Abstract
Purpose: The purpose of this study was to investigate the effect of local chemoimmunotherapy and hyperthermal intraperitoneal chemotherapy (HIPEC) in a mouse model of induced peritoneal carcinomatosis.
Material and methods: Peritoneal carcinomatosis in mice was produced by intraperitoneal implantation of MCa cells (5 × 103). Interleukin-2 (4.1 × 104 IU/mouse) was injected into the abdominal cavity of mice at day 7 and 3 before implantation of tumour cells. Immediately after implantation of MCa cells mice were treated twice with 2 ml of saline that was heated either at 37°C or 43°C and cytostatics (doxorubicin 20 mg kg−1, cisplatin 10 mg kg−1, mitomycin 5 mg kg−1, or 5-FU 150 mg kg−1). We followed the survival of animals and side effects appearing with different forms of treatment.
Results: Combined treatment with Interleukin-2 (IL-2) and cytostatics (5-FU, CIS or MIT) significantly affected the development of peritoneal carcinomatosis and increased the survival of mice (ILS% – 37°C = 29.88, 199.32, and 108.52, ILS% – 43°C = 62.69, 260.50, and 178.05, respectively). However, intraperitoneal chemotherapy on survival time of mice with DOX + IL-2 was ineffective as compared with DOX alone.
Conclusion: We would like to stress that treatment with IL-2 prior to tumour diagnosis is not clinically practical, rather, the manuscript attempts to describe an experimental proof of principle. Results suggest the synergistic effect of hyperthermia, chemotherapy and immunotherapy; IL-2 significantly increases antitumor activity of hyperthermic chemotherapy and survival rate of mice with peritoneal carcinomatosis.
Introduction
Peritoneal carcinomatosis (PC) represents an advanced form of cancer in patients with intestinal and stomach carcinoma as well as other gastrointestinal and gynaecologic tumours and is associated with a poor prognosis and quality of life. Advances in surgical techniques and improved anaesthesiology have made it possible to remove most or all macroscopic tumour in PC Citation1. However, the problem of microscopic residues still remains. Another problem is a markedly high PC risk during an advanced tumour surgery when tumour cells ‘drop off’ from the tumour surface affecting the serosa of the abdominal organ and causing iatrogenic PC. The median survival time after manifestation of peritoneal carcinomatosis is about six months and the five years survival period is less than 2%. If PC is present, normally there is no curative treatment available for any of the tumours in the abdominal cavity. Peritoneal seeding from gastrointestinal cancers is less responsive to either therapeutic approach. The main cytotoxic chemotherapy regimens include the use of doxorubicin, 5-fluorouracil and cisplatin Citation2–4, but their usage is limited by toxicity. Treatments such as combinations of chemoimmunotherapy and hyperthermic intraperitoneal chemotherapy (HIPEC), capable of combining high efficacy against peritoneal carcinomatosis with low toxicity, are needed to significantly affect the prognosis of patients with this disease. HIPEC is a treatment modality and is regarded as one of the best options for the therapy for peritoneal metastasis from gastrointestinal carcinoma. Hyperthermia is known to enhance the cytotoxicity of some chemotherapeutics and to increase the penetration depth, and is, therefore, considered to be a useful addition Citation5,Citation6. Intraperitoneal chemotherapy has the advantage of a high local concentration of the cytostatic drugs with less systemic exposure compared to conventional intravenous drug administration and limited systemic side effects Citation7,Citation8. The efficacy of intraperitoneal chemotherapy is synergistic with hyperthermia. Although the technique of hyperthermic intraperitoneal chemoperfusion in humans has been employed in cancer therapy, controlled studies using this approach are rare.
Literature reports lack objectivity and the adequate number of tests.
The aim of this study was to analyse and compare the validity of different models of intraperitoneal chemotherapy and HIPEC in an animal model of induced PC in order to find therapeutic as well as prophylactic modalities which would possibly ward off or at least slow down the occurrence and the development of peritoneal carcinomatosis.
