Abstract
Certain iron-based particle formulations have useful magnetic properties that, when combined with low toxicity and desirable pharmacokinetics, encourage their development for therapeutic applications. This mini-review begins with background information on magnetic particle use as MRI contrast agents and the influence of material size on pharmacokinetics and tissue penetration. Therapeutic investigations, including (1) the loading of bioactive materials, (2) the use of stationary, high-gradient (HG) magnetic fields to concentrate magnetic particles in tissues or to separate material bound to the particles from the body, and (3) the application of high power alternating magnetic fields (AMF) to generate heat in magnetic particles for hyperthermic therapeutic applications are then surveyed. Attention is directed mainly to cancer treatment, as selective distribution to tumors is well-suited to particulate approaches and has been a focus of most development efforts. While magnetic particles have been explored for several decades, their use in therapeutic products remains minimal; a discussion of future directions and potential ways to better leverage magnetic properties and to integrate their use into therapeutic regimens is discussed.
Introduction
The interaction between magnetic particles and externally applied magnetic fields encourages particle use in the detection, diagnosis, and treatment of various diseases. The following is a non-comprehensive survey of the scientific literature that from the author's viewpoint delineates key particle structural considerations and biological parameters that impact the utility of magnetic particles in therapeutic regimens.
Whether a material is magnetic or not, size plays a critical part in determining in vivo performance. Thus the effects of size reported for a variety of polymers and nanoparticles are introduced before delving specifically into magnetic particles. Particles are typically used with the intent to deliver a particular material to a cellular target in a particular tissue, but undesirably rapid kidney clearance and reticular endothelial system (RES) uptake mechanisms must be circumvented. Glomerular filtration will clear materials with a molecular weight of less than about 40 kDa, or about 10 nm Citation[1]. For particles larger than this the main mechanism of particle removal from the bloodstream is via the RES. Unless RES uptake is specifically desired, any systemically administered particle must be designed to avoid rapid opsonization (blood protein binding) and subsequent macrophage phagocytosis. Coating particles with polyethylene glycol (PEG) is frequently employed for retarding opsonization, but eventually even these ‘stealth’ particles are taken up by macrophages in the liver, spleen, marrow and lymph nodes, for example as demonstrated for stealth liposomes Citation[2].
Particles that remain at a high enough concentration in the blood stream for a sufficient length of time can drive a slow accumulation through the leaky vasculature found in a variety of lesions. Vascular leakiness is common to tumors and inflamed tissues characteristic of infections, arthritis, and artherosclerotic plaques Citation[3], Citation[4]. This ‘passive accumulation’ is perhaps the most cited attribute of macromolecular medicines and particulate drug delivery. In the case of tumors, in contrast to healthy tissues with tight junctions between endothelial cells, as tumors grow they develop a patchwork of defective endothelial cells which allow macromolecules and nanoparticles to extravasate Citation[5]. Leakiness develops with angiogenesis that occurs surprisingly early in tumor growth, with Dewhirst and colleagues observing angiogenic processes in masses as small as a few hundred cancer cells Citation[6].
In addition to dysfunctional blood vessels, tumors also have corrupted lymph vessels. Maeda documented what he termed the enhanced permeability and retention (EPR) effect, observing that macromolecules and other relatively large materials that leaked out of tumor vasculature remained for extended time in tumors compared to healthy tissue in part due to faulty lymph drainage Citation[7], Citation[8]. While many efforts have centered on leveraging the EPR effect, the advantage of large materials compared to small molecules in concentrating in the tumor's perivascular space becomes a disadvantage after extravasation. Due to the faulty lymph drainage, tumors have high interstitial pressures that retard permeation, and to reach cancer cells deep in tumor structures, material must permeate through a tumor's interstitial space. Additionally, tumors have heterogeneous, temporally dynamic structures with low porosity and dense extracellular matrices that provide substantial physical resistance to material permeation Citation[9]. Tumor extravasation and subsequent permeation has been assayed in depth for a wide range of materials. Using carbohydrate materials, Dreher et al. found that dextran of 2 MDa with a corresponding hydrodynamic diameter of about 50 nm had an apparent tumor permeability of almost 100-fold less than 3.3 kDa dextran Citation[10]. Dreher further observed that dextran polymers with molecular weights of 40 to 70 kDa, corresponding to a hydrodynamic size of 11 to 14 nm had maximum tumor concentration but remained close to the vessels from which they extravasated, while dextrans of molecular weight lower than 10 kDa penetrated deeply into tumors. In an earlier study examining tumor perfusion, Yuan et al. did not observe as large or straightforward an effect of tumor extravasation with material size as did Dreher. However, a variety of structures with different charges were surveyed by Yuan, a reminder that size is not the only important parameter influencing tumor penetration by macromolecules and particles Citation[11]. Generally, hydrophilic, neutrally charged or better yet uncharged materials will stick less to cell surfaces and interstitial biomolecules. Yuan et al. also modeled the extracellular volume available to materials using multiple pore sizes and connectedness in the tumor extracellular space to explain the loss in permeability with increasing molecular weight Citation[12].
Considering drug-conjugate polymer/particle structures, Lee et al. reported that small multivalent doxorubicin-PEG biodegradable ‘bowtie’ dendrimers with molecular weight in the 45 kDa range optimally treated tumors of a certain size. The authors speculated that the effectively treated tumors were large enough so that vascularization and concomitant vascular leakiness had developed, but not so large that the tumor could not be treated by the administered doxorubicin dose Citation[13]. This result emphasized that the utility for particulate systems is in treating tumors that are vascularized. Importantly, the hydrolytically labile structure also allowed for a ‘cluster bomb’ effect utilized in various macromolecular pro-drug constructs, where particles are first localized to the tumor via the EPR effect, and then subsequently degrade to smaller sizes, for instance through enzymatic or pH driven hydrolytic mechanisms, to small entities that are better able to penetrate the tumor mass and access cancer cells in hypoxic regions Citation[14]. Similarly for drug loaded liposomes, the liposome need not necessarily penetrate a tumor intact. Allen and coworkers found that relatively small liposomes of 80, 100 or 150 nm average diameter accumulated to a substantially greater level than 240 nm sized liposomes Citation[15]. While liposomes have been extensively studied and developed Citation[16], with doxorubicin loaded, PEGylated formulations one of the few systemically administered nanoparticles that have been commercialized, they are larger than desired for tumor penetration. However, as with pro-drug concepts, if the liposome payload can be released in the tumor vasculature or within the tumor space, the higher penetrability of small molecules compared to nanoparticles may improve therapeutic performance.
Magnetic iron oxide particles and their use as MRI contrast agents
For in vivo use, iron-based magnetic particles have an attractive combination of high magnetization, well-described metabolism and relatively low toxicity. The incorporation of iron oxide materials has been preferred over zero valent metallic iron in part because IO has better shelf stability and produces less oxidative stress induced toxicity in vivo. Also, IO particles under about 30 nm are superparamagnetic, with no magnetization in the absence of an externally applied magnetic field that can lead to aggregation. IO particles have been fabricated in sizes ranging from about 10 nm to 10 s of microns, with magnetic material, coating, and particle size all playing critical roles in determining particle magnetic properties and pharmacokinetics Citation[17]. For in vivo use IO particles are best known as contrast enhancing agents for magnetic resonance imaging (MRI). MRI with iron oxide contrast agents combines magnetic fields with iron particles and provides important background information for the medicinal use of magnetic particles. There are many articles and books that cover the theory and practice of MRI Citation[18] and contrast agent use in MRI Citation[19]. Briefly, under the influence of strong magnetic fields, water protons in different tissues produce different magnetic signals, and this difference in signals makes a given tissue lighter or darker than surrounding tissue in an MR image. When IO particles are located in a given tissue they alter the magnetic field experienced by water protons and thus substantially change the signal coming from that tissue, providing better contrast. As with all nanoparticles, the tissue distribution of IO contrast particles is heavily influenced by size. Small superparamagnetic iron oxide (SPIO) agents that are about 50 to150 nm are typically rapidly taken up by Kupffer cells (RES in the liver), when administered parenterally. SPIOs are thus used to enhance contrast for liver pathologies. Ultra-small superparamagnetic iron oxide (USPIO) agents are 10 to 40 nm in size and can be formulated with hydrophilic coatings that slow opsonization and monocyte and macrophage uptake Citation[20]. The extended residence time in the bloodstream of certain USPIO formulations makes them well-suited for vasculature imaging and also for imaging lesions where the particles can extravasate through leaky vasculature Citation[19]. In the latter capacity, USPIOs can provide valuable information on cancer stage and potential for metastasis; increased neovascularization and vascular leakiness with tumor malignancy Citation[21], Citation[22]. Feruglose, one of the smallest iron oxide-based MRI contrast agents with a size of 10–20 nm and a blood half-life of 3 to 4 hours was useful in characterizing tumor permeability by Daldrup-Link et al. Citation[23]. The particles were found to extravasate across the leaky vasculature of malignant tumors, but not across the intact endothelium of benign lesions. Similarly, in a rodent breast tumor model, feruglose enhanced MRI and provided accurate quantitative measures of tumor microvascular permeability that correlated with tumor grade Citation[24]. Vascular leakiness has been quantified using kinetic analysis of dynamic MRI data Citation[21], Citation[25], Citation[26].
