Abstract
Purpose: To evaluate and numerically score histological alterations observed in the acute phase in the esophagus after being exposed to a hyperthermic dosage and subsequently to correlate the scores obtained with the hyperthermic treatment parameters (i.e. temperature (T) and time (t)).
Material and methods: Esophagus samples obtained from New Zealand white rabbits were immersed in a temperature-controlled saline bath at 40, 50, 60 and 70°C for 30, 60 and 90 s. Samples were then processed for histological analysis (Masson Trichrome technique), and evaluated by searching for objective heat-damage signs. A numerical value was assigned to each sample for each finding.
Results: In general, all the layers were affected by the treatment, however, the greatest alterations were found in the epithelium and deeper muscular layers (circular and longitudinal). We found no damage (i.e. no differences to control) in all of the samples treated at 40°C, and severe damage in treatments at 60 and 70°C, regardless of exposure time. On the other hand, samples treated at 50°C did show different results related to time: no damage for 30 s, light damage for 60 s, and moderate damage for 90 s. We assigned a score value to each hyperthermic dosage, and obtained the fitted equation based on a logarithmic transformation of the Arrhenius equation: Score = 130.7 – 40,851/(T + 273) + log t, (R2 = 0.9326, P < 0.0001).
Conclusions: Hyperthermic treatment mainly affects the epithelium and deeper muscular layers. The results suggest a damage threshold of 50°C for treatments of 30–90 s. The proposed scoring system provides a good fit with the hyperthermic parameters.
Introduction
Ablation of cardiac tissue for the treatment of atrial fibrillation by any hyperthermic energy source (radiofrequency, microwave, laser or ultrasound) not only creates a thermal lesion in the atrial tissue, but may also potentially cause dangerous hyperthermic exposure of the proximal tissues (connective tissue and esophagus). Most living cells and tissues can tolerate and survive moderate temperature increments for a limited time, depending on the species and the metabolic status of the individual, but when thermal dosage (i.e. temperature × exposure time) reaches critical values, disorganization of tissue takes place, leading to focal necrosis. Thermal damage mechanisms are mostly responsible for injuries produced by cardiac ablation Citation1. Recent studies on radiofrequency cardiac ablation for the treatment of atrial fibrillation reported various cases of thermal injury to the esophagus Citation2–4. This type of injury may, in turn, lead to serious adverse effects including esophageal perforation and even death. In addition, this complication seems inherent in the use of any hyperthermic energy source Citation5. Different techniques have been proposed to minimize the risk of thermal injury, such as the use of a cooled intra-esophageal balloon in the esophagus Citation6,Citation7. However, to date, there are no histological studies which accurately characterize the morphological alterations in the esophagus after hyperthermic treatment. In particular, there is no work relating thermal dosage (i.e. temperature × exposure time) with the morphological changes observed in the different layers of the esophagus (epithelium, mucosa, submucosa and muscular). The objectives of this study were therefore: (1) To evaluate and numerically score the histological alterations observed in the acute phase in the esophagus after being exposed to a hyperthermic dosage, and (2) To correlate the scores obtained in the histological study with the hyperthermic treatment parameters (i.e. temperature and time).
Material and methods
Obtaining samples
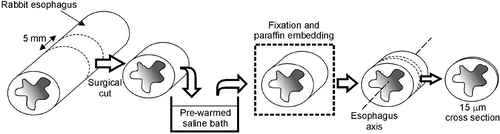
Esophagus tissue from six healthy, male, white New Zealand rabbits was used (1.8–1.9 kg). All animals received treatment in compliance with the guidelines of the European Union's directive for the protection of animals used for experiments and other scientific purposes (Normative No. 609) and the ‘Guide to the Management of Experimental Animals’ from the Spanish National Research Council (CSIC). Previous to surgery, animals were sedated by subcutaneous injection of 2-3 mL of xylacine hydrochloride (Rompun®, Bayer, Barcelona, Spain) and then sacrificed with an intra-cardiac sodium pentobarbital overdose (Dolethal®, Ventoquinol, Cedex, France). To facilitate esophageal extraction, a 3 mm diameter nasoqastric tube was inserted through the mouth. We conducted a medial thoracotomy to expose the thorax, and then extracted the esophagus along with the other organs of the mediastinum by the standard Virchow technique. The esophagus was then separated from the other organs by careful blunt dissection, greatly facilitated by the previously inserted nasogastric tube. The esophagus was then washed with saline at room temperature and transversal sections were performed by scalpel, obtaining pieces 5 mm in length (). These pieces of fresh excised esophagus were employed as samples for the hyperthermia treatment protocols that were always conducted less than 20 min after sacrifice. Although there are differences between distal and proximal esophagus with regard to muscle fibers in the human esophagus (smooth versus striated), we did not distinguish these zones in our study, since all the analysed rabbit samples showed only striated muscle.