Material and methods
Animals
Male and female CBA inbred mice two to three months old, weighing 20 to 25 g, obtained from the Department of Animal Physiology, Faculty of Science, University of Zagreb, were used in this study. The animals were kept in individual cages during the experiment and at 12 hours of light per day. They were fed a standard laboratory diet (4 RF 21, Mucedola, Settimo Milanese, Italy) and tap water ad libitum. Maintenance and care of all experimental animals were carried out according to the guidelines in force in Republic of Croatia (Law on the Welfare of Animals, N.N. 19, 1999) and carried out in compliance with the Guide for the Care and Use of Laboratory Animals, DHHS Publication (NIH) 86-123.
Tumour
A transplantable mammary carcinoma (MCa) of spontaneous origin in CBA mouse was used. The tumour is weakly immunogenic for syngeneic recipients as shown by skin grafting Citation9.
Tumour cell suspension
Single cell suspensions were prepared by digestion of tumour tissue with trypsin and DNase Citation10. Each suspension was passed through a stainless steel mesh (200 wires/inch), centrifuged three times at 24 g for 5 min in saline and then resuspended in medium RPMI 1640 (Institute of Immunology, Zagreb) supplemented with 5% serum from normal syngeneic mice. Viability of cells was determined in a haemocytometer by observing the ability of intact cells to exclude Trypan blue dye and by phase contrast microscopy; viability was over 95%.
Cytostatics
5-fluorouracil (Fluorouracil) was purchased from Hoffman-La Roche, Basel, Switzerland. It was injected into mice intraperitoneally at doses of 150 mg kg−1. Doxorubicin (Rastocin), product of Pliva, Zagreb, Croatia, was injected into mice intraperitoneally at doses of 20 mg kg−1. Mitomycin (Mutomycin) was obtained from Bristol Laboratories, Syracuse, New York, and was injected into mice intraperitoneally at doses 5 mg kg−1. Cisplatin (Platimid) was purchased from Pliva, Zagreb, Croatia, and was injected into mice intraperitoneally at doses 10 mg kg−1.
Animal treatments and experimental design
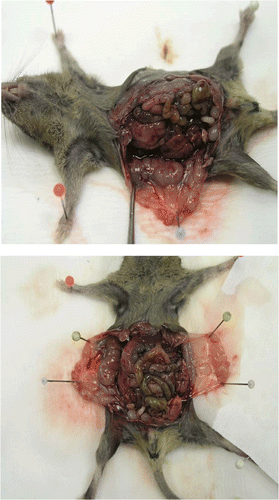
Peritoneal carcinomatosis () was generated by intraperitoneal injection of 5 × 103 viable tumour cells. At day 7 and 3 before implantation of MCa cells (5 × 103) mice were injected intraperitoneally with 4.1 × 104 IU/mouse of IL-2 (Boehringer, Manheim, Germany). Immediately after implantation of MCa cells, 2 ml of saline heated to either 37°C or 43°C (hyperthermal treatment) were injected intraperitoneally twice, 5 min apart, followed by cytostatics (doxorubicin 20 mg kg−1, cisplatin 10 mg kg−1, mitomycin 5 mg kg−1, 5-FU 150 mg kg−1) in 0.5 ml after the second hyperthermal treatment. The control animals were untreated tumour bearing mice.
Figure 1. Peritoneal carcinomatosis in CBA mice. Mice were injected intraperitoneally with 5 × 103 mammary carcinoma (MCa) cells.
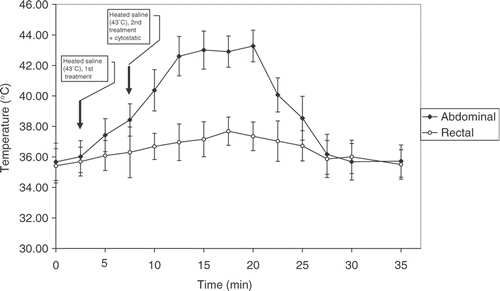
Systemic body temperature was determined using an electronic thermometer (BAT-10, Physitemp Instruments, New Jersey) with a rectal probe (RET-3, Physitemp Instruments) before and during hyperthermia procedure. Intra-abdominal temperature was measured by the needle probe introduced to a depth of 1 cm into peritoneum cavity of mice treated with hyperthermia, given alone or in combination with cytostatic. Drugs were given immediately after the last treatment of hyperthermia. The temperature was recorded, in peritoneal carcinomatosis-bearing mice in each group, every 2.5 min during the heating phase and every 2.5 min during the cooling phase. Hyperthermia with applied procedure was well tolerated by the animals. We followed the survival of animals and the side effects appearing after different treatments.