In summary, the use of IO particles as MRI contrast agents has provided a good deal of information on the distribution and pharmacokinetics of sub-100 nm sized iron oxide particles coated with various materials. To explore the potential to identify biomolecular signatures using MRI, USPIOs have been functionalized with targeting agents and have demonstrated binding to biomolecular targets in vitro Citation[27]. While targeting of USPIOs have been reported for tumor phenotyping in in vivo applications Citation[28], Citation[29], publications are relatively scarce. This is due in large part because with or without the display of targeting agents, particles passively accumulate and reside for extended times in diseased tissues as discussed above. The effects of a biomolecularly-specific targeting agent that are distinguishable from this passive, non-biomolecular specific tissue distribution are difficult to measure.
Magnetic particles used for therapeutic purposes
Therapeutic mechanisms of iron oxide-based nanoparticles can originate from the iron material in the particle, bioactive agents loaded into the particle coating or adsorbed on to the iron oxide surface, and from heat generated by the particles under the influence of alternating magnetic fields (AMFs). Also, the magnetic core may allow the particle to be concentrated in desired locations in the body by the application of a high strength magnetic field to those locations. Generally, relatively small particles less than about 100 nm are best for tissue penetration and AMF-driven heat generation applications, while larger particles can be more easily retained in static magnetic fields and are appropriate for magnetic capture. A variety of materials and methods have been used to coat ferrite materials to retard opsonization, improve biocompatibility, and enable or improve drug loading. For large micron-sized particles, cross-linked albumin Citation[30] and PLGA Citation[31] have been used, while for small iron particles, dextran or modified dextran coatings have been most often employed. PEG Citation[32], pluronics Citation[33], silanes Citation[34] and lipids Citation[35] are among other coating materials that have been used in attempts to provide formulations with good water dispersibility and stability in blood. PEG is particularly desirable due to its extensive track record for reducing opsonization and RES uptake for a broad spectrum of proteins and nanoparticles Citation[36], Citation[37]. A recent review of magnetic particle coatings is provided by Gupta Citation[38].
Incorporation of therapeutic material into ferrite particles
Various bioactive agents may be incorporated into ferrite particles through physical or chemical means. In the case of treating iron deficiency anemia, the bioactive agent is provided by the iron in the iron oxide core. Pathologies that may be addressed by iron oxide nanoparticle products include radiation and chemotherapy induced anemia, hematological malignancies, congestive heart failure, post partum anemia, and inflammatory bowel disease. For treating iron deficiency anemia, intravenous (IV) injections have reduced side effects than orally administered iron supplements. Magnetic particles have an extensive record of use in vivo, and while the rate of metabolism of a particular type of particle depends greatly on the size and coating composition, in general it proceeds along a well described path where uptake into the RES leads eventually, for the iron in the core that is, to incorporation into the body's iron stores, mostly into hemoglobin in erythrocytes and ferritin in other cells Citation[39]. Interestingly, animal ferritin is a supramolecular nanoparticle with an iron oxide mineral core that is similar in size to the smaller synthetic magnetic nanoparticles discussed below, but with low magnetization Citation[40]. Ferritin has a ferrihydrite-like iron crystal core about 8 nm in diameter encapsulated by proteins, with the entire complex being about 12 nm in size Citation[40], Citation[41]. Still, magnetic iron particles pose toxicity concerns, especially in the particles containing metallic iron, because upon metabolism the iron can generate oxidative stress through harmful reactive oxygen species Citation[42].
Many parenteral iron formulations have been used in people, with Venofer™ (American Reagent, Inc.) and Ferrlecit™ (Watson Pharmaceuticals) currently widely used in the United States. Venofer and Ferrlecit are both comprised of iron oxides, with Venofer an iron sucrose Citation[43] and Ferrlecit an iron gluconate formulation. In both cases the iron material is in the form of an iron oxyhydroxide colloid of about 2 nm in size for Venofer and 3 nm for Ferrlecit Citation[44]. Ferumoxytol, originally developed as an MRI contrast agent and now in development for treating anemia, has a bigger IO crystal and may be more easily administered and have less toxic side effects compared to currently commercialized formulations Citation[39].
Beyond the therapeutic use of iron in the core, a variety of small molecule drugs have been loaded into iron oxide particles. For example, chemotherapeutics have been loaded into pluronic-oleate coated iron oxide crystals Citation[33]. The ability to stabilize these constructs against rapid disintegration in the blood was viewed by the authors as a key to tumor accumulation when using IV injections. LHRH peptides have been incorporated into IO particles for tumor targeting, and lytic peptides have been incorporated into the same particles for membrane permeation and cell lysis Citation[45], Citation[46]. The anti-viral drug indinavir has been incorporated into magnetic particles that were taken up ex vivo by monocytes Citation[47], Citation[48]. The incorporation of small molecules and proteins into magnetic particles for capture by stationary, high-gradient magnetic fields localized to desired tissues is discussed below.
In addition to small molecule drugs, targeting agents may be attached to magnetic particles for therapeutic purposes. Potential targeting agents include vitamins like folate Citation[49], peptides, and antibodies and antibody fragments Citation[50]. Discussions of targeting agents used with nanoparticles have been widely published, for example Sapra and Allen have provided detailed reviews in terms of what has been accomplished for liposomes Citation[51], Citation[52], while a broader view of nanoparticle targeting is provided by Fahmy, et al. Citation[53]. Biomolecules to which particular targeting agents may bind include receptors that are overexpressed on the surface of cells, vascular targets, and extracellular proteins such as fibrin. In addition to targeting agents, enzymes, including collagenase for cutting through the extracellular matrix (ECM) of a tumor Citation[54], Citation[55] and urokinase for fibrin clot busting Citation[56], have also been attached to magnetic particle therapies.
Magnetic field capture of ferrite particles
Perhaps the most easily achieved magnetic manipulations of therapeutic value are ex vivo apheresis procedures where high strength, high gradient fields are readily applied to magnetic particles in biological fluids. In typical applications, magnetic particles are added to extracorporeal blood where they can bind to materials such as DNA, viruses Citation[57], Citation[58], stem cells Citation[59], or cancer cells Citation[60], Citation[61] and then be removed from the blood using a magnetic field prior to reintroduction to the blood stream. Particles for these applications can be large to aid in magnetic purification and they need not evade the RES because they are removed from the blood prior to its return to the body. More ambitiously, extracorporeal toxin removal after particles are injected into the body as a means of faster and more complete detoxification has been proposed Citation[62].
A more technically challenging application is to direct particles to tissues in vivo using high-gradient magnetic fields. As with size and biomolecular targeting, the objective of magnetic targeting is to better localize material to a lesion and avoid exposure of healthy tissues. With respect to cancer, while surgical resection is a first choice when tumor location is known, resection is not always possible or can be only incompletely accomplished, and magnetic targeting of drug-loaded particles to known tumor locations is potentially useful.
Among others, Driscoll, Widder, Senyei and colleagues Citation[63] and Gupta and Hung Citation[64] have performed experiments and presented calculations on field strengths and particle sizes necessary to retain particles in tissues and under the influence of blood flow. Force on a particle is a product of the externally applied magnetic field strength, magnetic gradient, particle volume (that is, the cube of the diameter), and particle magnetization. Thus the combination of large, high magnetization particles with high field gradients is necessary for retaining particles in magnetic fields. Strongly magnetic iron based particles can be either ferromagnetic or superparamagnetic. Ferromagnetic materials have strong remnant magnetization after a magnetizing field has been removed; metallic iron particles above a certain size fall into this category. Superparamagnetic materials on the other hand have strong magnetization under the application of an external magnetic field of sufficient strength but no net magnetization after the external field is removed; certain nanoparticulate iron oxide minerals, for example magnetite, can be superparamagnetic. In the absence of a magnetic field, superparamagnetic particles do not agglomerate through magnetic attraction; agglomeration leads to RES uptake as well as accumulation in healthy tissues like the lung and thus is generally not desired for systemically administered particles.
The magnetization for ferrite materials can range from about 100 emu/g for magnetite (Fe3O4) particles to over 200 emu/g for metallic iron, with values for a given material very sensitive to fabrication methods. A primary challenge, especially in deep tissues away from the magnet, in using magnetic fields to localize magnetic particles is in generating a strong enough magnetic force. Lubbe calculated that for permanent magnets, particles could only be concentrated in tissues that were within a few cm of an edge of typically available strong NdBFe permanent magnets Citation[65].