Figure 1. General set-up of the study. Esophagus samples from healthy male white New Zealand rabbits were employed. Once the esophagus was extracted it was washed with saline at room temperature and transversal sections were performed by scalpel, obtaining pieces 5 mm in length. These pieces of fresh excised esophagus were employed as samples for the hyperthermia treatment protocols (controlled dosage of constant temperature × exposure time) by submerging in a pre-warmed saline bath. The treated samples were then fixed by overnight immersion in 10% paraformaldehyde in PBS, and later a standard procedure was conducted based on paraffin embedding and 15 µm thick axial cross sections. These sections were perpendicular to the esophagus axis in order to assess all layers homogeneously. The samples were finally stained by the Masson Trichrome technique.
Hyperthermia treatments
In order to simulate acute hyperthermic injury in all the layers of the esophagus, we employed a model based on a controlled dosage of constant temperature × exposure time. We are aware that this heating model is different to that found in the clinical stage, where there is a thermal gradient in the muscular–mucosa. However, the objective of this study was to evaluate and score the histological alterations in the different esophagus layers after being exposed to the same hyperthermic dosage.
The protocol was based on submerging the esophagus samples in a pre–warmed saline bath. The bath temperature was controlled by means of a WB14 thermostat (Memmert GmbH & Co. KG, Schwabach, Germany). We chose four values of bath temperatures (40, 50, 60 and 70°C) and three treatment times (30, 60 and 90 seconds), hence we considered 12 groups. Samples of esophagus were randomly distributed throughout the 12 groups (3–4 samples by group). After each dosage, the treated sample was immediately submerged in a saline bath at room temperature in order to obtain a drastic drop in temperature. Finally, the control case was conducted by submerging esophagus samples in saline at 37°C for 60 seconds.
Histological analysis
The treated samples were fixed by overnight immersion in 10% paraformaldehyde in phosphate buffer saline (PBS). We conducted a standard procedure based on paraffin embedding and 15 µm thick axial cross sections. These sections were perpendicular to the esophagus axis in order to assess all the layers homogeneously (see ). The samples were then stained by the Masson Trichrome (TM) technique (Sigma Chemicals, St Louis, MO) using the manufacturer's standard protocol.
First, two authors (J.L.L. and E.S.) examined the samples for signs of tissue damage and classified them into four groups: undamaged, slightly damaged, moderately damaged and severely damaged. Although this first assessment was conducted independently and without imposing restrictions, it was considered too subjective and overview-oriented. Several techniques have been specifically developed to assess thermal damage in tissue, e.g. birefringence measurement by means of sirius red stain and subsequent examination under polarized light. This method has in fact been employed to assess thermal damage in arteries Citation8, and hence would probably be useful in assessing damage to the esophagus. However, we employed TM staining for conventional light microscopy, and then introduced a different histological scoring system, which was based on a similar system previously proposed for assessing thermal liver damage Citation9. Briefly, it consisted of assigning a numerical score for the following histological characteristics: alteration of tissue layer structure, focal destruction of tissues, integrity of sarcolemma, disruption of the fibers, tinctorial degeneration or necrosis of myocytes, intracellular and/or extracellular edema, presence of vacuoles and tearing and blurring of the epithelium. shows the histological alterations classified by location (whole piece, epithelium-submucosa, and muscular layers). Each alteration received a numerical score between 0 and the maximum points indicated in . The maximum score in a sample was 20 points. Additionally, two external pathologists who were unaware of the nature of the study also examined the same samples scoring the alterations according to the criteria detailed in .
