Abstract
Background. Peritoneal carcinomatosis is a stage of gynecological and gastrointestinal malignancies with poor prognosis. Options for enhancing the effect of standard chemotherapy, such as aggressive surgery and intraperitoneal chemotherapy, have limitations. In this phase I/II study, we evaluated regional hyperthermia of the pelvis and abdomen using the annular-phased-array technique as an adjunct to chemotherapy.
Methods. Forty-five patients with peritoneal carcinomatosis (with or without liver metastases) in colorectal cancer (CRC) (n = 16), ovarian cancer (OC) (n = 17), or gastric/pancreatic/biliary cancer (n = 12) underwent standard chemotherapy and regional hyperthermia. Most CRC patients received second-line chemotherapy. All OC patients were platinum resistant. Regional hyperthermia was applied using a SIGMA-60 applicator (OC), a SIGMA-Eye/MR applicator (CRC), or various ring applicators (gastric/pancreatic/biliary cancer).
Results. Abdominal regional hyperthermia was well tolerated, with acceptable acute discomfort and no long-term morbidity. The SIGMA-Eye/MR applicator achieved higher systemic temperatures (associated with higher systemic stress) and more effective heating of the upper abdomen; the SIGMA-60 applicator achieved higher temperatures (and power densities) in the pelvis. Three-year overall survival was encouraging for patients with CRC (22%) and OC (29%) but not gastric/pancreatic/biliary cancer. For the SIGMA-60 applicator (patients with OC), higher measured temperatures at the vaginal stump correlated with better outcome.
Conclusions. The SIGMA-60 and SIGMA-Eye/MR applicators are feasible for abdominal heating and have low toxicity. The SIGMA-60 applicator is specifically suitable for malignancies with high pelvic burden; the SIGMA-Eye/MR applicator better heats the upper abdomen, including the liver. Further randomized investigations are warranted.
Introduction
Gastrointestinal and gynecological malignancies are often regionally disseminated in the abdomen. Peritoneal carcinomatoses have a poor prognosis, regardless of the tumor entity. The standard treatment in these advanced stages is systemic chemotherapy, the regimen depending on the specific tumor entity. The clinical results of standard treatment are strongly affected by the specific malignancy and pretreatment, but generally are unsatisfactory.
Various strategies for improving the control of peritoneal carcinosis have been evaluated. One of the most effective approaches is aggressive surgery, which can be followed by hyperthermic intraperitoneal chemotherapy (HIPEC) Citation[1]. This expensive treatment can improve long-term follow-up, resulting in a 3-year overall survival of 20–30%. However, limited numbers of patients are suitable for this treatment, and unsatisfactory treatment-related mortality and complications have been reported Citation[2]. Intraperitoneal chemotherapy after colorectal surgery is generally inadequate due to the excessive number of adhesions. Therefore, an intraoperative approach is recommended Citation[3].
A surgical approach is common practice for selected patients with intraperitoneal carcinomatosis of colorectal cancer, but it is still controversial for gynecological malignancies. Recently, intraperitoneal chemotherapy was evaluated as first-line treatment for UICC stage III ovarian cancer Citation[4]. Although the outcome was slightly improved, grade 3-4 toxicity increased.
There is no standard treatment for the various kinds of recurrent cancer, and a method to intensify the regional effect of second- or third-line chemotherapy is greatly needed. Regional hyperthermia (RHT) of the abdomen with annular-phased-array applicators Citation[5], which is sometimes referred to as part-body hyperthermia, can intensify intraperitoneal or systemic chemotherapy in peritoneal carcinomatosis Citation[6], Citation[7]. Preclinical studies have shown that elevated temperatures enhance the effect of certain chemotherapeutic agents, particularly platinum-based cytostatics (cis-platinum, carboplatin, and oxaliplatin), alkylating agents (ifosfamide and treosulfan), and anthracyclines (doxorubicin and pegylated liposomal doxorubicin) Citation[8]. Interactions with temperature have also been found for other drugs (e.g. gemcitabine and topotecan). In addition, a variety of temperature-dependent physiological factors such as perfusion, tumor microenvironment, vessel penetration, and/or uptake of drugs can enhance effects of antitumor agents, including monoclonal antibodies and other innovative substances Citation[9].