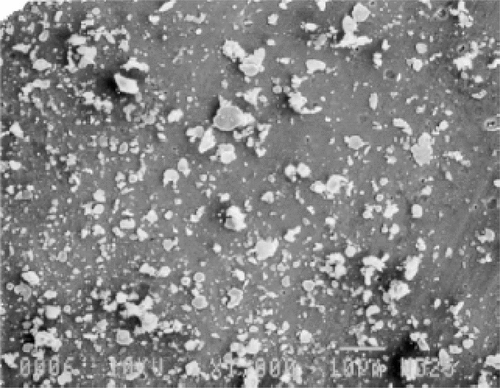
Large particles for magnetic capture can be made from ‘top down’ methods, that is, starting from larger particles and physically reducing their size. In the case of particles that advanced into late stage human clinical trials by the company FeRx, iron-activated carbon particles, termed magnetic targeted carriers (MTC), were combined with a variety of chemotherapeutic agents, including doxorubicin Citation[6]. As shown in , these iron-carbon particles had irregular shape and broad size distribution in the 0.5 to 5 micron range and were formed by mixing iron power and activated carbon using a ball mill mixer and ethanol as a lubricant, then homogenized and dried. The large particles were not amenable to systemic administration because they generally interact too rapidly with blood proteins and are rapidly removed from circulation; instead they were infused into arteries, for instance into the hepatic artery to treat liver cancer, with a high gradient achieved by placing high strength permanent magnets in close proximity to the liver to retain the particles and localize the release of the chemotherapeutic payload. For micron-sized iron-carbon particles with large magnetic susceptibilities of 175 Am2/kg, 100 gauss fields were sufficient to retain particles in flow fields representative of the bloodstream Citation[6]. Importantly, despite the large particle size, the magnetic forces were apparently strong enough to cause the particles to extravasate and embed in the tissue where the fields were applied Citation[66]. Besides infusion into the hepatic artery, other organs, including the lung after infusion into pulmonary artery, were tested in animal models using several chemotherapeutic drugs, including doxorubicin, mitomycin c, gemcitibine and oxaliplatin physically adsorbed on to MTCs Citation[67]. In human clinical trials, doxorubicin, the most fully investigated chemotherapeutic, was mixed with and physically adsorbed to the particles just before injection into patient Citation[68]. This material was called MTC-Dox, and later branded Magnetarg™. While the results of early clinical trials were promising, a late stage clinical trial yielded disappointing results that led to the discontinuation of development of chemotherapeutic MTC product candidates.
Figure 1. Scanning electron micrograph of iron-carbon microparticles prepared by FeRx for magnetic targeting of doxorubicin and other chemotherapeutics. 300 × magnification. Reprinted with permission from Elsevier from Biomaterials 21 (2000) 1411–1420.
Senyei and coworkers similarly used roughly micron-sized particles comprised of magnetite, cross-linked albumin and doxorubicin Citation[69], Citation[70] as well as other chemotherapeutic and bioactive agents to magnetically direct chemotherapeutic activity to tumors with significant improvement in therapeutic index in animal models. However, these particles and concomitant use of magnetic targeting did not proceed into human clinical trials.
Starch-coated particles about 100 nm in diameter loaded with epirubicin, an anthracycline chemotherapeutic, were used by Lubbe and colleagues in multiple clinical trials Citation[65], Citation[71]. While good tolerability was indicated in a phase I study in man, improvements in treatment protocols and materials, particularly the use of bigger particles, were deemed necessary before continuing human trials.
Smaller still, PEG-functionalized magnetite particles reportedly 30 to 60 nm in size were decorated with urokinase and magnetically directed to a fibrin clot in vitro using a 250 Oe magnet Citation[32], and this particle was further investigated in vivo Citation[56]. Due to the small size of the particles the magnetic field has to be very high with a pole in close proximity to targeted tissue for effective magnetic localization. The authors noted that because magnetic particles are attracted to one another in the presence of the magnetic gradient, particle clumping in tissues may aid in magnetic field sequestering.
Magnetoliposomes about 200 nm in diameter with very high magnetite loading were formulated by Lesieur and colleagues Citation[72] were magnetically targeted using a 0.3 T magnetic field and a reported gradient of 11 T/m applied externally to the tumors of subcutaneously inoculated tumor bearing mice Citation[73]. Liposomes were observed to embed in the capillary walls of the tumor and to be retained. While the particles were not loaded with chemotherapeutic, the authors propose that chemotherapy and/or AMF-driven hyperthermia are subsequently possible after localization.
For magnetic capture the magnetic field has typically been applied for short duration, usually less than an hour. Particle retention in the desired tissue after magnetic field removal has been a challenge; after a field is turned off, particles and their payloads are able to leave the tissue in which they were concentrated. This may occur through multiple mechanisms including diffusion of the particle back into the bloodstream, clearance by the lymph system, and uptake into phagocytosing cells and migration of the cells out of the tissue. To keep the particle in the desired location, a stealth particle that is not taken up by macrophages, that is sufficiently physically located away from the bloodstream in the perivascular space but that also is not easily cleared through lymph drainage are all desirable. It is notable that the MTC particles developed by FeRx were comparatively large and irregularly shaped compared to other magnetic targeted materials in clinical trial use and fared comparatively well. These particles were too large to penetrate further into the tumor after the field was turned off, but they also were effectively immobilized in the tumor where the payload could be released and penetrate effectively as a small molecule. However, in summary, the efforts of several academic and industrial organizations which began in the 1970s have yet to produce commercial products. The challenge of producing stable, stealthy, highly magnetic particles with sufficient payloads and high localized field gradients such that an improvement in therapeutic index is demonstrated in people have thus far been too difficult to overcome to commercialize products.
Therapy through particle heat generation
Targeting with static, high gradient magnetic fields discussed above is intended to direct the bioactivity of magnetic particles and thus is a delivery mechanism. The generation of heat by particles is intended primarily to have a therapeutic effect via hyperthermia. Particles containing ferromagnetic material such as iron or superparamagnetic iron oxide, typically a maghemite (γ-Fe2O3) or magnetite (Fe3O4) crystal structure, can be heated using high strength alternating magnetic fields (AMFs) Citation[74], Citation[75]. AMF-driven magnetic particle heating to provide localized hyperthermic treatment of cancers was proposed in the 1950s by Gilchrest Citation[74]. Hyperthermia via AMF-driven iron oxide particle heating has progressed into human phase II clinical trials in Europe for the treatment of glioblastoma and prostate cancer. In these trials the particles are injected intra-tumorally to treat localized disease Citation[76], due in part to the insufficient heating that can be achieved given the particle's Specific Absorption Rate (SAR) and limited tumor accumulation via systemic delivery as discussed below. The particles employed are narrowly dispersed iron oxide crystals in the 10 to 15 nm size range that are coated with aminosilane and the AMF applicator can reach 18 kA/m.
Before continuing the discussion of heat generation by particles, a few words on hyperthermic treatments in general are warranted. Hyperthermia can be applied using a variety of methods, including whole body heating, high intensity and frequency ultrasound, microwave–radiowave EM energy and infusion of warm water into the peritoneal cavity. Hyperthermic dose, that is both the tissue temperature profile and the duration of treatment, can be varied substantially and with these variations produce distinct and different outcomes. Thus, generalizations regarding physiological and biological effects should be made with caution. On the high temperature end, thermal ablative methods that heat tissues to above 50°C for even short duration directly kill cancer cells Citation[77]. At lower temperatures, mild temperature hyperthermia (MTH), that is a dose in the range of about 39° to 42°C for about one hour, produces more subtle effects, including induction of a heat shock response and immune system stimulation Citation[78]. Perhaps most relevant to the consideration of particle penetration, MTH increases tumor blood flow and vessel permeability, as reported by a number of researchers, including Vaupel and coworkers Citation[79–81]. MTH also generally increases tumor interstitial permeability and cancer cell uptake of a range of solutes, including oxygen and other low molecular weight solutes Citation[80], Citation[82], as well as antibody fragments Citation[83] and larger materials such as liposomes Citation[84], Citation[85]. In studies in mice, tumor permeability persisted up to about six hours after the initial hyperthermia application Citation[85]. Interestingly, in a study using liposomes, increased permeability was not seen in a heat-cool-heat cycle experiment with the second heat cycle applied within several hours of the initial heating, an effect called vascular thermotolerance Citation[85]. Thermotolerance is not observed if the heat cycles are spread out by sufficient time, for instance one week. Dosing schedules of hyperthermia and chemotherapeutic administration are thus critical to reaping benefits from combining modalities.
While systemic hyperthermia may be beneficial in treating metastatic disease, heat is a rather non-specific mechanism for cancer cell killing, with potential drawbacks similar to chemotherapeutics, and thus could benefit similarly by targeting its effects to pathological lesions. If particles capable of generating heat can be substantially localized in a tumor, tumor-specific heating may be achieved. Magnetic field heating of tissue embedded particles is attractive in part because magnetic fields in useful frequency ranges can penetrate the body deeply and are not reflected, in contrast with the problems of E-fields at the boundaries of materials with different conductivities. Ferromagnetic resonance (FMR) of iron's unpaired electron is one mechanism by which magnetic particles may be heated. Generally higher frequencies in the range on 1 to 1000 MHz may be useful for FMR. Larger particles may be heated through hysteretic effects using alternating magnetic fields in the range of 50 kHz to single digit low MHz. The most fully developed technology appears to be AMF-driven heating of small, highly crystalline particles presumably via Neel or Brownian mechanisms. Neel relaxation, that is, the fluctuation of a crystal's magnetic moment over an anisotropic energy barrier, and Brownian relaxation to viscous losses due to particle reorientation in solution, are two mechanisms typically cited for heat generation of iron oxide crystals less than about 30 nm in diameter. Theoretically, at resonant conditions when the particle's characteristic relaxation time (e.g. through Neel relaxation) matches the applied AMF frequency, the rate of heat generation, often referred to as the specific adsorption rate (SAR) is proportional to (Ms2 V) · (H2 f), where Ms is the particle magnetization, V is the particle volume, and H is the amplitude and f the frequency of the AMF Citation[86]. AMF amplitude and frequency are limited by the generation of eddy currents in the patient's body. Eddy currents increase with the square of the radius of the AMF-subjected volume. The volume of tissue to which a field is applied is thus an important consideration. An often cited, experimentally determined maximum safe AMF power level for application to humans is a product of the frequency times the magnetic field amplitude such that H · f < 4.85 × 108 A/(m·s) for one hour AMF application using a loop diameter of 30 cm Citation[87]. Additionally, frequencies above about 100 kHz and below about 500 KHz appear to be preferred for safety reasons. Using sub-MHz frequencies and amplitudes up to about 10 kA/m, synthetic IO particles with specific adsorption rates (SARs) greater than 500 W/g have been reported Citation[86]. Hergt and colleagues report that the SAR of a cobalt particle was about 720 W/g at the size where transition between superparamagnetic and ferromagnetic behavior, while magnetite of about 35 nm in size from bacteria have reported SARs of about 1000 W/g, which is the largest value reported to the author's knowledge Citation[86], Citation[88]. In the case of AMF heating of synthetic IO crystals, particle SAR appears to increase for monocrystalline IO particles with a diameter up to about 20 nm, not including coating. For a stealth particle, 30 nm or larger appears to be a reasonable estimate of minimum total particle size expected for maximum heat generation.