Table I. System of scoring the hyperthermic damage based on the histological findings found in the esophagus. Each histological characteristic received a score (between 1 and maximum) depending on its severity and evidence.
Statistical analysis
We compared the scores assigned by the authors (J.L.L. and E.S.) and the external pathologists by means of a factor variance analysis (ANOVA), using SPSS version 15.0 software (SPSS, Chicago, IL). Hypothesis of normality and homocedasticity of score sets were previously checked by the Sapiro–Wilk normality test. Homogeneity of variance was checked by the Levene test. Statistical significance was considered to be P < 0.05.
Finally, we studied the relationship between the assigned scores and the two parameters which define the hyperthermic dosage, i.e. temperature (T) and time (t). The aim was to obtain a thermal damage function by means of a regression analysis. We used the Sigma Plot v.8.02 statistical graphing software (Systat Software, Chicago, IL).
Results
General assessment
and show representative microphotographic cross sections of esophagus after hyperthermic treatment. In general the treatment affected all the layers, however we found the greatest alterations in the epithelium and deeper muscular layers (circular and longitudinal). shows the results of the preliminary exam conducted by the two authors (J.L.L. and E.S.) in order to achieve an overall impression of perceived thermal damage after examining the images of the treated esophagus samples. We found no damage (i.e. no differences to control) in all samples treated at 40°C, and severe damage when treatment was at 60 and 70°C, regardless of exposure time. On the other hand, samples treated at 50°C did show different results in accordance with the time of exposure: no damage for 30 s, light damage for 60 s, and moderate damage for 90 s.
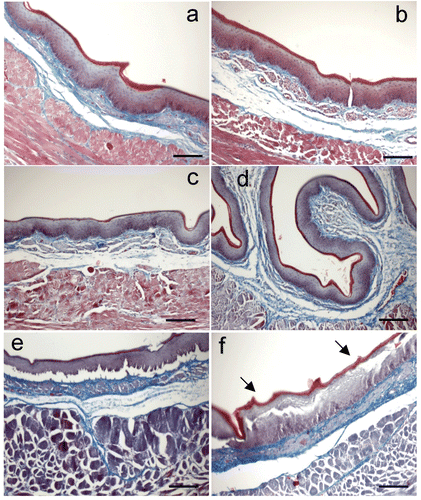
Figure 2. Microphotographs of esophageal epithelium (Masson Trichrome stained cross sections) exposed to different hyperthermic dosage: (a) 37°C × 60 s (i.e. control), (b) 40°C × 30 s, (c) 50°C × 30 s, (d) 60°C × 30 s, (e) 60°C × 90s, (f) 70°C × 30 s. Note that there were no alterations until thermal level of 60°C. These alterations mainly consisted of detachment of the epithelium at the level of the basal layer and tearing of upper layers (arrows). Bar: 100 µm.
Epithelium assessment
shows cross sections of the esophagus epithelia treated with different thermal dosages. Detachment between epithelium and submucosa unit was an artifact due to the different contractions of the tissue layers during fixation, and hence did not constitute a sign of thermal lesion. We found that temperatures of 40 and 50°C did not produce evidence of thermal damage in the epithelium (see ). In contrast, higher temperatures (60 and 70°C) caused disruption of the intercellular unions, especially at the prickle layer (stratum spinosum) (see f), and hence broke the continuous layer of epithelium. The upper layers of the epithelium (stratum lucidum and stratum granulosum) tolerated high temperatures much better and detached as a single unit. In addition, the outline of the cells tended to disappear, giving them a blurred appearance.
