Abstract
Purpose: To determine an effective triple-agent schedule combining fever-range whole body thermal therapy (FR-WB-TT) with cisplatin and gemcitabine by optimizing the timing of drug with heat, and drug with drug.
Materials and methods: Using an orthotopically implanted syngeneic breast adenocarcinoma in an immunologically normal female Fischer rat model, we investigated various schedules of a thermochemotherapy regimen combining FR-WB-TT with chemotherapy agents, cisplatin and gemcitabine. Differently timed combinations of a) cisplatin with FR-WB-TT, b) gemcitabine with FR-WB-TT, and c) cisplatin with gemcitabine were examined for anti-tumor efficacy and toxicity. A combination of the three agents based on the optimal two-agent schedules was then tested.
Results: The greatest primary tumor and axillary metastasis growth delay and lowest toxicity was induced with administration of cisplatin 24 h prior to gemcitabine and cisplatin 24 h prior to simultaneous gemcitabine and FR-WB-TT. Administering cisplatin 24 h prior to gemcitabine was more effective and less toxic than giving the two drugs simultaneously or gemcitabine prior to cisplatin. Survival was greatest when gemcitabine and FR-WB-TT were administered 24 h after cisplatin, even with reduced drug doses. One complete cure resulted from the triple agent treatment.
Conclusions: The relative timing of agents in multiple modality treatments is critically important in achieving tumor control or cures, and in reducing toxicity. Optimizing the relative timing of multiple agents in thermochemotherapy allows use of lower drug doses to achieve maximal anti-tumor efficacy and minimal toxicity.
Introduction
A major rationale for systemic (whole body) thermal therapy (hyperthermia) in cancer treatment is the ability to treat metastatic disease. Usually whole body hyperthermia is used as an adjunct to other cancer therapies, principally radiotherapy and chemotherapy. Thermo-chemotherapy using systemic fever-range (40°C) temperature for long durations (4-6 h) has better or equal anti-tumor efficacy compared to maximally-tolerated systemic temperatures (41.5°C–42°C), and generally results in less toxicity.
While it is important to recognize that whole body thermal therapy can enhance some of the toxicities associated with other treatments, the synergy of hyperthermia with several chemotherapy agents means that lower doses can be used, resulting in less toxicity. For example, the cardiotoxicity of doxorubicin and both the renal and hematological toxicities of platinum agents may increase under hyperthermia Citation[2], while the muscle and peripheral nervous system effects of radiation and some drugs can also be enhanced Citation[3]. Bone marrow suppression is the limiting toxicity of many chemotherapy drugs but there is little data to suggest that whole body hyperthermia exacerbates this effect. On the contrary, systemic hyperthermia combined with carboplatin achieved therapeutic results without increasing myelosuppression, and responses occurred at lower than maximally tolerated (MTD) doses Citation[4]. We have demonstrated for several chemotherapy agents (cisplatin, oxaliplatin, gemcitabine) that MTD-like efficacy, or greater, can be achieved at lower doses combined with thermal therapy Citation[5]. We further believe that it can be beneficial to combine several chemotherapy drugs at lower-than-usual doses with fever-range whole body thermal therapy (FR-WB-TT) in order to reduce the individual drug toxicities and distribute the toxicities amongst different organ systems (e.g. nephrotoxicity and myelotoxicity) while attacking the tumor by different mechanisms and boosting immune response.
Optimal combination of whole body hyperthermia with chemotherapy requires an understanding of the mechanisms of interaction of heat with individual drugs or drugs in combination. The timing of chemotherapy with respect to whole body hyperthermia is important in determining both the efficacy of the combination treatment and the therapeutic index. For example, we have previously shown that for cisplatin, the greatest therapeutic index is achieved if the drug is given 24 h before the start of whole body hyperthermia, thereby preventing thermal augmentation of cisplatin-induced nephrotoxicity Citation[6]. In a clinical investigation of multiple cycles of whole body hyperthermia combined with carboplatin, Ifosfamide, etoposide and granulocyte colony stimulating factor, it was found that toxicity was minimized when carboplatin was given during the plateau phase of heating, 10 min after target temperature was reached Citation[7].
In preclinical studies we also demonstrated a synergistic anti-tumor response to thermal therapy administered either simultaneously with gemcitabine, or given 48 h later. This therapeutic effect was completely negated, however, when hyperthermia was administered 24 h after gemcitabine, perhaps due to cell cycle effects Citation[8], Citation[9].
The purpose of this preclinical study was to establish an effective, but minimally toxic, tri-modality combination of cisplatin, gemcitabine, and fever-range whole body thermal therapy (FR-WB-TT; 40°C/104°F for 6 h duration) by first studying the efficacy and toxicity of two-modality drug-drug and drug-heat combinations administered according to various schedules in a rat mammary adenocarcinoma model. We examined different relative timings of cisplatin with gemcitabine, cisplatin with FR-WB-TT, and gemcitabine with FR-WB-TT and then tested the best schedule of the two drugs in combination with FR-WB-TT using lower doses than in the two drug and drug-heat combinations.
Methods
Animal tumor model
Experiments were performed using MTLn3 mammary tumors, orthotopically implanted in the left mammary fat pad of immunologically-normal female Fischer 344 rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN). The animals were allowed a 7-day environmental adaptation period before beginning the experimental studies. Tumors were implanted when the rat body weights ranged from 100 g to 120 g (6–7 weeks of age). Rats were fed standard laboratory chow, allowed free access to water, and housed under controlled conditions with a 12 hour light/dark cycle. The animal treatment protocol was reviewed and approved by the University of Texas Health Science Center Animal Care Committee.