Extraordinarily high power output is predicted for monodomain crystals with narrowly defined size when the size is matched to the AMF driving frequency. Narrow size dispersion may also be desirable for additional reasons, such as eliminating small particles that aren’t magnetic and/or eliminating larger particles that are unstable in solution and are poorly circulating. Commonly followed IO synthetic methods similar to that forwarded by Molday have often been employed Citation[89], where iron oxide nanoparticles were made by combining ferrous chloride and ferric chloride in a basic solution from which Fe3O4 precipitates. These precipitates are commonly coated prior to agglomeration by carbohydrates, particularly dextran or modified dextran, that are either present in the reaction mixture or introduced afterwards to form aqueous colloidal dispersions. Typical iron oxide particles made in this manner have a size in the 10 to 20 nm range. While particles made via iron chloride reduction do not have the monodispersed size that appears to be advantageous for AMF-driven hyperthermia, magnetic separation can be used to select particles of a desired size from mixtures with a broad distribution. Alternatively, recently developed thermal decomposition procedures similar to those published by Hyeon et al. Citation[91] produced iron oxide particles with standard deviation in size of typically less than about 5% of the particle diameter for particles of about 10 nm or less. Particle synthesis yielded iron oxide colloids with oleic acid coupled to the surface, presumably forming coordination bonds with iron atoms on the surface of the iron oxide core via the carboxylate moiety. These IO cores were solubilized in water either coating or replacing the oleate layer with a hydrophilic or amphiphilic polymer Citation[33]. Among other decomposition methods, ferrite nanoparticle cores with narrow size dispersion have also be prepared by reacting metal acetylacetonates (acac) with long chain alcohols in the presence of oleic acid and oleyl amine using procedures as described by Sun et al. Citation[92].
Returning to heat generation, the amount of heat needed to raise the temperature of tissue depends upon the volume, surface area, and vascularity of the tissue. Assuming a spherical tumor that has a certain concentration of particles, the amount of heat that can be generated scales with the number of particles, which equals the tumor volume multiplied by particle concentration. The heat can exit the tumors by convection and conduction through the tumor surface and through blood flow. In the case of the surface, the heat transport q = h · A · ΔT, where an average heat transport coefficient between the tumor and the surrounding tissue, A is the surface area, and ΔT is the temperature difference between the tumor and surrounding tissue. Heat removed through blood flowing through a tumor also scales with surface area. Thus heat generation scales with volume, or the cube of the diameter, while the heat dissipation scales with surface area, or with the square of diameter. Thus for a given concentration of particles, the temperature of a large tumor can be raised higher than a small tumor, and there is a minimum volume necessary to produce a temperature increase. At the lower end volume extreme, it appears that a single, isolated cell can not be heated even at high magnetic particle concentrations because the surface to volume ratio for a single cell is too great Citation[93].
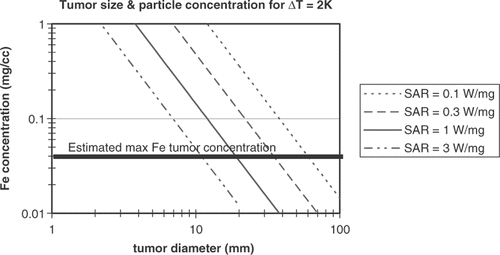
Andra, Hergt, Hilger and colleagues have measured and modeled AMF-driven magnetic particle heat generation in spherical tumors Citation[94]; is adapted from the calculated particle heating data for a range of SARs Citation[95], with the calculations modified to the lower particle concentrations in tumors expected for systemic dosing and to the lower tumor temperature increases needed for MTH. If about 2°C of temperature increase is produced in tissues by AMF application alone, then the particles need to supply enough heat to raise the temperature about 2°C for MTH. Particle dosing must be kept with in a safe range; previous USPIO MRI diagnostic agents were limited to 2.6 mg Fe/kg iron per injection. However, ferumoxytol, a newer formulation in late stage clinical trials for treating anemia, can be dosed at higher than 10 mg/kg Citation[39]. If a dose of double this, or about 20 mg Fe/kg was acceptable for treating cancer, this would correspond to 400 µg of Fe for a 20 g mouse. Concentrations of 40 µg Fe per gram of tumor may be realized if 10% of the injected dose is delivered per gram tumor, which is at the high end of what is possible. As seen in , in this particle concentration range, very high SARs of greater than 1 W/mg Fe are estimated to be needed to heat tumors of roughly 1 cc. Thus, while appearing to be eventually achievable with further gains in magnetic particle structure, a combination of lower toxicity and higher SAR than are currently available seem to be needed to treat tumors using systemically administered magnetic particles and AMF-driven heating.
Figure 2. Estimates of concentration of ferrite particles needed for a give tumor diameter to raise the temperature of the tumor 2°C. Both axes are log scale. Lines are shown for particles with specific loss power of 0.1, 0.3, 1, and 3 W/mg. The line indicating the maximum levels of ferrite particle tumor concentration anticipated to be achievable after systemic (e.g. IV) administration assumes an injection of approximately 20 mg/kg Fe. For a 20 g mouse this is 0.4 mg Fe injected and the line shows a tumor accumulation of about 10% of the injected dose per cc tumor. For IO particles with a given SAR, tumors of the size indicated by the intersection at the maximum concentration line or larger may be treatable. For example, IO particles concentrated at 0.04 mg Fe/cc tumor and with an SAR of 1 W/mg may heat tumors with diameter of roughly 20 mm or larger to a therapeutically useful temperature.
Success has been reported, however, using systemically administered magnetic particles and AMF-driven heating; in particular, a delay in tumor growth in a tumor bearing mouse study as reported by DeNardo et al. Citation[96]. Particles were about 20 nm in size and made of dextran coated magnetite decorated with tumor targeting monoclonal antibodies; Very high field strengths of 80 kA/m at a frequency of 153 kHz were used in these studies, two orders of magnitude higher than the safety limit observed by Bezovich Citation[87]. However, this high amplitude AMF was observed to be safe for mice when applied in pulses, for example with AMF field turned on for 90 seconds and then off for 90 seconds to allow for heat dissipation, with a total treatment time of up to 20 minutes. Denardo and colleagues speculated that isolated heating at a microenvironment level, perhaps at the cellular level, occurs to the extent that thermal ablation is achieved Citation[97]. As discussed above, Hilger et al. among others argue that heating of such small volumes is not possible Citation[98]. Perhaps some of the effects of AMF-particle interaction result in non-thermal effects on cell structure. For instance, Prasad and coworkers hypothesized that cell-killing ‘magnetocytolysis’ may occur through physical disruption of cellular membranes and perhaps other cellular structures Citation[99], Citation[100].
The above study notwithstanding, the SAR capabilities of currently available IO crystals, systemic administration of IO particles appear to at best approach the intra-tumoral concentrations needed for localized hyperthermia. Also problematic is that RES uptake is generally large, and the volume of the RES organs, especially the liver, is large. Due to lower surface to volume ratios larger volumes are heated more readily than smaller volumes as described above. Thus, avoiding undesirable heating of RES organs while still effectively heating small tumors may be a challenge. When the AMF application can be localized such that RES organs are avoided, undesirable organ heating may be avoided. Finally with regard to RES heating, RES accumulation suggests a potential for treating primary or metastatic disease of the RES organs, for instance liver cancer. However, the same problems with delivery of chemotherapeutic to RES macrophages is encountered–that is the macrophages are not the cells that we desire to treat.