Thus, a rationale exists for application of part-body hyperthermia and chemotherapy, and evaluation of hyperthermic chemotherapy appears worthwhile. The current study was designed to pursue this objective and compare the performance of the two most important applicators for abdominal heating and their extracted thermal parameters.
Methods
Patients
The target population was patients with peritoneal carcinomatosis which were treated according to a protocol of a phase I/II study which has been approved by the ethical committee of the Charité. Three patient groups were addressed and recruited between 2001 and 2005: patients with peritoneal metastases of colorectal cancer with or without liver metastases (n = 16, 12 allocated to second-line chemotherapy), patients with abdominally disseminated ovarian cancer (n = 17; all were platinum resistant), and patients with gastric, pancreatic, or biliary cancer (n = 12; allocated to first- or second-line treatments). Patients with platinum resistance either had recurred during six months after platinum-based first-line chemotherapy or had a progression among a platinum-based scheme.
Patients with liver metastases (see below) and/or with ascites were accepted for the study, but patients with extra-abdominal metastases (e.g. lung metastases) were excluded.
Treatment
All patients were treated by various chemotherapy regimens that were prescribed by the medical oncologist and considered to be standard chemotherapy (). The regimen was determined based on the tumor entity, and in particular on the nature of the pretreatment and patient factors (intolerances, morbidity, etc.). In the case of colorectal or ovarian cancer, more than one course was applied in conjunction with hyperthermia with varying schemes in more than half of the patients if a progression occurred again.
Table I. Characteristics of 45 patients with peritoneal carcinomatoses treated with systemic chemotherapy and abdominal regional hyperthermia (total number of 393 heat treatments). A significant portion of the patients were treated in more than one course with different chemotherapy schemes, if a progression developed again.
RHT was added to standard chemotherapy. The RHT schedule was adapted to the chemotherapy, i.e. RHT was performed weekly, every 3 weeks, or as otherwise prescribed by the regimen. A SIGMA-60 (S-60) applicator with four antenna pairs, a SIGMA-Eye/MR (S-Eye/MR) applicator with 12 antenna pairs, or the S-Eye applicator without MR-compatibility were used to apply RHT. All 17 patients with ovarian cancer were treated in the S-60 applicator; all 16 patients with colorectal cancer were treated in the S-Eye applicators, preferably under MR control. The rationale for this allocation has been derived from planning studies with HyperPlan Citation[22] predicting better pelvic heating in the S-60 applicator and better heating of the upper abdomen in the S-Eye applicator (see Discussion for further details).
Patients with gastric, pancreatic and biliary cancer were treated in different applicators such as the S-60, S-40, S-Eye/MR, or S-Eye. The S-Eye/MR applicator is constructed to be compatible with the MR-tomograph (Siemens Symphony, 1.5 Tesla); therefore, the antennas have a special design (see Citation[10] for further details).
Before starting heat treatments in the S-Eye/MR applicator we appointed a test, where we positioned the patient in the applicator and then acquired a MR-dataset for planning purposes. Patients not fitting into the applicator or suffering from claustrophobic attacks were excluded from the study. The minimum water distance between body surface and antennae was required to be 4 cm.
RHT was performed according to a standardized protocol. The mid-plane of the applicator was positioned on the patient's navel for abdominal heating. The heating-up period should require 30 minutes power-on at maximum to achieve a steady-state with 41-42°C in a reference point (see below) under ideal conditions. Then a therapeutic time of 60 min is aspired. If the therapeutic time is by any reason below 30 min we call it disruption.
Simulation studies have shown that this technique induces a temperature increase ΔT of 3-4°C the abdominal cavity, i.e. a mean temperature of 40.5-41.5°C, but can also heat the liver. The main contribution to the temperature elevation of the liver comes from the blood preheated in the abdomen and entering the portal vein. The mixture of portal-venous blood at 40.5-41.5°C, ΔT = 3.0–4.0°C (2/3) and arterial blood at 38-39°C, ΔT = 0.5–1.5°C (1/3) results in a mean blood temperature in the liver to a mean of 40-40.5°C:
Therefore at least weakly perfused liver metastases can be effectively heated if in addition sufficient SAR (specific absorption rate in W/kg) is deposited in the upper abdomen.