The MTLn3 mammary adenocarcinoma is a highly aggressive, spontaneously metastasizing syngeneic rat breast cancer cell line derived from a lung metastasis from the 13762NF rat mammary adenocarcinoma Citation[10]. When 5 × 105 MTLn3 cells are subcutaneously injected orthotopically into the mammary fat pad, all rats develop primary mammary tumors 50–150 mm3 in size by day 10. Metastatatic tumors develop soon thereafter. An earlier study showed that microscopic lymph node metastases are found on day 12 in all rats, and palpable axillary and inguinal lymph node metastases develop in all animals within 21 days of primary tumor implantation. All untreated animals develop macroscopic lung metastases on or before day 28 following tumor inoculation Citation[11]. For this study, rats were orthotopically inoculated with 5 × 105 MTLn3 cells in 0.25 mL of alpha-modified minimum essential medium (α-MEM, Invitrogen Corp., Carlsbad, CA) on day 0. The cells were inoculated into the left abdominal mammary fat pad beneath the nipple. Rats with palpable mammary tumors around 100 mm3 in volume were selected for the treatment and control groups. Animals with progressive disease were subsequently euthanized between days 35 to 38 to prevent suffering from bulky lymph nodes or metastatic lung tumors according to institutional animal welfare guidelines.
Therapy
Chemotherapy and/or thermal therapy was begun on day 10 after tumor inoculation, when the tumor was approximately 100 mm3 in volume. At this time, microscopic inguinal and/or axillary lymph node metastases would have been established, yet no microscopically visible metastatic lung deposits would have been present. The control and FR-WB-TT–alone groups received sham treatment of 0.9% NaCl administered on the same days after tumor inoculation as chemotherapy. Control animals received the same anesthesia protocol as the treated animals and were maintained at a normal body temperature by placement on a warmed water blanket to prevent hypothermia Citation[12]. The rats were randomized into groups of 6 per treatment.
Fever-range whole body thermal therapy (FR-WB-TT)
FR-WB-TT was administered by partially immersing halothane-anesthetized rats into a thermostatically controlled circulating water bath maintained at 40.0°C ± 0.1°C by a Haake Model E heater/circulator (as described previously) Citation[13]. Rectal temperature was measured continuously in all rats treated, and recorded every 5 min using YSI model 402 small animal rectal thermistor probes connected to a YSI model 4002 12-channel switch box, with rat body temperature displayed on a YSI model 49A digital tele-thermometer (Yellow Springs Instrument Co., Yellow Springs, OH) Citation[12]. The probes were calibrated against a mercury thermometer (Etrco ASTM 64°C) certified by the National Bureau of Standards. An average of 15 min was required for the rectal temperature to reach 40.0°C. The rats were then maintained for 6 h at a temperature of 40.0°C ± 0.01°C. Control animals were also anesthetized for 6 h with halothane. The optimal duration of FR-WB-TT in these rats was previously determined to be 6 to 8 h Citation[12]. For logistical reasons we opted for 6 h of hyperthermia treatment.
Anesthesia
All animals received halothane anesthesia during the 6 h hyperthermia treatments, as well as during the corresponding 6 h sham treatments as described previously Citation[14]. Lord et al. first showed the safety of halothane anesthesia in combination with WBH Citation[15] and our previous studies have shown that halothane anesthesia affects neither tumor growth Citation[14], Citation[16] nor normal tissue toxicity Citation[14]. The rats were first exposed to 3% halothane in pure oxygen in an induction chamber for approximately 10 min in order to induce surgical level anesthesia prior to the start of hyperthermia or sham treatment. 1.5% halothane mixed with pure oxygen in a standard hospital grade vaporizer was then applied to the rats through a custom-made polymethyl methacrylate mask throughout the warm-up time and 6 h treatments or equivalent sham treatment. The rats showed no evidence of discomfort during anesthesia and after treatment they recovered without ill effects, quickly becoming ambulatory.
Drugs
Cisplatin (Platinol-AQ) was obtained as an aqueous solution (Ben Venue Laboratories, Inc., Bedford, OH), and administered at the stock concentration of 1 mg/mL. Gemcitabine (Gemzar) was obtained as a lyophilized powder from Eli Lilly and Co., Indianapolis, IN, and was reconstituted according to the manufacturer's recommendations with United States Pharmacopeia (USP) standardized 0.9% NaCl, and further diluted with 0.9% NaCl USP to a final concentration of 2.7 mg/mL for administration to the rats. Cisplatin and gemcitabine were injected into the tail vein under light anesthesia as a bolus. Control animals received equal volumes of 0.9% NaCl USP solution vehicle alone under the same light ether anesthesia. The maximally tolerated dose (MTD) in our tumor-bearing rats is 7.5 mg/kg for cisplatin Citation[19] and ≥10 mg/kg for gemcitabine. We had previously determined that the MTD for the two drugs administered simultaneously was 3.5 mg/kg cisplatin + 6.0 mg/kg gemcitabine.
Combination therapy
Intravenous injection of each drug at the MTD was combined with thermal therapy in various relative timings (simultaneously, 24 h apart, or 48 h apart). The two drugs were administered by intravenous injection either simultaneously or 24 h apart at the two-drug MTD of 3.5mg/kg cisplatin + 6.0 mg/kg gemcitabine.