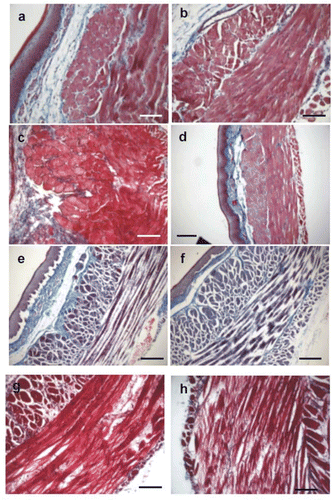
Figure 3. Microphotographs of muscular layers of esophagus (Masson Trichrome stained cross sections) exposed to different hyperthermic dosage: (a) control, 37°C × 60 s, (b) 40°C × 60 s, (c) 50°C × 30 s, (d) 50°C × 90 s, (e) 60°C × 30 s, (f) 60°C × 90 s, (g) 70°C × 60 s, and (H) 70°C × 90 s. First changes appeared at 50°C × 90 s (d) consisting mainly of variation of the tinctorial properties of the cells. Some areas appeared lighter than others. At thermal levels of 60 or 70°C, we observed signs of damage as alteration of architecture and integrity of sarcolemma, focal destruction of tissue, non-homogeneous coloration, alteration of staining characteristics, and disruption of the fibers and edema formation. Bars: 100 µm (a, b, c, g, h) and 200 µm (d, e, f).
Lamina propia and submucosa assessment
Regarding lamina propia and submucosa, it was very difficult to establish a pattern of thermal damage in this area. On occasions the architecture was preserved and at times not, but both patterns can be observed in specimens exposed to low temperatures as well as to high thermal dosages.
Table II. Subjective level of hyperthermic damage in the esophagus from the analysis of histological findings (n, no damage; +, light damage; ++, moderate damage; +++, severe damage).
Muscular layers assessment
shows cross sections of the esophagus muscular layers treated with different thermal dosages. We found striated muscle in all the samples studied. Visible striations appeared in the cytoplasm of the muscular cells of the circular layer, both in the control group and in samples heated at 70°C (data not shown). Control samples (a) and samples treated with low dosages () did not show significant changes in their architecture, either in the circular or longitudinal muscle layers. In particular, they presented two wide homogeneous pink continuous bands, corresponding to the longitudinal and circular layers, and separated by a thin layer of connective tissue. Nuclei were sharp and intensely basophilic. The separation between different cells was difficult to distinguish.
The first alterations appeared at a dosage of 50°C × 90 s (d) and consisted of a change in the tinctorial properties of the cells. Some areas appeared lighter than others (in this case, neighboring structures, such as the epithelium, were checked for uniform staining in order to avoid a bias due to irregular staining technique).
On the other hand, when samples were heated at temperatures of 60°C (f) or 70°C (h), damage signs gradually appeared in the muscular layer. The disorganization of the fibers due to hyperthermic damage changed the staining characteristics of individual or groups of cells in the form of loss of definition or increasing dye capture. Nuclei were difficult to distinguish. The general architecture of the muscular fiber was preserved, but thermal damage kept the fibers contracted inside the endomysium, reducing their diameter by about 50%. This effect could be clearly observed in the longitudinal layer when the fibers were cut axially (h). On occasions, the fibers presented a different aspect, when the small fibers of the circular layer inside the endomysium tended to separate and present a fringed appearance (h). These fibers did not show the tinctorial alteration tendency and appeared much lighter than the fibers described above. This effect was especially observed in the circular layer. Separation between these ‘fringed’ fascicles and total disorganization in the muscular layer was also occasionally detected.
Relationship between scored damage and hyperthermic dosage
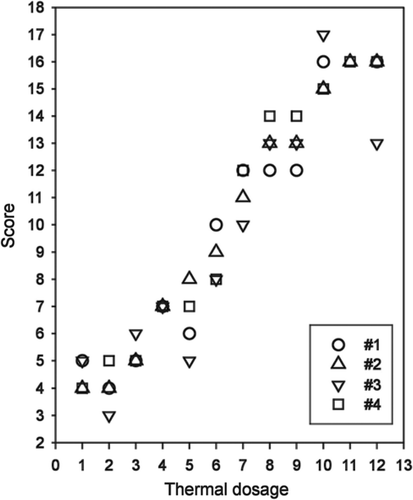
We studied the relationship between assigned scores and the two parameters which define the hyperthermic dosage, i.e. temperature (T) and time (t). Firstly, we employed the scoring system shown in to assign a general score value to each hyperthermic dosage. shows the score values obtained for each experimenter. The Shapiro–Wilk test for normality (P > 0.05) and the Levene test for homogeneity of variances (P = 0.904) confirmed the previous hypothesis of normality and homocedasticity. One-way analysis of variance (ANOVA) resulted in no statistical differences (P = 0.925) between score means, either between groups (two authors versus two outside pathologists) or within groups.