Survival analysis
Animal life span was evaluated by surveillance of spontaneous death or by elective killing of animals showing signs of pain or suffering according to established criteria. Long surviving mice (living more than 95 days) were euthanized on the 95th day by an overdose of anaesthetic and cervical dislocation. The results were expressed as a percentage of average life span of animals that were used (T) divided by average life span of animals in the control group (C) (T/C%). Percentage of increased life span (ILS%) was calculated with the formula ILS% = (T−C)/C × 100. According to the criteria of the National Cancer Institute, T/C above 125% and ILS above 25% means that treatment had significant anti-tumour effect Citation11.
Statistical analysis
Data were analysed using package STATA 7.0 (Stata Press, College Station, Texas) statistical software. The mean scores were calculated. Survival curves were computed according to the method of Kaplan and Meier Citation12, and comparison between survival curves was made by log rank test (α = 5%) Citation13. A p-value <0.05 was considered to be significant.
Results
IL-2 given alone with or without hyperthermia did not inhibit the development of peritoneal carcinomatosis. However, IL-2 combined with hyperthermal intraperitoneal chemotherapy (CIS, 5-FU, or MIT) significantly prolonged the survival time of mice (, ) as compared with single treatments of mice with cytostatics. Interestingly, single treatment with DOX was more effective than the combination DOX + IL-2 (). The most pronounced effect on the development of carcinomatosis was achieved by combination of IL-2, cisplatin and hyperthermia at 43°C; ILS% at 37°C = 199.32; ILS% at 43°C = 260.50 (); 5 mice treated with HIPEC versus 3 mice without HIPEC were long time survivors (p = 0.01852; p = 0.06232, Kaplan-Meier analysis).
Figure 2. Kaplan-Meier survival curves of CBA mice with peritoneal carcinomatosis treated with cytostatic under normothermic and hypertermic conditions. Mice were treated preventively intraperitoneally with interleukin-2 (IL-2; 4.1 × 104 IU) at day 7 and 3 before tumour cell inoculation (5 × 103 cells of MCa). Cytostatics were administered intraperitoneally immediately after tumour cell inoculation in the following concentration: CIS, 10 mg kg−1; DOX, 20 mg kg−1; 5-FU, 150 mg kg−1; MIT, 5 mg kg−1. The results of log rank test between test components and control at 37°C show (IL-2, p = 0.01224; IL-2 + CIS, p = 0.01852; CIS, p = 0.25614; IL-2 + DOX, p = 0.01816; DOX, p = 0.00873; IL-2 + 5-FU, p = 0.06418; 5-FU, p = 0.52018; IL-2 + MIT, p = 0.62784; MIT, p = 0.22411). The results of log rank test between test components and control at 43°C show (IL-2, p = 0.00591; IL-2 + CIS, p = 0.06234; CIS, p = 0.09850; IL-2 + DOX, p = 0.06442; DOX, p = 0.00917; IL-2 + 5-FU, p = 0.03304; 5-FU, p = 0.80534; IL-2 + MIT, p = 0.02554; MIT, p = 0.02554). Results of survival rate of mice are expressed as the means (n = 7) of each group and are representative of two independent experiments.
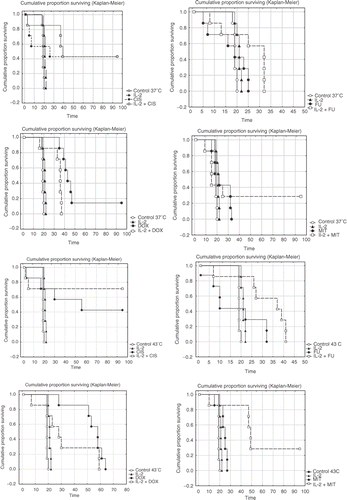
The most effective cytostatic treatment without HIPEC was achieved with doxorubicin (p = 0.00873).