The most efficacious two-drug schedule was then combined with FR-WB-TT using either 3.5mg/kg cisplatin + 6.0 mg/kg gemcitabine or 3.0 mg/kg cisplatin and 5.0 mg/kg gemcitabine since the MTD for cisplatin + gemcitabine + FR-WB-TT triple combination therapy had not been determined previously. The lower doses are approximately 50% of the single drug maximally tolerated dose. Drugs administered simultaneously with FR-WB-TT were injected when the rectal temperature first reached the target temperature of 40°C. The effect of each treatment on tumor growth, metastasis development, toxicity, and survival was measured.
Gemcitabine Combined With FR-WB-TT (experiment 1)
The following treatment groups were investigated: a) control = sham treatment, b) FR-WB-TT, c) gemcitabine (10 mg/kg), d) gemcitabine administered simultaneously with FR-WB-TT, e) gemcitabine 24 h prior to FR-WB-TT, f) gemcitabine 48 h prior to FR-WB-TT. We had previously determined that thermal therapy prior to gemcitabine was not more effective than gemcitabine alone.
Cisplatin combined with FR-WB-TT (experiment 3)
Cisplatin (3.5 mg/kg) was administered 24 h prior to FR-WB-TT since this had previously been determined to be the optimal cisplatin-TT schedule.
Cisplatin combined with gemcitabine (experiment 2)
The following treatment groups were investigated: a) control = sham treatment, b) cisplatin (3.5 mg/kg), c) gemcitabine (6.0 mg/kg), d) cisplatin administered simultaneously with gemcitabine, e) gemcitabine 24 h prior to cisplatin, and f) cisplatin 24 h prior to gemcitabine.
Cisplatin combined with gemcitabine and FR-WB-TT
Based on the superior efficacy of a) cisplatin 24 h before FR-WB-TT, b) gemcitabine with simultaneous FR-WB-TT, and c) cisplatin 24 h before gemcitabine, a triple-modality therapy of cisplatin followed 24 h later by simultaneous FR-WB-TT and intravenous injection of gemcitabine when the animal's core temperature first reached 40°C (CIS 24 h > GEM + TT) was tested. Two dose combinations were tested, namely CIS (3.5 mg/kg) 24 h > GEM (6.0 mg/kg) + TT and CIS (3.0 mg/kg) 24 h > GEM (5.0 mg/kg) + TT, and assessed for anti-tumor efficacy, metastasis development, toxicity, and survival.
Tumor assessment
Tumor size was measured every 2 days using a vernier caliper to determine three orthogonal dimensions (d), and the tumor volume (V) was calculated by using the formula V = (d1 × d2 × d3)/2. The incidence and size of axillary and inguinal lymph node metastases were recorded.
Toxicity assessment
Rat body weight was recorded every other day as a general indicator of toxicity. Drug-specific toxicities such as myelotoxicity, renal toxicity, neurotoxicity, etc. were not measured in this study.
Statistical analysis
The significance of differences in the primary tumor and metastatic lymph node tumor size, as well as changes in body weight among the experimental groups was calculated by the Student's t test. A P value of <0.05 was considered statistically significant. A Chi-squared analysis was used for nonparametric data. Survival curves were calculated using the Kaplan-Meier method Citation[17].
Results
Tumor growth
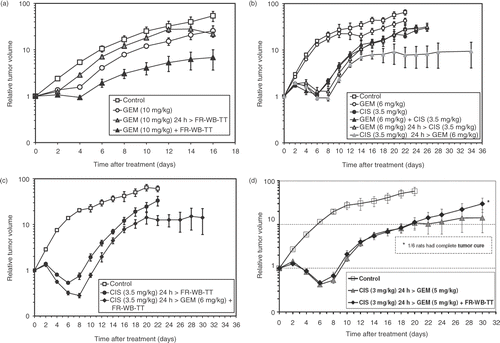
illustrates the schedule-dependency of primary tumor growth following 1) various schedules of gemcitabine and FR-WB-TT compared to gemcitabine alone; 2) various schedules of cisplatin and gemcitabine compared to each drug alone; 3) the triple agent combination of cisplatin (3.5 mg/kg) followed 24 h later by gemcitabine (6 mg/kg) with simultaneous FR-WB-TT compared to cisplatin (3.5 mg/kg) 24 h before FR-WB-TT; and 4) the lower dose triple agent combination of cisplatin (3.0 mg/kg) followed 24 h later by gemcitabine (5.0 mg/kg) with simultaneous FR-WB-TT compared to cisplatin 24 h before gemcitabine. All treatments caused a short-term tumor growth arrest between days 4 and 8, but this was followed by subsequent tumor regrowth. Cisplatin followed 24 h later by gemcitabine (at both dose combinations) and either of the triple-agent treatments resulted in a slowing of the tumor regrowth from day 12 to 14 onwards. The nadir of tumor volume appeared to be dose-dependent. Addition of thermal therapy to gemcitabine increased primary tumor growth delay when FR-WB-TT was administered simultaneously, but not when it was administered 24 h later (). Delaying heat treatment by 48 h also caused greater growth delay than gemcitabine alone (data not shown). Cisplatin given 24 h before gemcitabine resulted in substantial tumor suppression compared to either cisplatin or gemcitabine alone, and significantly greater tumor control than cisplatin simultaneously with gemcitabine or gemcitabine before cisplatin (). Primary tumor volume remained constant from day 16 to day 34. When cisplatin (3.5 mg/kg) was given 24 h before FR-WB-TT () there was substantial short term tumor regression to 0.53 of initial tumor volume but ultimate tumor regrowth leading to death by day 22 as in the control group. Addition of gemcitabine (6 mg/kg) caused greater early tumor regression to 0.28 of initial tumor volume, and although there was some subsequent tumor regrowth, 10x initial tumor volume was not reached until day 18, and tumor volume reached a plateau level at day 20 which was maintained in the long term without further regrowth (). No complete cures occurred, however, as the animals later succumbed to metastatic disease. When the drug doses were reduced to 50% of MTD, the primary tumors regressed to an average of 0.46 of initial volume, 10x initial tumor volume was reached on day 20, and one animal went on to be completely cured of all primary and metastatic tumors ().