Experimental data acquisition and evaluation
We acquired power and temperature data for annular-phased-array applicators of the S-60 and S-Eye family. Temperaturea˜ time curves were registered for each patient at a minimum of one reference point, either in the rectum near the recto-sigmoidal transition (12-15 cm ab ano) or at the end of the vagina (orifice or stump after hysterectomy), as well as sublingually (orally) to estimate the systemic temperature.
The following thermal parameters were derived Citation[11].
Tbasal [°C]: The temperature in the rectum or vagina before power-on, representative of the systemic temperature.
ΔTsyst = Toral (end) – Toral (start): The increase in sublingual temperature corresponding to an increase in systemic temperature.
Tsyst (steady-state) = Tbasal + ΔTsyst: Elevated systemic temperature (at the end of the RHT session).
ΔTref (steady state) = Tref (end) – Tbasal – ΔTsyst: The increase in temperature at the reference point caused by local power density (see SAR), that is, corrected for the increase in systemic temperature.
SAR (W/kg) (specific absorption rate) = −66.7 dT/dt (at power-off) (°C/min): The power density at the reference point derived from the tangent of the temperature-time curve at power-off (at the end of heat treatment).
Perfusion w [mL/100 g/min] = 1.4 (SAR/ΔTref): In RHT, the ratio of SAR and the corrected temperature increase corresponds to the perfusion, because the heated volumes are large and the thermal gradients are small.
ΔTbolus = Tbolus (power-on) – Tbolus (power-off): The increase in the bolus temperature in the S-Eye/MR applicator.
In addition, side effects and intolerances, including every disruption of heat treatment (and its reason) were documented.
Mann-Whitney U tests were used for comparisons between groups with quantitative and ordinal variables. Analyses of survival were carried out by the Kaplan-Meier method with a median follow-up time of 44 months (18 to 161 months). Comparisons between patient groups were calculated by log-rank tests. All analyses were performed by the statistical package SPSS 10 (SPSS Inc., Chicago, IL).
Results
Forty-five patients with a total number of 67 courses entered the study. presents the patient characteristics according to the tumor type. The mean ages are slightly below the expected ages of the respective cancer population, which are in the range of 67–70 years (colorectal, gastric, pancreatic cancer) or 65 years (ovarian cancer). This selection bias is in accordance with other studies Citation[2], Citation[4], because younger people are more vigorously looking for alternative treatment options.
Data regarding the feasibility and tolerance of abdominal heating with the annular-phased-array technique are summarized in and . Three hundred ninety-three heating sessions were performed. In 357 sessions the S-60 or S-Eye/MR applicator were used. Disruption (<30 minutes therapeutic time) was necessary in 30 of these 357 sessions (8.4%); these disruptions occurred by request from the patient or (rarely) for technical reasons. The overwhelming majority of the heating sessions took 30 to 60 minutes to complete; generally they lasted for approximately 60 minutes (time at steady state temperature plateau) (). Disruptions occurred more frequently in the S-Eye applicator (10.6% versus 5.9%) indicating the more aggressive approach using the S-Eye applicator (see Discussion). The reasons for disruptions are also listed – systemic and local side effects roughly equally common.
Table II. Therapeutic durations and disruptions in 359 evaluated abdominal heating sessions considering the most important applicators SIGMA-60 and SIGMA-Eye/MR. Note that more than one reason is possible in case of a disruption. The SIGMA-Eye (without MR) applicator (25 sessions) and the SIGMA-40 applicator (9 sessions) are not included in this analysis.
Interestingly, local problems dominate in the S-Eye applicator. This is probably due to the higher power level (see below) and the proximity to the dipole antennae (compared with the S-60 applicator). In particular, applicator edge phenomena are more often observed in the S-Eye applicator.
Table III. Occurrence* of acute side effects and intolerances in 393 abdominal regional hyperthermia sessions in 45 patients according to applicator.
For the S-60 applicator, side effects were reported only in approximately 10% of the sessions. In contrast, some intolerance or side effects were reported by patients in most heating sessions with the S-Eye applicator, but without early termination (). Usually the physician in charge was able to address the problem by reducing the total power, changing the phases of the channels, repositioning the patient, cooling the bolus water, using water bags, or administering medication – especially in case of edge effects.