Table I. Preventive treatment of peritoneal carcinomatosisa with IL-2b and cytostaticsc.
It is important to mention that during the heating phase intra-abdominal temperature was stable, ranging from 42.5–43°C, while rectal temperature was not significantly affected. During the cooling phase the temperature in the peritoneal cavity of treated mice decreased to normal values approximately 15 minutes after hyperthermal procedure ().
Discussion
This study was designed to answer the question of whether the combination of immunotherapy and HIPEC improves the survival of mice and which therapeutic model would prevail in the prevention of PC in mice. It is known that cytostatics is frequently implicated in immunological disorders and mostly decreases host immunity contributing to the increased risk of cancer. Immunotherapy (IT) has become an accepted therapeutic modality. IL-2 is a potent stimulator of lymphocyte proliferation and augments the activity of cytotoxic T lymphocytes (CTLs) Citation14. IL-2 has a broad range of immunologic effects, such as induction of specific T helper cells, natural killer cells and lymphokine-activated killer cells Citation15. Due to these effects, IL-2 is widely used in cancer therapy to enhance cellular immunity and the cytotoxic activity of effector cells Citation16. IL-2 preventive treatment of mice with intraperitoneal carcinomatosis was chosen in accordance with our previous findings where the most effective immunomodulatory treatment was found to be day 7 and 3 before tumour cell inoculation Citation10,Citation17 which is in accordance with findings described in papers by Ben Salah-Abbès et al. and Nanni et al. Citation18,Citation19.
IL-2 alone did not inhibit the PC. This is in correlation with the study of Ehrke et al. Citation20 showing that IL-2 alone at very high doses has limited clinical efficacy against certain tumour types. On the other hand, they observed that at lower, more physiologic doses, IL-2 stimulated potential anticancer immune responses Citation20, but it was unlikely that it had a direct effect on the tumour. This suggested that the prolonged IL-2 treatment in the combination therapy would decrease the percentage of mice that relapse rather than to increase the percentage of mice that respond.
This study confirmed that preventive applications of IL-2 at day 7 and 3 before tumour cell application in order to activate the immune anticancer response) in combination with certain cytostatic exerted remarkable anticancer effect. The most pronounced effect on animal survival was achieved with the combination of IL-2 and cisplatin when combined with hyperthermia; enhancement of cytotoxicity in combination with hyperthermia and synergistic activity of IL-2 and cisplatin should be considered to be the case as suggested by Citation21–23. According to findings described in Citation3 the synergistic effect between IL-2 and cisplatin is the highest during simultaneous application as was also shown in this study. From our results it is obvious that hyperthermia increased sensitivity of tumour cells to cisplatin (the chemo-sensitisation effect).
Single treatment with cisplatin or doxorubicin without IL-2 also exerted remarkable effects in the prevention of PC in treated mice. Similar findings were presented by de Bree et al. Citation24. Nevertheless, though a couple of mice treated with cisplatin and IL-2 with or without hyperthermal treatment unexpectedly died in the early phase after treatment, we considered this treatment to be the most successful since the early mortality in this experimental group could be prescribed to the cytostatic toxicity. This is particularly important for HIPEC since this treatment increases activity of the drugs (especially cisplatin), giving strong anticancer effect on one hand, while on the other hand rapidly increasing toxicity. This suggests the benefit of decreasing drug dosage, or its sequential application during long period of time as suggested in Lindegaard et al. and Marmor Citation3,Citation25. This is consistent with understandings that hyperthermia is an effective way of local drug delivery.
The combination of mitomycin and IL-2 prolonged survival of animals. The combination of intraperitoneal IL-2 immunotherapy and thermochemotherapy could promote the Th1 immune response and enforce the anti-tumour activity, which plays a positive role in preventing PC as suggested by Fu et al. Citation26. However, in our study, mitomycin showed very low anticancer effect in treated mice (). Opposite to our findings some authors reported that monotherapy with mitomycin influenced survival of patients Citation27,Citation28.