Figure 1. Tumor volume versus time after treatment with A) gemcitabine (GEM), 10 mg/kg, plus fever-range whole body thermal therapy (FR-WB-TT)⁁; B) cisplatin (CIS), 3.5 mg/kg, combined with GEM, 6 mg/kg, in different schedules*; C) CIS (3.5 mg/kg) 24 h > FR-WB-TT and CIS (3.5. mg/kg) 24 h > GEM (6 mg/kg) + simultaneous FR-WB-TT**; D) the best two-drug schedule, CIS 24 h > GEM at the lower doses of 3 mg/kg and 5 mg/kg, respectively, and the lower dose three agent combination of CIS (3 mg/kg) followed 24 hours later by GEM (5 mg/kg) plus simultaneous FR-WB-TT#. The relative timing of treatments significantly affects antitumor effect. Data points are mean ± SEM of 6 rats per group. Data from four experiments are presented: ⁁experiment 1, *experiment 2, **experiment 3, #experiment 4. Tumor response is schedule-dependent.
Toxicity
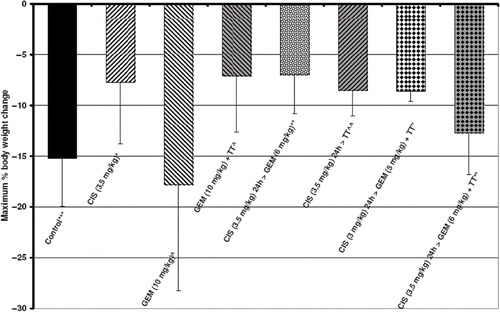
Body weight is an indicator of general health and treatment-induced toxicity, typically falling to a nadir a number of days after treatment and rising again if the animal recovers. A small increase in total body weight occurs due to tumor growth, but is much less than the decline in body weight due to toxicity. plots the nadir of post-treatment body weight as maximum percentage body weight change for each drug alone, the most effective two-agent combinations, and the two triple agent treatments. All treatments except gemcitabine (10 mg/kg) resulted in significantly less body weight loss than that of control animals (p ≤ 0.011). 10 mg/kg of gemcitabine resulted in substantial toxicity, causing an average of 17.8%, and as much as 35% loss of body weight. Combining FR-WB-TT with gemcitabine appeared to reduce the toxicity of the drug, resulting in significantly less body weight loss than gemcitabine alone (p = 0.025). The combination of cisplatin (3.5 mg/kg) 24 hours before gemcitabine (6 mg/kg) also led to less body weight loss than gemcitabine alone (p < 0.02). There was no significant difference at the 5% level in body weight loss between gemcitabine (10 mg/kg) + FR-WB-TT, cisplatin (3.5 mg/kg) 24 h before FR-WB-TT, and either of the two triple-agent treatments, though the higher dose triple agent treatment may be somewhat less toxic than the lower dose triple agent treatment (p < 0.062). Control of normal tissue toxicity is as important as increased tumor cytotoxicity in determining the therapeutic index and patient outcome.
Figure 2. Maximum body weight loss after treatment (a measure of toxicity) for cisplatin (CIS) and gemcitabine (GEM) with and without fever-range whole body thermal therapy (FR-WB-TT, abbreviated to TT in the figure), the best dual drug schedule of CIS (3.5 mg/kg) followed 24 h later by GEM (6 mg/kg), and a triple agent combination of CIS followed 24 h later by GEM plus simultaneous TT at two dose levels: CIS (3.5 mg/kg) 24 h > GEM (6 mg/kg) + TT and CIS (3 mg/kg) 24 h > GEM (6 mg/kg) + TT. The relative timing of agents, and chemotherapy dose, affect toxicity. Data points are mean ± standard deviation of 6 rats per group. Chart displays data from four experiments: ⁁experiment 1, *experiment 2, **experiment 3, #experiment 4, ***average of experiments 2, 3, and 4. Toxicity is schedule-dependent.
Metastasis development
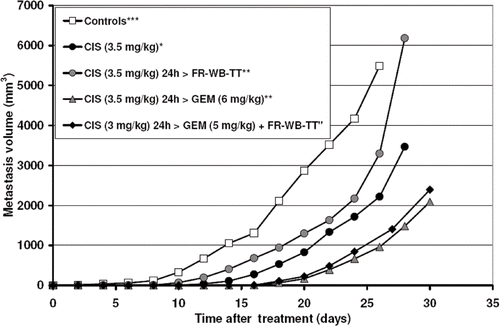
Combination treatment delayed the growth of lymph node metastases. Addition of FR-WB-TT to gemcitabine (10 mg/kg) delayed the first appearance of measurable inguinal metastases by 4 days and of axillary metastases by 8 days. Cisplatin (3.5 mg/kg) administered 24 hours before gemcitabine (6 mg/kg), delayed the first appearance of measurable inguinal metastases by 3 days compared to cisplatin alone (3.5 mg/kg) and axillary metastases by 8 days. With the lower dose triple combination of cisplatin (3 mg/kg) 24 h before gemcitabine (5 mg/kg) plus FR-WB-TT inguinal metastases first appeared on day 12 and axillary metastases on day 14, compared to day 10 (inguinal) and day 12 (axillary) in CIS 24 h > GEM treated rats. plots the growth of axillary metastases in selected cisplatin-treated groups. Cisplatin (3.5 mg/kg) and cisplatin (3.5 mg/kg) 24 h before FR-WB-TT resulted in an approximately 7 day growth delay of axillary metastases over control animals. Both cisplatin (3.5 mg/kg) 24 h before gemcitabine (6 mg/kg) and the lower dose triple agent treatment further increased the delay in growth of axillary metastases, resulting in an approximately 12 day growth delay over controls.