We found no evidence for heat-specific subacute or chronic toxicities such as thermal burns or tissue damage. Chemotherapy toxicities were in the expected range and not readily identified as increased by the heat treatments.
provides the temperatures and derived thermal parameters for the evaluated applicators. For the S-60 applicator, lower total power (approximately 600 W versus 1250 W for the S-Eye) achieved a higher SAR at the reference point (approximately 15 W/kg versus 8 W/kg). The systemic temperature was higher for the S-Eye applicator (approximately 39°C versus 38.3°C), but the achieved temperature at a reference point (vagina or rectum) was more than 1°C lower in the S-Eye/MR applicator (39.3°C versus 40.6°C).
Table IV. Thermal parameters in 327/393 heating sessions according to applicator. Nine RHT sessions in the SIGMA-40 applicator were excluded. For 57 RHT sessions thermal data were not evaluable due to hardware errors. In the case of patients with ovarian cancer the reference point was selected at the vaginal stump. Otherwise, the tip of the catheter in the rectum (>12 cm) typically was used as reference point.
Vital parameters are shown in . Typically, the pulse rate increases during heating, in parallel to the increased perfusion of the exposed regions. Pulse rate increases were somewhat higher when using the S-Eye applicator. This is in accordance with the higher systemic burden ΔTsyst or Tsyst with this applicator. Slight applicator-dependent changes in systolic and diastolic blood pressures were also observed during hyperthermia. We also examined changes in blood pressure after stratifying the patients into a larger group of patients who were normotensive before treatment (n = 32 patients, mean 120/70 mmHg) and a small group of patients who were hypertensive before treatment (n = 12, mean 150/90 mmHg). For the normotensive patients, there was a trend for blood pressures to slightly increase during heating sessions, whereas for hypertensive patients, blood pressures slightly decreased during the heating sessions.
Table V. Vital parameters registered in 327/393 heating sessions according to applicator. Nine RHT sessions in the SIGMA-40 applicator were excluded. For 57 RHT sessions data sets with vital parameters were not evaluable due to hardware errors.
We evaluated the response for every single course (). Reduction of symptoms (palliation) was designated as clinical response, e.g. improvement of general condition (11 cases), decrease of tumor size (3 cases), improved bowel functions (4 cases) or ingestion (2 cases), decrease of abdominal pressure and circumference (4 cases), reduction of pain and medication (8 cases) and resuming of vocation (1 case). In addition, tumor marker decreases (20 cases) and a decrease of tracer activity in a PET scan (1 case) were classified as biochemical responses. Both categories of responses summed up to an overall response rate of 68.7% for all chemotherapy courses. Women with ovarian cancer sometimes insisted on a further chemotherapy course, if the tumor marker CA-125 increased, in the absence of clinical symptoms. Under these circumstances a clinical response could not occur.
Table VI. Overall response rates differentiated in clinical and biochemical response rates according to tumor entity. See for further details.
Table VII. Comparison of literature on second-line single-agent chemotherapy in recurrent platinum-resistant ovarian cancer.
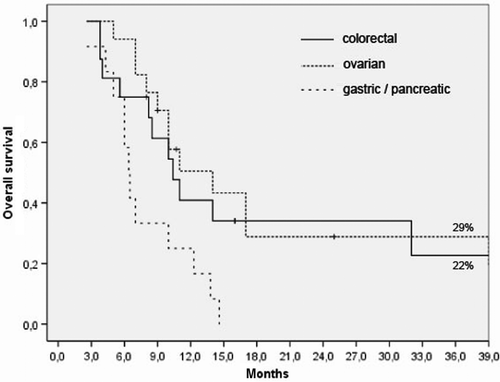
shows overall survival according to the tumor entity. For colorectal cancer that spread to the peritoneum and liver (n = 16 patients), the 3-year survival was 22% (solid line). Note that the majority of these patients underwent second-line chemotherapy (12/16). Because all 17 patients with ovarian cancer had a platinum-resistant recurrence of peritoneal carcinomatosis, second-line chemotherapy (and often even third-line chemotherapy or more) was administered in conjunction with RHT. A 3-year overall survival of 29% (dotted line, ) was achieved. In the 12 patients with metastatic gastric and pancreatic/biliary cancer, the 1-year overall survival was 25%, and the median survival time was 7 months.