Figure 4. Score values obtained for each experimenter (#1–#4). Key for combinations of temperature and time (hyperthermic parameters): (1) 40°C × 30 s, (2) 40°C × 60 s, (3) 40°C × 90 s, (4) 50°C × 30 s, (5) 50°C × 60 s, (6) 50°C × 90 s, (7) 60°C × 30 s, (8) 60°C × 60 s, (9) 60°C × 90 s, (10) 70°C × 30 s, (11) 70°C × 60 s, (12) 70°C × 90s.
We tried to fit the scored damage to the two parameters which define the hyperthermic dosage, i.e. temperature (T) and time (t). Since thermal damage is known to be a first order kinetic process, we chose a fitted equation based on a logarithmic transformation of the well known Arrhenius equation to model the thermal damage Citation10. The Arrhenius equation is as follows:where Ω is the value of thermal damage value, T is the temperature (°C) calculated at each time τ, t is the total duration of the heating, R is the gas constant (8.3134 J/(mole · °C)−1), A (s−1) is the frequency factor (a measure of molecular collisions), and ΔE (J/mole) is an activation energy barrier which tissue constituents must surmount to denature. In our research, we employed a hyperthermic protocol based on a temperature value which was uniform throughout the entire tissue sample and constant for the duration of heating (t). Hence, Equation 1 can be integrated and simplified Citation11 to:
If we now apply logarithmic transformation (natural log), we get the new equation:
We chose a fitted equation following this structure and hence obtained:
by means of a regression analysis. This analysis showed an adjusted R2 of 0.9326 (P < 0.0001), which is somewhat lower than a linear fit. The power of the performed test (with α = 0.05) was 1.00, the Durbin–Watson statistic was 2.08, the K-S statistic by normality test was 0.1288 (significance level of 0.4053) and constant variance test passed (P = 0.2561). shows the plot of Equation 4. As can be observed, the temperature parameter had a greater influence on thermal damage (i.e. score) than the time parameter.
Figure 5. Plot of the fitted equation relating scored damage (obtained from the histological analysis) with the parameters of the hyperthermic treatment (time t and temperature T). The fitted equation was Scored damage = 130.7 − 40,851/(T + 273) + ln t (R2 = 0.9326, P < 0.0001), which is a logarithmic transformation of the well known Arrhenius equation to model thermal damage Citation10.
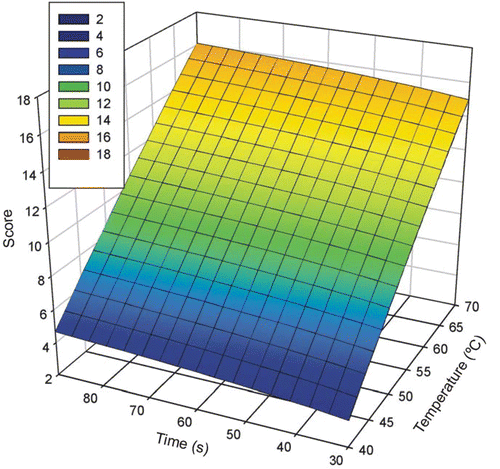
We next tried to fit the score to a plane fitted equation, obtaining:by means of a regression analysis. We used this linear fit due to its simplicity. This analysis showed an adjusted R2 of 0.9346 (P < 0.0001). The power of performed test (with α = 0.05) was 1.00, the Durbin–Watson statistic was 2.0778, the K-S statistic by normality test was 0.1506 (significance level of 0.2279) and constant variance test passed (P = 0.7899). Note that the data do not deviate from a linear regression, and also, as can be observed in Equation 5, the temperature parameter had a greater influence on thermal damage (i.e. score) than the time parameter, which is characteristic of Equation 2.