Figure 3. Axillary metastasis volume versus time after treatment with cisplatin (CIS), gemcitabine (GEM), and fever-range whole body thermal therapy (FR-WB-TT). Properly scheduled combination treatments result in a substantial growth delay of metastases. Data points are mean ± SEM of 6 rats per group. Chart displays data from four experiments: ⁁experiment 1, *experiment 2, **experiment 3, #experiment 4, ***average of experiments 2, 3, and 4. Axillary metastasis development was delayed with carefully scheduled combination treatment.
Survival
As shown in nimal survival was also highly dependent on the relative timing of the treatment agents. Cisplatin administered 24 h before gemcitabine resulted in a significant increase in survival compared to cisplatin given simultaneously with gemcitabine or gemcitabine before cisplatin (). Comparable short-term survival was seen in the lower dose triple agent treatment group, but one animal went on to be completely cured of primary and metastatic tumors (). , which lists the median survival for all the tested treatments, also illustrates the schedule-dependency of thermochemotherapy treatment combinations. Gemcitabine with simultaneous FR-WB-TT produced the longest survival of the three GEM-TT schedules while cisplatin 24 h before FR-WB-TT resulted in comparable survival. An approximately 50% increase in median survival over control was seen with the two triple agent treatments and cisplatin (3.5 mg/kg) 24 h before gemcitabine (6 mg/kg).
Figure 4. Percentage survival versus time after treatment with different schedules of A) cisplatin (CIS) and gemcitabine (GEM), and B) lower dose two-drug and two-drug plus fever-range whole body thermal therapy (FR-WB-TT) combinations. Careful scheduling produced significant increases in survival. The triple agent treatment of CIS (3 mg/kg) 24 h > GEM (5 mg/kg) + FR-WB-TT resulted in 1 complete cure (17%). Data points are mean of 6 rats per group: *experiment 2, #experiment 4. Survival is schedule-dependent.
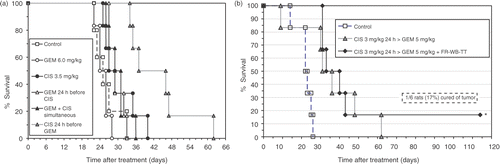
Table I. Median survival is schedule-dependent. Data from four experiments: ⁁experiment 1, *experiment 2, **experiment 3, #experiment 4, ***pooled data from experiments 1, 2, and 3.
Discussion
The importance of timing when radiation is combined with local-regional thermal therapy has been well documented Citation[18], Citation[19], Citation[20], Citation[21]; however, the timing of chemotherapy drugs with thermal therapy has received less attention. A few studies do show that the timing of chemotherapy drugs with one another, and with heat, is critically important. The data presented here demonstrate that the timing of cisplatin or gemcitabine with systemic heat, and the timing of cisplatin with gemcitabine, affects both primary and metastatic antitumor efficacy as well as toxicity, which in turn translates into survival. Our previous studies demonstrated that cisplatin combined simultaneously with WB-TT (41.5°C/107°F for 2 h duration) induces a synergistic antitumor response. However, simultaneous administration of cisplatin with 41.5°C WB-TT also induces severe acute, as well as chronic, renal tubule damage resulting in kidney failure Citation[22], Citation[23], Citation[24]. On the other hand, cisplatin administered 48 h to 1 h prior to WB-TT induced a supra-additive antitumor response, and renal tubular toxicity was no different than that induced by cisplatin as a single agent Citation[13]. The same schedule-dependent tumor response and renal tubular toxicity occurred when cisplatin was combined with FR-WB-TT Citation[13], Citation[25].
Interestingly, carboplatin, a second generation platinum drug with a similar spectrum of antitumor activity to cisplatin, has a very different toxicity profile. Carboplatin induces much less severe proximal renal tubule toxicity compared to cisplatin Citation[26], however it induces acute myelosuppression, while cisplatin does not. The antitumor effect of combining carboplatin with systemic thermal therapy is maximal when the two treatments are administered simultaneously, and normal-tissue toxicity is tolerable Citation[1], Citation[2], Citation[27], Citation[28]. We have also found that FR-WB-TT reduces the myelotoxicity of oxaliplatin, a third generation platinum chemotherapy drug (data not shown).
Doxorubicin has been shown to have the best antitumor effect, and the least toxicity, when administered before thermal therapy, compared to simultaneous administration Citation[29], Citation[30]. Liposomal doxorubicin (Doxil) induces a greater antitumor effect and tolerable toxicity if administered 1 h to 2 h after thermal therapy Citation[31], Citation[32]. 5-Fluorouracil (5-FU) demonstrates no enhanced antitumor activity if administered simultaneously with thermal therapy, yet its tumor growth delay is supra-additive when continuous intravenous infusion of 5-FU is followed 24 h later by thermal therapy Citation[33].