Beyond ferrites containing only iron, thermal decomposition methods also facilitated the incorporation of divalent cations such as Zn, Mn, Ni, or Co into spinel crystal structures which has been shown to increase magnetization and SAR Citation[101]. While gains in magnetization may be achieved, the complexity of synthesis and potential increase in toxicity, for instance when cobalt or nickel are employed, lessen the desirability of mixed cation ferrite particles. In another alternative to typical IO structures, iron core particles with iron oxide shell particles could be more effective in heating due to the higher magnetization of the metallic core. However core/shell structures are difficult to synthesize and to achieve the desired magnetic performance, large particles are often necessary. For instance Qiang and coworkers fabricated relatively large iron/iron oxide particles with the core and shells over a range of sizes in the 10 to 100 nm range, with particles larger than about 40 nm approaching the magnetization of metallic iron Citation[102]. These particles are relatively large even without coating and thus will likely be difficult to solubilize and will have tumor penetration challenges.
Looking towards additional ways to leverage AMF-driven hyperthermia of magnetic particles, reports on the use of hyperthermia to improve chemotherapeutic delivery via phase change of a carrier material are instructive. Chilkoti and colleagues used a polymer exhibiting a Lower Critical Solution Temperature (LCST) to affect a hydrophilic to hydrophobic transition at 40°C; when a tumor is locally heated, the hydrophobic material adheres to the tumor tissue and is better retained Citation[103], Citation[104]. Particularly attractive is the combination of thermally labile liposomes with hyperthermia, with liposome disruption occurring below temperatures where healthy cells are harmed Citation[105]. Heat-triggered release of liposomal doxorubicin has advanced into clinical trials for ovarian patients Citation[106]. Combining concepts of thermally labile liposomes and magnetic liposomes, magnetoliposomes have been formed with thermally labile lipid bilayers Citation[107]. Doxorubicin loaded into these particles was reportedly released in vitro with the application of alternating magnetic fields at 3.5 MHz and 1.5 mT field strength Citation[108].
Improving particle penetration: Dynamic particle size and increasing tumor permeability
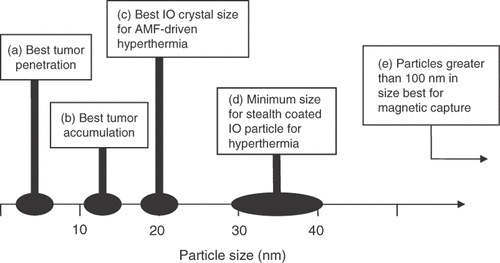
As has been emphasized throughout this review, the constraints that particle size place on the strength of magnetic interactions and extravascular tissue penetration confound the development and utility of therapeutic magnetic particles. summarizes nanoparticle size and structure effects on distribution and tumor penetration; particle size desirable for tumor accumulation is smaller than what is possible for long circulating magnetic particles with high SAR and far smaller than what is useful for magnetic localization.
Figure 3. Approximate particle sizes that appear to be best suited for (a) tumor penetration, (b) tumor accumulation, and (c) AMF-Neel relaxation-driven hyperthermia. Also shown are the size ranges for (d) making stealth IO particles for hyperthermia, and (e) particle size needed for magnetic field capture.
The in vivo modulation of material size is a means by which tumor penetration may be improved. This is part of the rationale for thermally labile liposomes, where chemotherapeutics are incorporated into liposomes and heat is applied to both increase tumor permeability and release the liposome payload Citation[105]. AMF has been applied to labile magnetoliposomes as well to drive chemotherapeutic release Citation[108]. However, if particle disintegration is to be driven by AMF-IO heat generation, then the concentration of particles in a tumor needed for the requisite temperature increase is likely to be too high for what can be achieved using large particles like liposomes.
In exploring ways to improve performance, it may be useful to view magnetic hyperthermia as a ‘pre-targeting’ concept. In drug or radioactive isotope pre-targeting, a binding agent is localized to a tumor prior to administration of a cell killing agent that binds to the pre-targeting agent Citation[109]. In the magnetic particle case, particles are localized to the tumor prior to administration of the AMF. The more material that can be selectively loaded into the tumor prior to being energized by the AMF, the more heat can be generated. It may thus be beneficial to employ smaller magnetic particles than those with higher SAR so that the particles concentrate and deeply penetrate tumors.
In contrast to the size reduction concepts used in labile liposomes, if size could be increased to better adsorb AMF energy selectively within the tumor, this would allow a tumor specific platform to provide better absorption of magnetic energy for heating purposes. However, the application of particles prior to AMF application would not take advantage of increasing tumor porosity and particle localization through hyperthermia application immediately prior to particle administration, as shown by Kong et al. Citation[84], Citation[85]. Perhaps hyperthermia applied through non-AMF mechanisms immediately prior to particle parenteral dosing followed at some later time by AMF application would improve both particle localization and tumor specific heating, but such a regimen is perhaps too complicated to warrant investigation.
Instead of, or in addition to, manipulating particle size by employing polymer precursors or particle components to facilitate interstitial permeation, decreasing tumor permeability barriers is also conceptually appealing. For instance by displaying certain enzymes, particles may cut their way through the tumor ECM. Increased particle permeation through ECM material in in vitro experiments has been recently reported for nanoparticles displaying collagenase Citation[54], Citation[55].
Looking forward: Magnetic particle use in multimodal regimens
Multi-modal approaches are now the norm in treating cancer, with mono-modal approaches increasingly rare. Surgery is used to remove readily accessed and identifiable cancer lesions, followed by one or more of radiation, radiation sensitizers, chemotherapeutic ‘cocktails’ and targeted therapeutic agents like monoclonal antibodies. Specific combinations of these modalities produce superior outcomes for certain cancers and cancer stages; that is, certain modalities synergize with one another. For instance, platinum therapeutics have demonstrated synergy with the targeted therapeutics such as trastuzumab Citation[110], Citation[111]. Paraplatin in combination with paclitaxel has demonstrated promise as neoadjuvant and adjuvant in treating NSCLC. Platinum agents are also excellent radiation sensitizers Citation[112]. Hyperthermia synergizes with a variety of materials, including arsenic trioxide Citation[113], tirapazamine Citation[114], cisplatin Citation[115], and with radiation therapy Citation[116]. Often, hyperthermia equipment is housed within radiation oncology facilities at major medical centers. Radiation produces oxygen radicals, which then produce double-strand breaks in cancer cell DNA which may lead to cancer cell destruction. Cancer cells can mitigate this damage by oxygen radicals by first detecting double strand breaks, then producing mediators that repair the breaks Citation[117]. Multiple studies have shown that hyperthermia can substantially increase oxygenation levels in tumors, thereby facilitating radiation-induced oxygen radical formation. Additionally, hyperthermia reportedly both hinders the ability of cancer cells to sense DNA destruction and impedes DNA repair mediators. In a triple combination, hyperthermia, platin chemotherapeutics and radiation are particularly synergistic Citation[118], Citation[119]. The better localization of these modalities to the tumor environment may substantially improve the treatment of cancer.
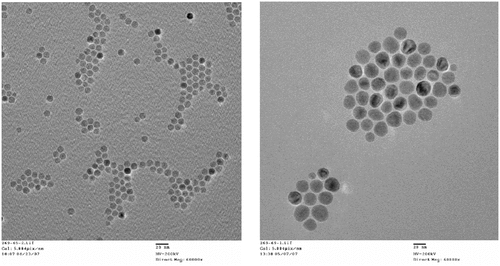
Consideration of the enabling features of iron-based magnetic particles, attributes and capabilities of the various materials and methods that can be used to coat particles, and the loading of bioactive agents into the coating, has led to the fabrication of MagNaGel™ nanoparticles by the author and coworkers Citation[120]. With size tunable in the 20 to 50 nm range and high hydrophilicity, MagNaGel nanoparticles avoid RES uptake and increase tumor penetration. Iron oxide cores were synthesized to optimize AMF-driven hyperthermia and allow MRI tracking. Examples of IO nanoparticle cores fabricated by Alnis are shown in . SARs of 670 W/g of Fe (at 400 kHz and about 8 kA/m) have been achieved, approximately matching the highest reported values of synthesized IO in the literature; as discussed above, further improvements in SARs are desired. Platin chemotherapeutics were incorporated at greater than 10 wt%, and targeted agents were incorporated for targeting vasculature or target cancer associated growth factor receptors. These heat-generating, platin-loaded nanoparticles thus combine hyperthermia and platinum chemotherapeutics, and may improve radiation efficacy 121–123 and are being evaluated in in vivo studies.
Figure 4. Transmission electron micrographs of iron oxide particles made by Alnis BioSciences, Inc. The smaller particle shown on the left had an average size of 10 nm, stdev 0.8 nm demonstrating the tight size control currently achievable in IO particle fabrication, while the particles on the right had an average size of 23 nm, stdev 4 m and generated about 670 W/g when subject to a 400 KHz alternating magnetic field generated using a 2 turn, 2.5 cm diameter copper coil driven by Ameritherm Hotshot 5000 power supply supplying 200 amps (estimated magnetic field amplitude of 8 kA/m).
Conclusions
Interactions with localizing or energizing magnetic fields are integral to therapeutic applications of magnetic particles, and particle size plays a central role in determining the strength of these interactions. Iron or iron oxide cores with a minimum size of 100 nm for practical use with strong permanent magnetic fields appear to be needed for high gradient magnetic drug targeting, but this size is larger than desired for (tumor) tissue perfusion. The large particle sizes required may have limited the utility of high gradient magnetic field approaches to localizing drug delivery. For hyperthermia applications, smaller magnetic particles can be employed; coated magnetic particles that can be efficiently heated the 30 to 100 nm or larger. While such particles are larger than desirable for optimized tumor perfusion, the size still takes advantage of the EPR effect. According to theory and recently acquired data, AMF driven heating appears to be substantially improved by matching a particular frequency with a monocrystalline ferrite particle of a narrowly defined size. Further advances in particle design and construction that improve AMF-driven magnetic particle therapy to the extent that metastatic disease can be treated using systemically administered particles appears to be achievable.
