld PJM, Van Der Zee J. A 433 MHz Lucite cone waveguide applicator for superficial hyperthermia. Int J Hyperthermia 1998; 14: 13–27
- Gelvich EA, Mazokhin VN. Contact flexible microstrip applicators (CFMA) in a range from microwaves up to short waves. IEEE Trans Biomed Eng 2002; 49: 1015–1023
- Lee WM, Gelvich EA, van der Baan P, Mazokhin VN, van Rhoon GC. Assessment of the performance characteristics of a prototype 12-element capacitive contact flexible microstrip applicator (CFMA-12) for superficial hyperthermia. Int J Hyperthermia 2004; 20: 607–624
- Samulski TV, Fessenden P, Lee ER, Kapp DS, Tanabe E, McEuen A. Spiral microstrip hyperthermia applicators: Technical design and clinical performance. Int J Radiat Oncol Biol Phys 1990; 18(1)233–242
- Lee ER, Wilsey TR, Tarczy-Hornoch P, Kapp DS, Fessenden P, Lohrbach AW, Prionas SD. Body conformable 915 MHz microstrip array applicators for large surface area hyperthermia. IEEE Trans Biomed Eng 1992; 39: 470–483
- Juang T, Stauffer P, Neuman D, Schlorff J. Multilayer conformal applicator for microwave heating and brachytherapy treatment of superficial tissue disease. Int J Hyperthermia 2006; 22: 527–544
- Stauffer PR, Schlorff JL, Juang T, Neuman DG, Johnson JE, Maccarini PF, Pouliot J. Progress on system for applying simultaneous heat and brachytherapy to large-area surface disease 2005; 82–96, 5698, Proc SPIE Int Soc Opt Eng
- Johnson J, Neuman D, Maccarini P, Juang T, Turner P, Stauffer P. Evaluation of a dual arm Archimedean spiral array for microwave hyperthermia. Int J Hyperthermia 2006; 22: 475–490
- Lee E, Kapp D, Lohrbach A, Sokol J. Influence of water bolus temperature on measured skin surface and intradermal temperatures. Int J Hyperthermia 1994; 10: 59–72
- Rossetto F, Diederich CJ, Stauffer PR. Thermal and SAR characterization of multi-element DCC microwave applicators for hyperthermia, a theoretical investigation. Med Phys 2000; 27(4)745–753
- Hartov A, Colacchio TA, Strohbehn JW, Ryan TP, Hoopes PJ. Performance of an adaptive MIMO controller for a multiple-element ultrasound hyperthermia system. Int J Hyperthermia 1993; 9: 563–579
- Van Baren P, Ebbini ES. Multipoint temperature control during hyperthermia treatments: Theory and simulation. IEEE Trans Biomed Eng 1995; 42: 818–827
- Zhou LJ, Fessenden P. Automation of temperature control for large-array microwave surface applicators. Int J Hyperthermia 1993; 9: 479–490
- Stauffer PR, Jacobsen S, Neuman D, Rossetto F. Microwave array applicator for radiometry controlled superficial hyperthermia 2001; 19–29, 4247, Proc SPIE Int Soc Opt Eng
- Stauffer PR, Jacobsen S, Neuman D, Rossetto F. Progress toward radiometry controlled conformal microwave array hyperthermia applicator. Proc 22nd Annual EMBS International Conference. Chicago, IL July 23–28, 2000
- Kapton® Polyimide film, http://www2.dupont.com/kapton/en_US/index.html
- Juang T, Neuman D, Schlorff J, Stauffer PR. Construction of a conformal water bolus vest applicator for hyperthermia treatment of superficial skin cancer. International Conference of the IEEE Engineering in Medicine and Biology Society, 2004. IEEE Press, San Francisco 2004
- Waterman FM. Determination of the temperature artifact during ultrasound hyperthermia. Int J Hyperthermia 1990; 6: 131–142
- Waterman FM, Nerlinger RE, Leeper JB. Catheter induced temperature artifacts in ultrasound hyperthermia. Int J Hyperthermia 1990; 6: 371–381
- Underwood HR, Peterson AF, Magin RL. Electric-field distribution near rectangular microstrip radiators for hyperthermia heating: Theory versus experiment in water. IEEE Trans Biomed Eng 1992; 39: 146–153
- Stauffer P, Maccarini P, Juang T, Jacobsen S, Gaeta C, Schlorff J, Milligan AJ. Progress on conformal microwave array applicators for heating chestwall disease, Proc SPIE;6440:64400E1–13
- Lamaitre G, Van Dijk JDP, Gelvich EA, Wiersma J, Schneider CJ. SAR characteristics of three types of contact flexible microstrip applicators for superficial hyperthermia. Int J Hyperthermia 1996; 12: 255–269
- Sherar MD, Clark H, Cooper B, Kumaradas J, Liu FF. A variable microwave array attenuator for use with single-element waveguide applicators. Int J Hyperthermia 1994; 10: 723–731
- Johnson JE, Maccarinil PF, Neuman D, Stauffer PR. Automatic temperature controller for multielement array hyperthermia systems 2006; 1006–1015, IEEE Trans Biomed Eng:53(6)
- Arunachalam K, Maccarini PF, Stauffer PR. A thermal monitoring sheet with low influence for adjacent water bolus for tissue surface thermometry during clinical hyperthermia, In Press, IEEE Trans Biomed Eng