Testing drugs with heat in vivo rather than only in vitro is important for clinical translation. For example, in contrast to our findings, a previous in vitro study suggested that a simultaneous administration of gemcitabine with high temperature heat (43°C) was less cytotoxic than gemcitabine administration prior to heat Citation[34]. While this may be due to the fact that the temperature used in these experiments was 43°C not 40°C, however, it is a critical principal that in vivo findings are often quite different from in vitro results. Interestingly, another in vivo study of thermal therapy at 41.5°C combined with several drugs demonstrated enhanced tumor cytotoxicity of gemcitabine, docetaxel, irinotecan (at lower doses), and oxaliplatin (at high dose) with heat, however, the study examined only simultaneous administration of each drug with heat Citation[35]. It would appear to be important to test different schedules of administration of each drug with thermal therapy.
In addition to the significant influence of timing of chemotherapy drug with heat on tumor response, combining two drugs together also clearly affects antitumor efficacy and toxicity Citation[36]. In addition to the data presented here showing the schedule dependency of cisplatin plus gemcitabine, we have also shown that epirubicin administered 24 h after CPT-11 resulted in increased survival compared to control and other schedules of the two drugs Citation[37]. Generally, however, there has been little interest in optimizing the relative timing of administration of more than one chemotherapy drug. The schedule dependency of the therapeutic index of multiple drug combinations, and drugs combined with thermal therapy, can be linked to the mechanisms of action of the drugs, the toxicities induced by the drugs, and the effect of heat on drug delivery, drug targets, and damage repair. For example, thermal enhancement of liposomally encapsulated drugs (e.g. Doxil) is at least in part due to hyperthermia increasing the size of endothelial gaps, thereby allowing more liposomes to penetrate the tumor. We had postulated that heat inhibition of DNA repair could be responsible for thermal enhancement of gemcitabine therapy, however we found no significant difference in DNA repair between various schedules of administration of gemcitabine with FR-WB-TT (data not shown). The schedule-dependency of gemcitabine combined with FR-WB-TT (as seen in ) may instead be a function of the cell-cycle dependency of the metabolic action of the drug, and thermal increase of gemcitabine-induced apoptosis Citation[38]. Similarly, the long-term tumor control seen when FR-WB-TT was added to gemcitabine given 24 h after cisplatin () may be due to the additional apoptosis caused by heat treatment. Gemcitabine, when administered after cisplatin, inhibits the repair of cisplatin-induced DNA damage (platinum adducts). On the other hand, cisplatin decreases the cellular accumulation of the anti-metabolite gemcitabine triphosphate, thus diminishing the effect of gemcitabine when administered simultaneously or before gemcitabine Citation[39]. These effects may be responsible for the schedule-dependency of anti-tumor effect seen in . A prior study of ours had also demonstrated substantially increased efficacy against primary tumor and axillary lymph node metastases when cisplatin was administered 48 h prior to gemcitabine compared to simultaneously with, or 48 h after Citation[27].
Kroep et al. investigated several two-drug schedules of cisplatin and gemcitabine and concluded that thrombocytopenia was not schedule-dependent but that leukopenia was significantly more serious when cisplatin preceded gemcitabine Citation[40]. Nonetheless, they recommended this schedule since it produced the best pharmacological profile and the toxicities were manageable. In the studies presented here, cisplatin 24 h prior to gemcitabine did not result in greater toxicity than cisplatin alone, at least as indicated by body weight (). Addition of thermal therapy did not significantly increase body weight loss, though the higher dose triple agent treatment may be somewhat more toxic than the lower dose triple agent treatment. Control of normal tissue toxicity is as important as increased tumor cytotoxicity in determining the therapeutic index and patient outcome.
The most effective schedules of thermochemotherapy with cisplatin, gemcitabine, and FR-WB-TT against the primary tumor were also effective in delaying the development of inguinal and axillary metastases (). Together with manageable toxicity, such a delay translates into superior quality of life and increased survival. The survival curves shown in demonstrate that maximally tolerated doses are not needed for maximum survival; shows significantly increased survival in the triple agent group using lower doses (≤50% of MTD), and this was also the only group in which a cure occurred. Indeed, when combining drugs, lower doses and delayed schedules are preferable in order to reduce the critical toxicities and preserve immune function. Thermal enhancement of antigen presentation and effector cell function is increasingly being recognized as an important component of fever-range thermal therapy Citation[41], Citation[42] and probably also plays an important role in the successful scheduling of combination thermochemotherapy.
Conclusion
The timing of chemotherapy with respect to heat, and of two drugs relative to each other, is critical in determining antitumor efficacy, toxicity, and survival. The significant sequence-mediated differences in antitumor response demonstrated when cisplatin is administered with gemcitabine suggest that optimizing the administration schedule of other multidrug chemotherapy regimens may also prove to be important. Preclinical optimization of the timing of chemotherapy drugs relative to each other, and drugs relative to heat, in multi-agent thermochemotherapy regimens could significantly increase tumor response while minimizing toxicity. It is important that in vivo testing, not only in vitro testing, precede translation of thermochemotherapy to the clinic. The multi-agent thermochemotherapy regimen presented here demonstrates that rationally combining drug with drug, and drug with heat, into an optimal schedule transforms the treatment-induced anticancer activity and toxicity from ineffective and toxic to highly successful and tolerable, leading to long-term survival. Furthermore, use of relatively low drug doses appears to be beneficial. These preclinical studies suggest the utility of a clinical trial.
Acknowledgements
We thank Ms Maude Veech for her valuable editorial work.