In addition to size, particle stability and drug retention in vivo has been cited as a key for drug delivery using magnetic particles, and the lack of coating stability has hindered therapeutic product development Citation[33]. Similarly, magnetic core coating stability has been cited as the key for improving hyperthermia performance Citation[86]. So coating is forwarded as a key for both delivery and heating, and generally the formation of a stable, long circulating ferrite particle remains a technical challenge. Additionally, even for relatively simpler polymeric/supramolecular structures compared to magnetic particle constructs, the difficulties of building a stable structure to a desirable size, size tolerance, and disintegration profile are under-appreciated Citation[13]. However, supramolecular assembly methods and materials are improving at the same time that biological knowledge is rapidly accruing, informing upon what materials can and should be combined to construct magnetic medicines. Looking to the future, the synergies gained by combining magnetic particles and AMF-driven hyperthermia with molecular targeting, drug delivery, and radiation may greatly improve treatments of certain cancers. Additionally, modality combinations that include magnetic particles may be beneficial in the treatment of inflammatory diseases and infections where the advantages of magnetic particle localization, particle heating and MRI tracking of disease location are desirable.
Acknowledgements
The author thanks Christopher Sunderland for synthesizing magnetic particles, obtaining the image shown in and for proofreading the manuscript. Also, the National Cancer Institute's support of Alnis BioSciences’ efforts under contract # HHSN261200411004C to develop magnetic particles for the detection, diagnosis and treatment of cancer is greatly appreciated.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Oliver JD, 3rd, Deen WM. Random-coil model for glomerular sieving of dextran. Bull Math Biol 1994; 56: 369–389
- Huang SK, Lee KD, Hong K, Friend DS, Papahadjopoulos D. Microscopic localization of sterically stabilized liposomes in colon carcinoma-bearing mice. Cancer Res 1992; 52: 5135–5143
- Tang T, Howarth SP, Miller SR, Trivedi R, Graves MJ, King-Im JU, Li ZY, Brown AP, Kirkpatrick PJ, Gaunt ME, Gillard JH. Assessment of inflammatory burden contralateral to the symptomatic carotid stenosis using high-resolution ultrasmall, superparamagnetic iron oxide-enhanced MRI. Stroke 2006; 37: 2266–2270
- Yancy AD, Olzinski AR, Hu TC, Lenhard SC, Aravindhan K, Gruver SM, Jacobs PM, Willette RN, Jucker BM. Differential uptake of ferumoxtran-10 and ferumoxytol, ultrasmall superparamagnetic iron oxide contrast agents in rabbit: Critical determinants of atherosclerotic plaque labeling. J Magn Reson Imaging 2005; 21: 432–442
- Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol 2000; 156: 1363–1380
- Rudge SR, Kurtz TL, Vessely CR, Catterall LG, Williamson DL. Preparation, characterization, and performance of magnetic iron-carbon composite microparticles for chemotherapy. Biomaterials 2000; 21: 1411–1420
- Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul 2001; 41: 189–207
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release 2000; 65: 271–284
- Jang SH, Wientjes MG, Lu D, Au JL. Drug delivery and transport to solid tumors. Pharm Res 2003; 20: 1337–1350
- Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J Natl Cancer Inst 2006; 98: 335–344
- Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. Vascular permeability in a human tumor xenograft: Molecular size dependence and cutoff size. Cancer Res 1995; 55: 3752–3756
- Yuan F, Krol A, Tong S. Available space and extracellular transport of macromolecules: Effects of pore size and connectedness. Ann Biomed Eng 2001; 29: 1150–1158
- Lee CC, Gillies ER, Fox ME, Guillaudeu SJ, Frechet JM, Dy EE, Szoka FC. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc Natl Acad Sci USA 2006; 103: 16649–16654
- Satchi-Fainaro R, Hailu H, Davies JW, Summerford C, Duncan R. PDEPT: Polymer-directed enzyme prodrug therapy. 2. HPMA copolymer-beta-lactamase and HPMA copolymer-C-Dox as a model combination. Bioconjug Chem 2003; 14: 797–804
- Charrois GJ, Allen TM. Rate of biodistribution of STEALTH liposomes to tumor and skin: Influence of liposome diameter and implications for toxicity and therapeutic activity. Biochim Biophys Acta 2003; 1609: 102–108
- Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev 1999; 51: 691–743
- Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J Phys D: Applied Phys 2003; 36: 81
- McRobbie DW. MRI from picture to proton. Cambridge University Press, Cambridge 2003
- Corot C, Robert P, Idee JM, Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev 2006; 58: 1471–1504
- Oude Engberink RD, van der Pol SM, Dopp EA, de Vries HE, Blezer EL. Comparison of SPIO and USPIO for in vitro labeling of human monocytes: MR detection and cell function. Radiology 2007; 243: 467–474
- Daldrup H, Shames DM, Wendland M, Okuhata Y, Link TM, Rosenau W, Lu Y, Brasch RC. Correlation of dynamic contrast-enhanced magnetic resonance imaging with histologic tumor grade: Comparison of macromolecular and small-molecular contrast media. Pediatr Radiol 1998; 28: 67–78
- Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis - Correlation in invasive breast carcinoma. N Engl J Med 1991; 324: 1–8
- Daldrup-Link HE, Rydland J, Helbich TH, Bjornerud A, Turetschek K, Kvistad KA, Kaindl E, Link TM, Staudacher K, Shames D, Brasch RC, Haraldseth O, Rummeny EJ. Quantification of breast tumor microvascular permeability with feruglose-enhanced MR imaging: initial phase II multicenter trial. Radiology 2003; 229: 885–892
- Turetschek K, Huber S, Floyd E, Helbich T, Roberts TP, Shames DM, Tarlo KS, Wendland MF, Brasch RC. MR imaging characterization of microvessels in experimental breast tumors by using a particulate contrast agent with histopathologic correlation. Radiology 2001; 218: 562–569
- Shames DM, Kuwatsuru R, Vexler V, Muhler A, Brasch RC. Measurement of capillary permeability to macromolecules by dynamic magnetic resonance imaging: A quantitative noninvasive technique. Magn Reson Med 1993; 29: 616–622
- Brasch RC, Shames DM, Cohen FM, Kuwatsuru R, Neuder M, Mann JS, Vexler V, Muhler A, Rosanau W. Quantification of capillary permeability to macromolecular magnetic resonance imaging contrast media in experimental mammary adenocarcinomas. Invest Radiol 1994; 29: S8–S11
- Funovics MA, Kapeller B, Hoeller C, Su HS, Kunstfeld R, Puig S, Macfelda K. MR imaging of the her2/neu and 9.2.27 tumor antigens using immunospecific contrast agents. Magn Reson Imaging 2004; 22: 843–850
- Choi H, Choi SR, Zhou R, Kung HF, Chen IW. Iron oxide nanoparticles as magnetic resonance contrast agent for tumor imaging via folate receptor-targeted delivery. Acad Radiol 2004; 11: 996–1004
- Montet X, Montet-Abou K, Reynolds F, Weissleder R, Josephson L. Nanoparticle imaging of integrins on tumor cells. Neoplasia 2006; 8: 214–222
- Widder KJ, Senyei AE. Magnetic microspheres: A vehicle for selective targeting of drugs. Pharmacol Ther 1983; 20: 377–395
- Cheng J, Teply BA, Jeong SY, Yim CH, Ho D, Sherifi I, Jon S, Farokhzad OC, Khademhosseini A, Langer RS. Magnetically responsive polymeric microparticles for oral delivery of protein drugs. Pharm Res 2006; 23: 557–564
- Inada Y, Ohwada K, Yoshimoto T, Kojima S, Takahashi K, Kodera Y, Matsushima A, Saito Y. Fibrinolysis by urokinase endowed with magnetic property. Biochem Biophys Res Commun 1987; 148: 392–396
- Jain TK, Morales MA, Sahoo SK, Leslie-Pelecky DL, Labhasetwar V. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol Pharm 2005; 2: 194–205
- Kohler N, Fryxell GE, Zhang M. A bifunctional poly(ethylene glycol) silane immobilized on metallic oxide-based nanoparticles for conjugation with cell targeting agents. J Am Chem Soc 2004; 126: 7206–7211