In this study we observed that intraperitoneal application of 5-FU alone did not show any significant anticancer effect in treatment of PC in mice. Although 5-FU alone or combined with leukovorin is used as a way of treatment of patients with PC Citation27,Citation29. This raises a question whether to use this drug as monotherapy in clinical practice. The reason for such a low effect of 5-FU could be the intraperitoneal route of application, because for the activity of this drug and for the anticancer effect, biotransformation of the drug is required. Doxorubicin as a single treatment was equally effective concerning the survival of mice at 37°C or 43°C (ILS = 168.20 or 178.42). One or two injections of doxorubicin at moderate doses increases the activity of macrophages, natural killer cells, lymphokine-activated killer cells and increases the production of IL-2, both in mice and in humans Citation20,Citation30,Citation31. Results from these studies suggest that the combination of hyperthermia and DOX may be in agreement with the studies by Newman et al. Citation32 concerning plasma drug clearance, and for this does not account for the significant increase in thermochemotherapy-mediated cytotoxicities to tumour cells. Other explanations may include an impaired mechanism of DOX activity to inhibit DNA topoisomerase II which is critical to DNA function Citation33. Doxorubicin damages DNA by intercalation of the anthracycline portion, metal ion chelation, generation of free radicals or by inhibition of DNA topoisomerase II. However, when doxorubicin was combined with IL-2, there was no effect on survival time of mice as compared to doxorubicin alone (). These results are not in agreement with studies of Ewens et al. Citation4 showing that doxorubicin plus IL-2 immunomodulation-based anticancer therapy is curative in C57BL/6 mouse model of breast cancer. Ehrke et al. Citation34 also suggested that effective anticancer immune response depended on the dose and the schedule of both doxorubicin and IL-2, and required prolonged IL-2 administration.
This study represents a contribution in evaluation of local chemoimmunotherapy on prevention of PC in mice. Also it showed how some locoregional treatment could provide a good value in prevention of PC. This is in concordance with the findings that intraperitoneal application of high concentration of cytostatics directly to tumour side insures better antitumor effectiveness which is not possible with systematic cytostatics application Citation2,Citation35,Citation36. In our opinion it is likely that stimulative effect of IL-2 on immunomodulation may be a possible mechanism which protects mice from development of peritoneal carcinomatosis and reduction of side effects of chemotherapy, increasing life span of mice.
Our study showed that the treatment with HIPEC did not affect the systemic (rectal) temperature in treated mice (), while intraperitoneal temperature after treatment with 4 ml (2 + 2 ml) of heated (43°C) saline elevated during the period of approximately 15 minutes after the completition of the treatment. It is also of importance to point out that the cytostatic in 0.5 ml of solution at room temperature did not affect the duration and the stability of the temperature in the peritoneal cavity of treated animals. The main reason for elevation of intraperitoneal temperature without significant raising of systemic temperature after application of 4 ml of 43°C saline over ten minutes in a mouse may be the conductivity of body tissues. Tissues with higher conductivity reach higher temperature when exposed to the heated saline. Because the conductivities of fat, skin, and blood are all low (0.04, 0.1, and 0.7, respectively), the excess elevation in temperature during exposure to the heated saline was minimal and not significant. Nevertheless, the conductivity of the body could have had some influence on rectal temperature, which was elevated to a small and variable degree during exposure to the heated saline, although it remained at approximately 37°C in treated mice. The delay in reaching the systemic temperature in vivo could reflect heat loss caused by blood flow in the peritoneal cavity of a mouse as well as the rate of heart pumping and breathing (the mouse heart rate per minute is 300–800 beats and respiratory rate is 215–230 per minute as compared to 70 and 12 in humans, respectively.
Conclusion
Absolutely, cisplatin alone, or in combination with IL-2 and hyperthermia was the most successful in this treatment of PC in mice. Results from these studies suggest that combination of hyperthermia and cytostatic drugs offers the possibility of reduction of dose of chemotherapeutics by which the toxic effects of the drug could be avoided, still keeping anticancer effect. It is of importance that treatment with IL-2 prior to tumour diagnosis is not clinically practical, rather, the manuscript attempts to describe an experimental proof of principle.