Figure 1. Kaplan-Meier survival curves for the three tumor types evaluated for abdominal dissemination: colorectal cancer, ovarian cancer, and gastric/pancreatic/biliary cancer.
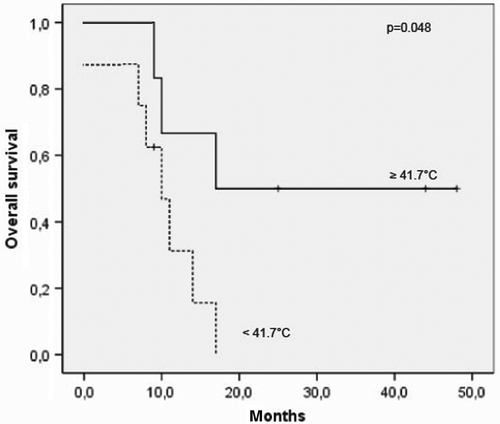
Quality control is a key issue regarding hyperthermia. In order to limit risk and burden to the patients in a palliative situation, invasive thermometry in the peritoneal cavity was not used. Instead, thermal data were acquired, as reported in . We grouped the clinical outcome according to thermal parameters. For patients with ovarian cancer, all of whom were treated in the S-60 applicator, a relationship was found between survival and temperatures measured in the vaginal stump (), a measurement that has a relationship to the mean temperature in the peritoneal cavity. Patients with mean steady-state temperatures ≥ 41.7°C (37.5°C + 4.2°C) at the vaginal reference point had a significantly greater long-term survival of > 50% after 3 years in comparison to patients with lower temperatures. For patients with colorectal cancer, who were treated in the S-Eye applicator, no correlation was found between survival and local temperature in the rectum or vagina or systemic temperature. Note that the local temperature was approximately 1°C lower and the systemic temperature was more than 0.5°C higher than typically measured in the S-60 applicator ().
Figure 2. Relationship between temperature at the reference point and survival in ovarian cancer patients treated with the SIGMA-60 applicator. Survival depends on the steady-state temperatures achieved at the reference point (vaginal stump). In patients with ovarian cancer, the survival was improved for steady state temperatures ≥ 41.7°C.
Discussion
RHT has been used to enhance the effect of systemic chemotherapy in the treatment of tumor diseases with peritoneal carcinomatoses. Compared to other methods for enhancing the effects of systemic chemotherapy, such as cytoreductive surgery or intraperitoneal chemotherapy, abdominal heating is particularly well tolerated, and it is not associated with relevant acute or long-term morbidity. In the discussion to follow, we interpret the specific results of this phase I/II study in terms of the outcome for different tumor entities and the suitability of the RHT applicators.
Peritoneal carcinomatosis of colorectal cancer
Peritoneal carcinomatosis occurs in only 8% of patients with recurrent colorectal cancer Citation[3]. In approximately 20% of these patients, liver metastases are coexistent Citation[12]. The survival time of patients with peritoneal carcinomatosis is shorter than for patients with liver metastases alone. The 5-year survival rate with standard chemotherapy at 0% is very unsatisfactory Citation[12], Citation[13], and the median survival time poor (5–6 months).
A few surgeons have performed aggressive cytoreductive surgery followed by HIPEC in selected patients with peritoneal carcinomatosis (absent other manifestations) Citation[1], Citation[2], Citation[14], Citation[15]-Citation[16]. A 3-year survival rate of 20–30% was achieved with a first-line approach, but there was substantial perioperative morbidity (WHO 3: 65%, WHO 4: 45%) Citation[2] and mortality (approximately 10%). In the one randomized trial comparing cytoreductive surgery plus HIPEC followed by chemotherapy with chemotherapy alone Citation[2], no patient receiving only standard chemotherapy survived after 3 years. This is in accordance with other reports Citation[17].