- Laconte LE, Nitin N, Zurkiya O, Caruntu D, O'Connor CJ, Hu X, et al. Coating thickness of magnetic iron oxide nanoparticles affects R(2) relaxivity. J Magn Reson Imaging 2007; 26(6)1634–1641
- Gref R, Luck M, Quellec P, Marchand M, Dellacherie E, Harnisch S, Blunk T, Muller RH. ‘Stealth' corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B Biointerfaces 2000; 18: 301–313
- Immordino ML, Dosio F, Cattel L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine 2006; 1: 297–315
- Gupta AK, Naregalkar RR, Vaidya VD, Gupta M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomed 2007; 2: 23–39
- Spinowitz BS, Schwenk MH, Jacobs PM, Bolton WK, Kaplan MR, Charytan C, Galler M. The safety and efficacy of ferumoxytol therapy in anemic chronic kidney disease patients. Kidney Int 2005; 68: 1801–1807
- Theil EC. Ferritin: At the crossroads of iron and oxygen metabolism. J Nutr 2003; 133: S1549–S1553
- Cowley JM, Janney DE, Gerkin RC, Buseck PR. The structure of ferritin cores determined by electron nanodiffraction. J Struct Biol 2000; 131: 210–216
- Glickstein H, El RB, Link G, Breuer W, Konijn AM, Hershko C, Nick H, Cabantchik ZI. Action of chelators in iron-loaded cardiac cells: Accessibility to intracellular labile iron and functional consequences. Blood 2006; 108: 3195–3203
- Yee J, Besarab A. Iron sucrose: The oldest iron therapy becomes new. Am J Kidney Dis 2002; 40: 1111–1121
- Kudasheva DS, Lai J, Ulman A, Cowman MK. Structure of carbohydrate-bound polynuclear iron oxyhydroxide nanoparticles in parenteral formulations. J Inorg Biochem 2004; 98: 1757–1769
- Kumar CS, Leuschner C, Urbina M, Ozkaya T, Hormes J. Glutaric acid as a spacer facilitates improved intracellular uptake of LHRH-SPION into human breast cancer cells. Int J Nanomedicine 2007; 2: 175–179
- Zhou J, Leuschner C, Kumar C, Hormes JF, Soboyejo WO. Sub-cellular accumulation of magnetic nanoparticles in breast tumors and metastases. Biomaterials 2006; 27: 2001–2008
- Gorantla S, Dou H, Boska M, Destache CJ, Nelson J, Poluektova L, Rabinow BE, Gendelman HE, Mosley RL. Quantitative magnetic resonance and SPECT imaging for macrophage tissue migration and nanoformulated drug delivery. J Leukoc Biol 2006; 80: 1165–1174
- Dou H, Destache CJ, Morehead JR, Mosley RL, Boska MD, Kingsley J, Gorantla S, Poluektova L, Nelson JA, Chaubal M, Werling J, Kipp J, Rabinow BE, Gendelman HE. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood 2006; 108: 2827–2835
- Sonvico F, Mornet S, Vasseur S, Dubernet C, Jaillard D, Degrouard J, Hoebeke J, Duguet E, Colombo P, Couvreur P. Folate-conjugated iron oxide nanoparticles for solid tumor targeting as potential specific magnetic hyperthermia mediators: Synthesis, physicochemical characterization, and in vitro experiments. Bioconjug Chem 2005; 16: 1181–1188
- Ito A, Kuga Y, Honda H, Kikkawa H, Horiuchi A, Watanabe Y, Kobayashi T. Magnetite nanoparticle-loaded anti-HER2 immunoliposomes for combination of antibody therapy with hyperthermia. Cancer Lett 2004; 212: 167–175
- Allen TM, Sapra P, Moase E, Moreira J, Iden D. Adventures in targeting. J Liposome Res 2002; 12: 5–12
- Sapra P, Allen TM. Ligand-targeted liposomal anticancer drugs. Prog Lipid Res 2003; 42: 439–462
- Fahmy TM, Fong PM, Goyal A, Saltzman WM. Targeted for drug delivery. Materials Today 2005; 8: 18–26
- Goodman TT, Olive PL, Pun SH. Increased nanoparticle penetration in collagenase-treated multicellular spheroids. Int J Nanomedicine 2007; 2: 265–274
- Kuhn SJ, Finch SK, Hallahan DE, Giorgio TD. Proteolytic surface functionalization enhances in vitro magnetic nanoparticle mobility through extracellular matrix. Nano Lett 2006; 6: 306–312
- Yoshimoto T, Ohwada K, Takahashi K, Matsushima A, Saito Y, Inada Y. Magnetic urokinase: Targeting of urokinase to fibrin clot. Biochem Biophys Res Commun 1988; 152: 739–743
- Pichl L, Heitmann A, Herzog P, Oster J, Smets H, Schottstedt V. Magnetic bead technology in viral RNA and DNA extraction from plasma minipools. Transfusion 2005; 45: 1106–1110
- Veyret R, Elaissari A, Marianneau P, Sall AA, Delair T. Magnetic colloids for the generic capture of viruses. Anal Biochem 2005; 346: 59–68
- Williams SF, Lee WJ, Bender JG, Zimmerman T, Swinney P, Blake M, Carreon J, Schilling M, Smiths S, Williams DE, Oldham F, Van Epps D. Selection and expansion of peripheral blood CD34+ cells in autologous stem cell transplantation for breast cancer. Blood 1996; 87: 1687–1691
- Denis MG, Lipart C, Leborgne J, LeHur PA, Galmiche JP, Denis M, Ruud E, Truchaud A, Lustenberger P. Detection of disseminated tumor cells in peripheral blood of colorectal cancer patients. Int J Cancer 1997; 74: 540–544
- Zigeuner RE, Riesenberg R, Pohla H, Hofstetter A, Oberneder R. Isolation of circulating cancer cells from whole blood by immunomagnetic cell enrichment and unenriched immunocytochemistry in vitro. J Urol 2003; 169: 701–705
- Chen H, Kaminski MD, Liu X, Mertz CJ, Xie Y, Torno MD, Rosengart AJ. A novel human detoxification system based on nanoscale bioengineering and magnetic separation techniques. Med Hypotheses 2007; 68: 1071–1079
- Driscoll CF, Morris RM, Senyei AE, Widder KJ, Heller GS. Magnetic targeting of microspheres in blood flow. Microvasc Res 1984; 27: 353–369
- Gupta PK, Hung CT. Magnetically controlled targeted micro-carrier systems. Life Sci 1989; 44: 175–186
- Lubbe AS, Alexiou C, Bergemann C. Clinical applications of magnetic drug targeting. J Surg Res 2001; 95: 200–206
- Goodwin S, Peterson C, Hoh C, Bittner C. Targeting and retention of magnetic targeted carriers (MTCs) enhancing intra-arterial chemotherapy. J Magn Magn Mat 1999; 194: 132–139
- Rudge S, Peterson C, Vessely C, Koda J, Stevens S, Catterall L. Adsorption and desorption of chemotherapeutic drugs from a magnetically targeted carrier (MTC). J Control Release 2001; 74: 335–340
- Goodwin SC, Bittner CA, Peterson CL, Wong G. Single-dose toxicity study of hepatic intra-arterial infusion of doxorubicin coupled to a novel magnetically targeted drug carrier. Toxicol Sci 2001; 60: 177–183
- Widder K, Flouret G, Senyei A. Magnetic microspheres: Synthesis of a novel parenteral drug carrier. J Pharm Sci 1979; 68: 79–82
- Senyei AE, Driscoll CF, Widder KJ. Biophysical drug targeting: Magnetically responsive albumin microspheres. Methods Enzymol 1985; 112: 56–67
- Lubbe AS, Bergemann C, Riess H, Schriever F, Reichardt P, Possinger K, Matthias M, Dorken B, Herrmann F, Gurtler R, Hohenberger P, Haas N, Sohr R, Sander B, Lemke AJ, Ohlendorf D, Huhnt W, Huhn D. Clinical experiences with magnetic drug targeting: A phase I study with 4'-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res 1996; 56: 4686–4693
- Lesieur S, Grabielle-Madelmont C, Menager C, Cabuil V, Dadhi D, Pierrot P, Edwards K. Evidence of surfactant-induced formation of transient pores in lipid bilayers by using magnetic-fluid-loaded liposomes. J Am Chem Soc 2003; 125: 5266–5267
- Fortin-Ripoche JP, Martina MS, Gazeau F, Menager C, Wilhelm C, Bacri JC, Lesieur S, Clement O. Magnetic targeting of magnetoliposomes to solid tumors with MR imaging monitoring in mice: Feasibility. Radiology 2006; 239: 415–424
- Gilchrest R, Medal R, Shorey W, Hanselman R, Parrott J, Taylor C. Selective inductive heating of lymph nodes. Ann Surg 1957; 146: 596–606
- Jordan A, Scholz R, Wust P, Fahling H, Felix R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J Magn Magn Mat 1999; 201: 413–419
- Johannsen M, Gneveckow U, Eckelt L, Feussner A, Waldofner N, Scholz R, Deger S, Wust P, Loening SA, Jordan A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int J Hyperthermia 2005; 21: 637–647
- Merkle EM, Boll DT, Boaz T, Duerk JL, Chung YC, Jacobs GH, Varnes ME, Lewin JS. MRI-guided radiofrequency thermal ablation of implanted VX2 liver tumors in a rabbit model: Demonstration of feasibility at 0.2 T. Magn Reson Med 1999; 42: 141–149
- Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia 2005; 21: 779–790
- Bicher HI, Hetzel FW, Sandhu TS, Frinak S, Vaupel P, O'Hara MD, O′Brien T. Effects of hyperthermia on normal and tumor microenvironment. Radiology 1980; 137: 523–530