References
- Sakaguchi Y, Makino M, Kaneko T, Stephens LC, Strebel FR, Danhauser LL, Jenkins GN, Bull JM. Therapeutic efficacy of long duration-low temperature whole body hyperthermia when combined with tumor necrosis factor and carboplatin in rats. Cancer Res 1994; 54: 2223–2227
- Ohno S, Strebel FR, Stephens LC, Siddik ZH, Baba H, Makino M, Khokhar AR, Bull JM. Haematological toxicity of carboplatin and cisplatin combined with whole body hyperthermia in rats. Br J Cancer 1993; 68: 469–474
- Haveman J, Van Der Zee J, Wondergem J, Hoogeveen JF, Hulshof MC. Effects of hyperthermia on the peripheral nervous system: A review. Int J Hyperthermia 2004; 20: 371–391
- Calvert AH, Harland SJ, Newell DR, Siddik, ZH, Jones AC, McElwain TJ, Raju S, Wiltshaw E, Smith IE, Baker JM, Peckham MJ, Harrap KR. Early clinical studies with cis-diammine-1,1-cyclobutane dicarboylate platinum II. Cancer Chemother Pharmacol 1982; 9: 140–147
- Strebel FR, Proett JM, Rowe RW, Deng W, Bull JMC. Effect of oxaliplatin combined with fever-range whole body thermal-therapy in a rat mammary adenocarcinoma in vivo. Proc Amer Assoc Cancer Res 2006; 47: 905, (Abstract 3847)
- Baba H, Siddik ZH, Strebel FR, Jenkins GN, Bull JM. Increased therapeutic gain of combined cis-diamminedichloroplatinum (II) and whole body hyperthermia therapy by optimal heat/drug scheduling. Cancer Res 1989; 49: 7041–7044
- Katschinski DM, Wiedemann GJ, Mentzel M, Mulkerin DL, Touhidi R, Robins HI. Optimization of chemotherapy administration for clinical 41.8°C whole body hyperthermia. Cancer Lett 1997; 115: 195–199
- Bull JMC, Loo CH, Strebel FR, Jenkins GN, Tomoda M, Rowe RW. Treatment of a highly metastatic mammary adenocarcinoma with fever-range, long-duration, low-temperature (40°C for 6 h) whole body hyperthermia (LL-WBH) combined with gemcitabine (dFdC) in rats. Proc Amer Assoc Cancer Res 1999; 40: 552, (Abstract 3643)
- Bull JMC, Strebel FR, Jenkins GN, Tomoda M, Rowe RW. Sequence dependency of antitumor effect of gemcitabine combined with cisplatin in the highly metastatic MTLN3 rat mammary adenocarcinoma. Proc Amer Assoc Cancer Res 2000; 41: 410, (Abstract 2609)
- Evans DP, Meyn RE, Tomasovic SP. Survival of rat mammary tumor cell clones and DNA strand damage following adriamycin treatment. Cancer Chemother Pharmacol 1986; 18: 137–139
- Matsuda H, Strebel FR, Kaneko T, Stephens LC, Danhauser LL, Jenkins GN, Toyota N, Bull JM. Apoptosis and necrosis occurring during different stages of primary and metastatic tumor growth of a rat mammary adenocarcinoma. Anticancer Res 1996; 16: 1117–1121
- Matsuda H, Strebel FR, Kaneko T, Danhauser LL, Jenkins GN, Toyota N, Bull JM. Long duration-mild whole body hyperthermia of up to 12 hours in rats: Feasibility, and efficacy on primary tumour and axillary lymph node metastases of a mammary adenocarcinoma: Implications for adjuvant therapy. Int J Hyperthermia 1997; 13: 89–98
- Baba H, Siddik ZH, Strebel FR, Jenkins GN, Bull JM. Increased therapeutic gain of combined cis-diamminedichloroplatinum (II) and whole body hyperthermia therapy by optimal heat/drug scheduling. Cancer Res 1989; 49: 7041–7044
- Wondergem J, Strebel FR, Siddik ZH, Newman RA, Bull JM. The effects of anaesthetics on cisplatinum-induced toxicity at normal temperatures and during whole body hyperthermia: The influence of NaCl concentration of the vehicle. Int J Hyperthermia 1988; 4: 643–654
- Lord PF, Kapp DS, Morrow D. Increased skeletal metastases of spontaneous canine osteosarcoma after fractionated systemic hyperthermia and local X-irradiation. Cancer Res 1981; 41: 4331–4334
- Wondergem J, Siddik ZH, Strebel FR, Bull JM. Effect of whole body hyperthermia on cis-diamminedichloroplatinum (II)-induced antitumour activity and tissue Pt-distribution: Do anaesthetics influence the therapeutic ratio?. Eur J Cancer 1993; 29A: 549–554
- Shwartz M, Pliskin JS, Grondahl HG, Boffa J. Use of the Kaplan-Meier estimate to reduce biases in estimating the rate of caries progression. Community Dent Oral Epidemiol 1984; 12: 103–108
- Peschke P, Heimburg S, Wolber G, Zuna I, Hahn EW. Improved therapeutic response by distinct timing of multiple heat treatments during interstitial radiation in the Dunning R3327 prostate tumor model. J Cancer Res Clin Oncol 1998; 124: 172–178
- Baker DG, Sager H, Constable WC, Goodchild NT. Influence of ultrasound-induced hyperthermia and X-irradiation on the incidence of metastases from a solid tumor. Invasion Metastasis 1984; 4: 111–124
- Kampinga HH. Cell biological effects of hyperthermia alone or combined with radiation or drugs: A short introduction to newcomers in the field. Int J Hyperthermia 2006; 22: 191–196