Thus, the data in our study concerning the long-term follow-up with chemotherapy plus abdominal RHT deserve closer attention. While the median survival time of approximately 12 months is similar to that for the control group in the study by Verwaal Citation[2], we identified a subgroup of patients that benefited in the form of a long remission and, as a consequence, long-term survival. Our survival data for patients with peritoneal carcinomatoses of colorectal origin are remarkable in that most patients (75%) had already received a course of chemotherapy and were administered a second-line chemotherapy in conjunction with RHT. In contrast, Verwaal Citation[2] and other investigators used surgery and HIPEC as first-line treatments. Moreover, the toxicities of hyperthermic chemotherapy ( and ) were low and very acceptable compared to the morbidity and mortality with multimodal surgical approaches. Nevertheless, the long-term survival in our study is comparable to a 20–30% 3- to 5-year survival rate.
Peritoneal carcinomatosis of ovarian cancer
The ovarian cancer patients in our study were platinum-resistant (see Methods). Such patients usually have a poor prognosis. One of the earliest trials by Trimble et al. of patients with refractory ovarian cancer reported a median survival duration of only 9 months and a 1-year survival rate of 37% Citation[18].
There is no standard treatment for platinum-resistant relapse in ovarian cancer. However, several single agents show some activity; these include paclitaxel, topotecan, pegylated liposomal doxorubicin (e.g. Caelyx), oxaliplatin, gemcitabine, and etoposide. Combination chemotherapy is not superior and might be more toxic. A survey by Gonzalez-Martin et al. reported median survival durations of 9–14 months with paclitaxel-based regimens Citation[19]. Note, that in our patients paclitaxel was used as part of the first-line chemotherapy; therefore, other agents were typically administered (). A variety of studies were performed comparing single agents for platinum-resistant recurrent ovarian cancer. Generally, the results are poor, with median survival times of 9–14 months Citation[18], Citation[20], Citation[21], and only in one study Citation[21] a 3-year overall survival of 10–15%. A summary of our findings with respect to earlier studies is provided in Table VIII. In the context of this other work, the outcome in our study is quite favorable.
Peritoneal carcinomatosis of gastric and pancreatic cancer
While the survival data for peritoneal carcinomatoses in colorectal and ovarian cancer encourage further evaluation of abdominal hyperthermia, the survival data for gastric and pancreatic cancer () are not better than expected for chemotherapy alone. In particular, there were no long-term survivors, and after 14 months all patients were deceased. Therefore, we could not find a group of patients which might have some benefit from the intensified treatment with RHT.
Applicators for abdominal heating
Abdominal heating with the S-60 and S-Eye annular-phased-array applicators proved well tolerable and feasible. This approach might enhance the effectiveness of standard chemotherapy. There were no serious complications of RHT. Some systemic and/or local burden to the patient occurred in approximately half of the heating sessions (). This seems acceptable for oncological treatment, and the disruption rate was very low (2.5%, ).
Both applicators (S-60 and S-Eye) were determined suitable for abdominal heating. However, there were slight differences in performance that might favor a particular applicator for specific indications. It appears that systemic stress (systemic temperatures, disruption rate, and pulse rate) is greater in the S-Eye applicator (Tables ). In particular, the total power was higher for the S-Eye applicator, but this is partly due to a lower efficiency and a higher volume of the patient's body being exposed to the antennae. The efficiency of the S-60 was near 80%; that is, for a total amplifier power of 600 W, 480 W is deposited in the patient. The S-Eye/MR applicator with its 24 short antennae specifically designed for MR-compatibility has a lower efficiency of approximately 50%; for an amplifier power of more than 1,200 W, the deposited power was more than 600 W. Therefore, the accumulated power in the patient is higher for the S-Eye applicator – but this is not necessarily the case for the local SAR, because the exposed volumes and the SAR distributions are different. However, due to the shortness and proximity of the antennae more local discomfort is induced in the S-Eye/MR applicator (see ).