Many questions remain unanswered. This experimental study combined two treatment elements: chemoimmunotherapy and hyperthermia. Whether the combination of these treatment modalities was required for the survival benefit is unclear, but it seems certainly promising for further outcome improvement.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003; 21: 3737–3743
- Sugarbaker PH, Gianola FJ, Speyer JL, Wesley R, Barofsky I, Myers CE. Prospective randomized trial of intravenous versus intraperitoneal 5-FU in patients with advanced primary colon or rectal cancer. Semin Oncol 1985; 12: 101–111
- Lindegaard JC, Radacic M, Khalil AA, Horsman MR, Overgaard J. Cisplatin and hyperthermia treatment of a C3H mammary carcinoma in vivo. Importance of sequence, interval, drug dose, and temperature. Acta Oncol 1992; 31: 347–351
- Ewens A, Luo L, Berleth E, Alderfer J, Wollman R, Hafeez BB, Kanter P, Mihich E, Ehrke MJ. Doxorubicin plus interleukin-2 chemoimmunotherapy against breast cancer in mice. Cancer Res 2006; 66: 5419–5426
- Hahn GM, Braun J, Har-Kedar I. Thermochemotherapy: Synergism between hyperthermia (42-43 degrees) and adriamycin (of bleomycin) in mammalian cell inactivation. Proc Natl Acad Sci USA 1975; 72: 937–940
- Storm FK. Clinical hyperthermia and chemotherapy. Radiol Clin North Am 1989; 27: 621–627
- Los G, Mutsaers PH, van der Vijgh WJ, Baldew GS, de Graaf PW, McVie JG. Direct diffusion of cis-diamminedichloroplatinum(II) in intraperitoneal rat tumors after intraperitoneal chemotherapy: A comparison with systemic chemotherapy. Cancer Res 1989; 49: 3380–3384
- Los G, Sminia P, Wondergem J, Mutsaers PH, Havemen J, ten Bokkel Huinink D, Smals O, Gonzalez–Gonzalez D, McVie JG. Optimisation of intraperitoneal cisplatin therapy with regional hyperthermia in rats. Eur J Cancer 1991; 27: 472–477
- Basic I, Varga E. Immunogenicity of mammary carcinoma and a fibrosarcoma of CBA mice. Period Biol 1979; 81: 335–337
- Oršolić N, Bašić I. Immunomodulation by water-soluble derivative of propolis (WSDP) a factor of antitumor reactivity. J Ethnopharmacol 2003; 84: 265–273
- Plowman J, Dykes DJ, Hollingshead M. Anticancer drug development guide: Preclinical screening, clinical trials, and approval. Humana, Totowa, NJ 1995; 101
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–465
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22: 719–748
- Farrar WL, Johnson HM, Farrar JJ. Regulation of the production of immune interferon and cytotoxic T lymphocytes by interleukin 2. J Immunol 1981; 126: 1120–1125
- Rosenberg SA, Grimm EA, McGrogan M, Doyle M, Kawasaki E, Koths K, Mark DF. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science 1984; 223: 1412–1414
- Hadden JW. Recent advances in the preclinical and clinical immunopharmcology of interleukin-2: Emphasis on IL-2 as an immunorestorative agent. Cancer Detect Prev 1988; 12: 537–552
- Oršolić N, Horvat Knežević A, Šver L, Terzić S, Bašić I. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. J Ethnopharmacol 2004; 94: 307–315
- Ben Salah-Abbès J, Abbès S, Houas Z, Abdel-Wahhab MA, Oueslati R. Zearalenone induces immunotoxicity in mice: Possible protective effects of radish extract (Raphanus sativus). J Pharm Pharmacol 2008; 60(6)761–770