- Vaupel P, Muller-Klieser W, Otte J, Manz R. Impact of various thermal doses on the oxygenation and blood flow in malignant tumors upon localized hyperthermia. Adv Exp Med Biol 1984; 169: 621–629
- Vaupel PW. The influence of tumor blood flow and microenvironmental factors on the efficacy of radiation, drugs and localized hyperthermia. Klin Padiatr 1997; 209: 243–249
- Song CW, Park H, Griffin RJ. Improvement of tumor oxygenation by mild hyperthermia. Radiat Res 2001; 155: 515–528
- Cope DA, Dewhirst MW, Friedman HS, Bigner DD, Zalutsky MR. Enhanced delivery of a monoclonal antibody F(ab')2 fragment to subcutaneous human glioma xenografts using local hyperthermia. Cancer Res 1990; 50: 1803–1809
- Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res 2000; 60: 4440–4445
- Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res 2001; 61: 3027–3032
- Hergt R, Dutz S, ller R, Zeisberger M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J Phys Condensed Matter 2006; 18: S2919–S34
- Brezovich I. Low frequency hyperthermia. Med Phys Mono 1988; 16: 82–111
- Hilger I, Hergt R, Kaiser WA. Use of magnetic nanoparticle heating in the treatment of breast cancer. IEE Proc-Nanobiotechnol 2005; 152: 33–38
- Molday RS. inventor, Canadian Patents and Development, Ltd, assignee, Magnetic iron dextran microspheres 1984, USA patent 4452773
- Palmacci S, Josephson L. inventors, Advanced Magnetics, Inc., assignee, Synthesis of polysaccharide covered superparamagnetic oxide colloids 1993, USA patent 5262176
- Hyeon T, Lee S, Park J, Chung Y, Bin Na H. Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process. J Am Chemical Society 2001; 123: 12798–12801
- Sun S, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li G. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J Am Chem Soc 2004; 126: 273–279
- Rabin Y. Is intracellular hyperthermia superior to extracellular hyperthermia in the thermal sense?. Int J Hyperthermia 2002; 18: 194–202
- Andra W, d'Ambly CG, Hergt R, Hilger I, Kaiser WA. Temperature distribution as function of time around a small spherical heat source of local magnetic hyperthermia. J Magn Magn Mat 1999; 194: 197–203
- Hergt R, Hiergeist R, Hilger I, Kaiser WA, Lapatnikov Y, Margel S, Richter U. Maghemite nanoparticles with very high AC-losses for application in RF-magnetic hyperthermia. J Magn Magn Mat 2004; 270: 345–357
- DeNardo SJ, DeNardo GL, Natarajan A, Miers LA, Foreman AR, Gruettner C, Adamson GN, Ivkov R. Thermal dosimetry predictive of efficacy of 111In-ChL6 nanoparticle AMF-induced thermoablative therapy for human breast cancer in mice. J Nucl Med 2007; 48: 437–444
- Ivkov R, DeNardo SJ, Daum W, Foreman AR, Goldstein RC, Nemkov VS, De Nardo GL. Application of high amplitude alternating magnetic fields for heat induction of nanoparticles localized in cancer. Clin Cancer Res 2005; 11: S7093–7103
- Bergey E, Levy L, Wang X, Krebs L, Lal M, Kim K, Pakatchi S, Liebow C, Prasad P. DC magnetic field induced magnetocytolysis of cancer cells targeted by LH-RH magnetic nanoparticles in vitro. Biomedical Microdevices 2002; 4: 293–299
- Halbreich A, Roger J, Pons JN, Geldwerth D, Da Silva MF, Roudier M, Bacri JC. Biomedical applications of maghemite ferrofluid. Biochimie 1998; 80: 379–390
- Lee JH, Huh YM, Jun YW, Seo JW, Jang JT, Song HT, Kim S, Cho EJ, Yoon HG, Suh JS, Cheon J. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med 2007; 13: 95–99
- Qiang Y, Antony J, Sharma A, Nutting J, Sikes D, Meyer D. Iron/iron oxide core-shell nanoclusters for biomedical applications. J Nanoparticle Res 2006; 8: 489–496
- Meyer DE, Shin BC, Kong GA, Dewhirst MW, Chilkoti A. Drug targeting using thermally responsive polymers and local hyperthermia. J Control Release 2001; 74: 213–224
- Bae Y, Buresh RA, Williamson TP, Chen TH, Furgeson DY. Intelligent biosynthetic nanobiomaterials for hyperthermic combination chemotherapy and thermal drug targeting of HSP90 inhibitor geldanamycin. J Control Release 2007; 122: 16–23
- Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia: Characterization and testing in a human tumor xenograft model. Cancer Res 2000; 60: 1197–1201
- Alvarez Secord A, Jones EL, Hahn CA, Petros WP, Yu D, Havrilesky LJ, Soper JT, Berchuck A, Spasojevic I, Clarke-Pearson DL, Prosnitz LR, Dewhirst MW. Phase I/II trial of intravenous Doxil and whole abdomen hyperthermia in patients with refractory ovarian cancer. Int J Hyperthermia 2005; 21: 333–347
- Babincova M, Altanerova V, Altaner C, Cicmanec P, Babinec P. In vivo heating of magnetic nanoparticles in alternating magnetic field. Med Phys 2004; 31: 2219–2221
- Babincova M, Cicmanec P, Altanerova V, Altaner C, Babinec P. AC-magnetic field controlled drug release from magnetoliposomes: Design of a method for site-specific chemotherapy. Bioelectrochemistry 2002; 55: 17–19
- Li GP, Zhang H, Zhu CM, Zhang J, Jiang XF. Avidin-biotin system pretargeting radioimmunoimaging and radioimmunotherapy and its application in mouse model of human colon carcinoma. World J Gastroenterol 2005; 11: 6288–6294
- Colbern GT, Hiller AJ, Musterer RS, Working PK, Henderson IC. Antitumor activity of Herceptin in combination with STEALTH liposomal cisplatin or nonliposomal cisplatin in a HER2 positive human breast cancer model. J Inorg Biochem 1999; 77: 117–120
- Muggia FM, Fojo T. Platinums: Extending their therapeutic spectrum. J Chemother 2004; 16: 77–82
- Livingston RB. Combined modality therapy of lung cancer. Clin Cancer Res 1997; 3: 2638–2647
- Griffin RJ, Lee SH, Rood KL, Stewart MJ, Lyons JC, Lew YS, Park H, Song CW. Use of arsenic trioxide as an antivascular and thermosensitizing agent in solid tumors. Neoplasia 2000; 2: 555–560
- Masunaga S, Ono K, Hori H, Kinashi Y, Suzuki M, Takagaki M, Kasai S, Nagasawa H, Uto Y. Modification of tirapazamine-induced cytotoxicity in combination with mild hyperthermia and/or nicotinamide: Reference to effect on quiescent tumour cells. Int J Hyperthermia 1999; 15: 7–16
- Akaboshi M, Tanaka Y, Kawai K, Akuta K, Masunaga S, Ono K. Effect of hyperthermia on the number of platinum atoms binding to DNA of HeLa cells treated with 195mPt-radiolabelled cis-diaminedichloroplatinum(II). Int J Radiat Biol 1994; 66: 215–220
- Masunaga S, Nagata K, Suzuki M, Kashino G, Kinashi Y, Ono K. Inhibition of repair of radiation-induced damage by mild temperature hyperthermia, referring to the effect on quiescent cell populations. Radiat Med 2007; 25: 417–425
- Xu M, Myerson RJ, Xia Y, Whitehead T, Moros EG, Straube WL, Roti JL. The effects of 41°C hyperthermia on the DNA repair protein, MRE11, correlate with radiosensitization in four human tumor cell lines. Int J Hyperthermia 2007; 23: 343–351
- Raaphorst GP, Maio J, Ng CE, Stewart DJ. Concomitant treatment with mild hyperthermia, cisplatin and low dose-rate irradiation in human ovarian cancer cells sensitive and resistant to cisplatin. Oncol Rep 1998; 5: 971–977
- Raaphorst GP, Miao J, Stewart D, Ng CE. Interactions of mild hyperthermia, cisplatin and split dose irradiation in human ovarian carcinoma cells. Cancer Chemother Pharmacol 1998; 41: 491–496
- Sunderland CJ, Steiert M, Talmadge JE, Derfus AM, Barry SE. Targeted nanoparticles for detecting and treating cancer. Drug Development Research 2006; 67: 70–93
- Douwes F, Bogovi CJ, Douwes O, Migeod F, Grote C. Whole-body hyperthermia in combination with platinum-containing drugs in patients with recurrent ovarian cancer. Int J Clin Oncol 2004; 9: 85–91
- Kusumoto T, Holden SA, Ara G, Teicher BA. Hyperthermia and platinum complexes: Time between treatments and synergy in vitro and in vivo. Int J Hyperthermia 1995; 11: 575–586
- Westermann AM, Grosen EA, Katschinski DM, Jager D, Rietbroek R, Schink JC, Tiggelaar CL, Jager E, Zum Vorde sive Vording P, Neuman A, Knuth A, Van Dijk JD, Wiedemann GJ, Robins HI. A pilot study of whole body hyperthermia and carboplatin in platinum-resistant ovarian cancer. Eur J Cancer 2001; 37: 1111–1117