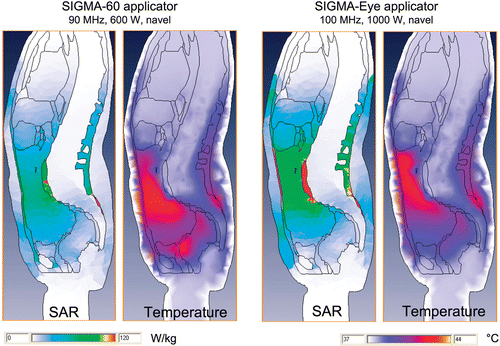
We performed simulation studies with the planning system HyperPlan Citation[22] for both applicators. The results were in accordance with our clinical experience. The modeling calculations showed that when centered at the patient's navel, the applicators produced different SAR and temperature distributions (). In fact, higher temperatures were achieved in the pelvic region with the S-60 applicator (, left), while SAR and temperatures in the upper abdomen were more homogeneous with the S-Eye applicator. These differences are attributable to different directions of electric fields relative to electrical boundaries in the pelvis for the S-applicators. The S-60 applicator has outside the mid-plane a higher percentage of E-field components perpendicular to the patient axis, which deposit more power at the electrical boundaries in the pelvis due to the complex pelvic anatomy.
Figure 3. SAR (specific absorption rate) and temperature distributions for the SIGMA-60 and SIGMA-Eye/MR applicator. Both applicators were positioned at the patient's navel. Abdominal perfusions were assigned to 20 mL/100 g/min. Left (S-60): Using the SIGMA-60 applicator, the SARs and temperatures were increased in the pelvic region. Right (S-Eye/MR): Using the SIGMA-Eye/MR applicator, the SARs and temperatures were higher in the upper abdomen (increased systemic temperature is not shown and further improves performance).
Direct measurements of temperature distributions in the abdomen, specifically in the upper abdomen, are difficult. Non-invasive thermometry is presently not available in the abdomen. Therefore, we have to rely on planning studies, which are extensively verified by phantom studies Citation[23], Citation[24] and by the method of matched planning. With this approach an agreement between SAR and temperature measurements in reference points (rectum, vagina) and calculations (after parameter adaption) is achieved. These planning studies predict a mean temperature in the abdomen of T50 ≈ 41°C, and T90 ≈ 39°C, Tmax ≈ 43°C, for typical adjustments used in clinical practice (unpublished results). In conclusion, a mean temperature of 40.5–41.5°C is assumed in the portal-venous blood (see Methods). Further improvements are possible by parameter optimization.
In summary, the S-Eye applicator radiated more power (in Watts) into the abdomen, particularly the upper abdomen, than the S-60 applicator. It is therefore more adequate for homogeneous heating of the entire abdomen, including the liver. This is consistent with the higher systemic temperature and pulse rate as well as the slightly higher perfusion level with this applicator (). Thus, the S-Eye applicator is appropriate for colorectal cancer, wherein the upper abdomen is at greater risk. In contrast, the S-60 applicator deposits higher SAR in the pelvis with the standard set-up, the temperature being approximately 1°C higher near the rectosigmoidal junction compared with the S-Eye applicator. Thus, we used the S-60 applicator for peritoneal carcinomatosis in ovarian cancer, where a higher tumor burden in the pelvis is expected.
The pelvic temperatures measured in ovarian cancer patients with the S-60 applicator correlated with outcome. This is shown in , where the survival curves split with respect to the vaginal temperatures at a separating value of 41.7°C. No relationship of outcome to measured temperatures was found for the S-Eye applicator in colorectal cancer; this is because the lower temperatures at the pelvic reference points are not representative of the effectiveness of heating for this applicator.
In conclusion, both applicators are suitable for abdominal heating, but they have slightly different optimal indications. These data provide strong support for combining chemotherapy with abdominal RHT. Further evaluation in a randomized study is desirable.
Acknowledgements
This work has been supported by grants of the Helmholtz Gemeinschaft (VH-VI-140, Project ‘Virtual Institute for Clinical Hyperthermia and Related Technology’) and the Berliner Sparkassenstiftung Medizin (Project ‘Oxygenation in Oncology’). We are grateful for their support.
The technical support of our co-workers Dr Jacek Nadobny and Horst Fähling is gratefully acknowledged.