- Nanni P, Nicoletti G, Palladini A, Croci S, Murgo A, Antognoli A, Landuzzi L, Fabbi M, Ferrini S, Musiani P, Jezzi M, De Giovanni C, Lollini PL. Antimetastatic activity of a preventive cancer vaccine. Cancer Res 2007; 67(22)11037–11044, 12034
- Ehrke MJ. Immunomodulation in cancer therapeutics. Int Immunopharmacol 2003; 3: 1105–1119
- Sugarbaker PH. A curative approach to peritoneal carcinomatosis from colorectal cancer. Semin Oncol 2005; 32: S68–73
- Freedman RS, Lenzi R, Kudelka AP, Lawrence DD, Rosenblum M, Platsoucas CD. Intraperitoneal immunotherapy of peritoneal carcinomatosis. Cytokines Cell Mol Ther 1998; 4: 121–140
- Zylberberg B, Dormont D, Janklewicz S, Darai E, Bretel JJ, Poncelet C, Guillet JL, Madelenat P. Response to neo-adjuvant intraperitoneal and intravenous immunochemotherapy followed by interval secondary cytoreduction in stage IIIc ovarian cancer. Eur J Gynaecol Oncol 2001; 22: 40–45
- de Bree E, Witkamp AJ, Zoetmulder FA. Intraperitoneal chemotherapy for colorectal cancer. J Surg Oncol 2002; 79: 46–61
- Marmor JB. Interactions of hyperthermia and chemotherapy in animals. Cancer Res 1979; 39: 2269–2276
- Fu QG, Meng FD, Shen XD, Guo RX. Efficacy of intraperitoneal thermochemotherapy and immunotherapy in intraperitoneal recurrence after gastrointestinal cancer resection. World J Gastroenterol 2002; 8(6)1019–1022
- Comella P, Crucitta E, De Vita F, De Lucia L, Farris A, Del Gaizo F, Palmeri S, Lannelli A, Mancarella S, Tafuto S, et al. Addition of either irinotecan or methotrexate to bolus 5-fluorouracil and high-dose folinic acid every 2 weeks in advanced colorectal carcinoma: A randomised study by the Southern Italy Cooperative Oncology Group. Ann Oncol 2002; 13: 866–873
- Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, Ramanathan RK, Hart LL, Gupta S, Garay CA, Burger BG, et al. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: Interim results of a phase III trial. J Clin Oncol 2003; 21: 2059–2069
- Esquivel J, Vidal-Jove J, Steves MA, Sugarbaker PH. Morbidity and mortality of cytoreductive surgery and intraperitoneal chemotherapy. Surgery 1993; 113: 631–636
- Ehrke MJ, Mihich E, Berd D, Mastrangelo MJ. Effects of anticancer drugs on the immune system in humans. Semin Oncol 1989; 16: 230–253
- Mihich E. Historical overview of biologic response modifiers. Cancer Invest 2000; 18: 456–466
- Newman RA, Dogramatzis D, Benvenuto JA, Trevino M, Stephens LC, Wondergem J, Strebel R, Baba H, Bull JM. Effect of whole-body hyperthermia on pharmacokinetics and tissue distribution of doxorubicin. Int J Hyperthermia 1992; 8(1)79–85
- Momparler RL, Karon M, Siegel SE, Avila F. Effect of adriamycin on DNA, RNA and protein synthesis in cell-free systems and intact cells. Cancer Research 1976; 36: 2891–2895
- Ehrke MJ, Verstovsek S, Zaleskis G, Ho RL, Ujhazy P, Maccubbin DL, Mihich E. Specific anti-EL4-lymphoma immunity in mice cured 2 years earlier with doxorubicin and interleukin-2. Cancer Immunol Immunother 1996; 42: 221–230
- Nomura E, Niki M, Fujii K, Shinohara H, Nishiguchi K, Sonoda T, Tanigawa N. Efficacy of intraperitoneal and intravenous chemotherapy and left upper abdominal evisceration for advanced gastric cancer. Gastric Cancer 2001; 4: 75–82
- McQuellon RP, Loggie BW, Fleming RA, Russell GB, Lehman AB, Rambo TD. Quality of life after intraperitoneal hyperthermic chemotherapy (IPHC) for peritoneal carcinomatosis. Eur J Surg Oncol 2001; 27: 65–73