- Peschke P, Hahn EW, Wenz F, Lohr F, Braunschweig F, Wolber G, Zuna I, Wannenmacher M. Differential sensitivity of three sublines of the rat Dunning prostate tumor system R3327 to radiation and/or local tumor hyperthermia. Radiat Res 1998; 150: 423–430
- Wondergem J, Bulger RE, Strebel FR, Newman RA, Travis EL, Stephens LC, Bull JM. Effect of cis-diamminedichloroplatinum(II) combined with whole body hyperthermia on renal injury. Cancer Res 1988; 48: 440–446
- Wondergem J, Strebel FR, Stephens LC, Siddik ZH, Bull JM. Chronic effect of whole body hyperthermia combined simultaneously with cis-diamminedichloroplatinum (II) on normal tissue in rat. Int J Hyperthermia 1995; 11: 37–47
- Wondergem J, Bulger RE, Siddik ZH, Leygraaf JW, Strebel FR, Alonso M, Travis EL, Bull JM. A comparison of thermal enhancement of cis-diamminedichloroplatinum (II) induced renal and intestinal toxicities by whole body hyperthermia in the rat. Int J Radiat Oncol Biol Phys 1989; 16: 1551–1556
- Toyota N, Strebel FR, Stephens LC, Matsuda H, Bull JM. Long-duration, mild whole body hyperthermia with cisplatin: Tumour response and kinetics of apoptosis and necrosis in a metastatic rat mammary adenocarcinoma. Int J Hyperthermia 1997; 13: 497–506
- Richel O, Zum Vörde Sive Vörding PJ, Rietbroek R, Van der Velden J, Van Dijk JD, Schilthuis MS, Westermann AM. Phase II study of carboplatin and whole body hyperthermia (WBH) in recurrent and metastatic cervical cancer. Gynecol Oncol 2004; 95: 680–685
- Ohno S, Siddik ZH, Baba H, Stephens LC, Strebel FR, Wondergem J, Khokhar AR, Bull JM. Effect of carboplatin combined with whole body hyperthermia on normal tissue and tumor in rats. Cancer Res 1991; 51: 2994–3000
- Cohen JD, Robins HI, Tapazoglou E, Schmitt CL, Sapareto SA, Khatana A. Whole body hyperthermia and carboplatin: cytotoxicity for murin leukaemia and normal marrow. Br J Cancer 1991; 64: 528–30
- Baba H, Maehara Y, Takeuchi H, Inutsuka S, Sugimachi K. Optimal scheduling increases therapeutic gain of adriamycin combined with hyperthermia. Anticancer Res 1993; 13: 651–654
- Wondergem J, Stephens LC, Strebel FR, Baba H, Ohno S, Siddik ZH, Newman RA, Bull JM. Effect of adriamycin combined with whole body hyperthermia on tumor and normal tissues. Cancer Res 1991; 51: 3559–3567
- Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res 2000; 60: 4440–4445
- Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res 2001; 61: 3027–3032
- Sakaguchi Y, Stephens L, Danhauser L, Makino M, Strebel FR, Jenkins GN, Bull JMC. Enhancement of antitumor effect and induction of apoptosis by a combination of 5-fluorouracil and whole body hyperthermia in rats. Proc Amer Assoc Cancer Res 1993; 34: 361, (Abstract 215)
- Haveman J, Rietbroek RC, Geerdink A, van Rijn J, Bakker PJl. Effect of hyperthermia on the cytotoxicity of 2',2'-difluorodeoxycytidine (gemcitabine) in cultured SW1573 cells. Int J Cancer 1995; 62: 627–630
- Mohamed F, Marchettini P, Stuart OA, Urano M, Sugarbaker PH. Thermal enhancement of new chemotherapeutic agents at moderate hyperthermia. Ann Surg Oncol 2003; 10: 463
- Katschinski DM, Jacobson EL, Wiedemann GJ, Robins HI. Modulation of VP-16 cytotoxicity by carboplatin and 41.8°C hyperthermia. J Cancer Res Clin Oncol 2001; 127: 425–432
- Strebel FR, Sumiyoshi K, Jenkins GN, Rowe RW, Bull JMC. Drug sequence effect on therapeutic outcome (tumor growth, toxicity, and survival) of Irinotecan combined with Epirubicin in the in vivo MTLn3 rat mammary adenocarcinoma. Proc Amer Assoc Cancer Res 2002; 43: 587
- Vertrees RA, Das GC, Popov VL, Coscio AM, Goodwin TJ, Legrono R, Zwischenberger JB, Boor PJ. Synergistic interaction of hyperthermia and gemcitabine in lung cancer. Cancer Biol Ther 2005; 4: 1144–1153
- Van Moorsel CJ, Pinedo HM, Smid K, Comijn EM, Voorn DA, Veerman G, Lakerveld B, Van der Vijgh WJ, Giaccone G, Postmus PE, Peters GJ. Schedule-dependent pharmacodynamic effects of gemcitabine and cisplatin in mice bearing Lewis lung murine non-small cell lung tumours. Eur J Cancer 2000; 36: 2420–2429
- Kroep JR, Peters GJ, van Moorsel CJA, Catik A, Vermorken JB, Pinedo HM, van Groeningen CJ. Gemcitabine–cisplatin: A schedule finding study. Annals of Oncology 1999; 10: 1503–1510
- Ostberg JR, Repasky EA. Emerging evidence indicates that physiologically relevant thermal stress regulates dendritic cell function. Cancer Immunol Immunother 2005; 55: 292–298
- Milani V, Noessner E. Effects of thermal stress on tumor antigenicity and recognition by immune effector cells. Cancer Immunol Immunother 2005; 55: 312–319