References
- Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg 1995; 221: 124–132
- Verwaal VJ, van Ruth S, de Bree E, van Slooten GW, van Tinteren H, Boot H, Zoetmulder FAN. Randomized trial of cytoreduction and hyperthermic introperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003; 21: 3737–3743
- De Bree E, Witkamp AJ, Zoetmulder FAN. Intraperitoneal chemotherapy for colorectal cancer. J Surg Oncol 2002; 79: 46–61
- Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. New Engl J Med 2006; 354: 34–43
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002; 3: 487–497
- Jones E, Secord AA, Prosnitz LR, Samulski TV, Oleson JR, Berchuck A, Clarke-Pearson D, Soper J, Dewhirst MW, Vujaskovic Z. Intra-peritoneal cisplatin and whole abdomen hyperthermia for relapsed ovarian carcinoma. Int J Hyperthermia 2006; 22: 161–172
- Hildebrandt B, Rau B, Gellermann J, Wust P, Riess H. Hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinosis. J Clin Oncol 2004; 22(8)1527–1529
- Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 2002; 43: 33–56
- Dewhirst MW, Vujaskovic Z, JNones E, Thrall D. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia 2005; 21: 779–790
- Gellermann J, Wlodarczyk W, Ganter H, et al. A practical approach to perform the thermography in a hyperthermia/MR hybrid system - validation in an anthropomorphous phantom. Int J Radiat Oncol Biol Phys 2005; 61: 267–277
- Wust P, Cho CH, Hildebrandt B, Gellermann J. Thermal monitoring: Invasive, minimal-invasive and non-invasive approaches. Int J Hyperthermia 2006; 22: 255–262
- Sadeghi B, Arvieux C, Glehen O, Beaujard A, Rivoire M, Beaulieux J, et al. Peritoneal carcinomatosis from non-gynecological malignancies. Results of the EVOCAPE 1 multi-centric prospective study. Cancer 2000; 88: 58–63
- Chu DZJ, Lang NP, Thomson C, Osteen PK, Westbrook KC. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer 1989; 63: 374–377
- Fujimura T, Yonemura Y, Fujita H, Michiwa Y, Kawamura T, Nojima N, et al. Chemohyperthermic peritoneal perfusion for peritoneal dissemination in various intraabdominal malignancies. Int Surg 1999; 84: 60–66
- Witkamp AJ, de Bree E, Kaag MM, Boot H, Beijnen JH, van Slooten GW, et al. Extensive cytoreductive surgery followed by intraoperative hyperthermic intraperitoneal chemotherapy with mitomycin C for colorectal cancer with peritoneal metastases. Eur J Cancer 2001; 37: 979–984
- Elias D, Blot F, El Otmani A, Antoun S, Lasser P, Boige V, et al. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer 2001; 92: 71–76
- Gertsch P. A historical perspective on colorectal liver metases and peritoneal carcinomatosis: Similar results, different treatments. Surg Oncol Clin N Am 2003; 12: 531–541
- Trimble EL, Adams JD, Vena D, Hawkins MJ, Friedman MA, Fisherman JS, et al. Paclitaxel for platinum refractory ovarian cancer: Results from the first 1,000 patients registered to National Cancer Institute Referral center 9103. J Clin Oncol 1993; 11: 2405–2410
- González-Martin A. Treatment of recurrent disease: Randomized trials of monotherapy versus combination chemotherapy. Int J Gynecol Cancer 2005; 15(Suppl. 3)241–246
- Piccart MJ, Green JA, Lacave AJ, Reed N, Vergote I, Benedetti-Panici P, Bonetti A, et al. Oxaliplatin or paclitaxel in patients with platinum-pretreated advanced ovarian cancer: A ranzomized phase II study of the European Organization for Res. and Treatment of Cancer Gynecology Group. J Clin Oncol 2000; 18: 1193–1202
- Gordon AN, Tonda M, Sun S, Rachoff W. 2004; 95: 1–8, on behalf of the Doxil Study 30–49 investigators. Gyn Oncol
- Sreenivasa G, Gellermann J, Rau R, et al. Clinical application of the hyperthermia treatment planning system HyperPlan - comparison of algorithms and clinical observables. Int J Radiat Oncol Biol Phys 2003; 55: 407–419
- Gellermann J, Weihrauch M, Cho CH, et al. Comparison of MR-thermography and planning calculations in phantoms. Med Phys 2006; 33: 3912–3920
- Weihrauch M, Wust P, Weiser M, et al. Adaptation of antenna profiles for control of MR guided hyperthermia (HAT) in a hybrid system. Med Phys 2007; 34: 4717–4725