Discussion
Studies on the relationship between temperatures reached in the esophagus during ablation and histological alterations caused by thermal damage are scarce. In the published studies on histological alterations in the esophagus after cardiac ablation Citation5, the esophagus was always non-uniformly heated (i.e. an unknown thermal gradient was established across the esophagus cross section), hence an accurate value of the thermal dosage (temperature × time) received by each layer of the esophagus could not be determined. We believe that in order to develop procedures and devices to minimize esophageal thermal damage, it is first necessary to determine exactly how different hyperthermic dosages can affect the different esophageal layers. The purpose of our study was therefore to quantify the histological findings observed in esophagus samples exposed to different hyperthermic dosages under controlled conditions of temperature and time.
From our first assessment (see ), we established a thermal damage threshold at about 50°C. Below this temperature value (40°C), we did not find appreciable pathological damage. On the other hand, over 50°C (60 and 70°C in our experiments), the histological findings suggest severe damage, regardless of exposure time. These results are in agreement with previous studies on hyperthermic tissue treatments. For instance, Thomsen et al. Citation10 studied the decrease of birefringence and the coagulation of collagen and found a threshold of 60–70°C for these phenomena. Likewise, Whittaker et al. Citation12 found a threshold of 60°C associated with swollen mitochondria containing dense granules and multiple breached sarcolemma, which was compatible with irreversible injuries in cardiac tissue.
In previous studies on cardiac ablation, in vivo experiments using endocardial unipolar radiofrequency produced esophageal alteration in numerous cases affecting periesophageal connective tissue, muscular layers and vessels, but not the epithelium Citation5. Unfortunately, this study did not record the temperature values reached in the esophageal tissue, except at the lumen (which was measured by inserting an esophageal temperature probe).
In another study on an in vivo porcine model, Meeson et al. Citation13 examined the esophageal thermal damage caused by a microwave applicator designed to treat Barrett's esophagus. In their experiments they observed that an activation temperature around 55°C for less than 1 min (i.e. a thermal dosage of 55°C × 60 s) was needed to produce tissue injury, resulting in superficial damage to the epithelium while sparing subjacent tissues. In this respect, it is noteworthy that the temperature needed to cause damage to the epithelium and deeper layers in this experiment (55°C) strongly matches the temperature found in our study (50–60°C). However, the main difference between the two experiments lies in how heat was applied to the esophagus. In Meeson et al. Citation13 the esophagus temperature was gradually increased from normal body temperature to 55°C heterogeneously from the esophageal lumen towards deeper layers; whereas in our study we heated the esophagus samples rapidly and homogenously by means of immersion in a preheated bath.
The findings of the present study cover part of the scarcity of experimental results showing the relationship between the hyperthermic dosage received in the esophagus and histological alterations in each esophageal layer. In this respect, our results could be useful not only for the study of techniques and devices to minimize esophageal thermal injury during cardiac ablation, but also to design or improve esophageal heating techniques, such as in the treatment of Barrett's esophagus.
Finally, we found a good correlation between thermal damage (scored by histological alterations) and the programmed parameters in the hyperthermic treatment (i.e. temperature and time). We consider that this fitted equation could be useful in validating computer models Citation14. Briefly, by using the temperature–time profiles obtained from computer simulations, it might be possible to estimate the histological impact on the esophagus, and vice versa, the histological characteristics of a thermally damaged esophagus might be used to determine the hyperthermic history in terms of temperature and time. In addition, from the numerical data of Equation 4 we can estimate the values of the parameters A and ΔE in Equation 1. The value of the frequency factor was A = 5.8 × 1056 s−1, and the activation energy barrier was ΔE = 3.4 × 105 J/mole. Both values are in very good agreement with those reported by Thomsen et al. Citation10 to describe collagen denaturization (6.3 × 1052 s−1 and 3.6 × 105 J/mole respectively).
Limitations of the study
The limitations of this study are inherent in the nature of the ex vivo samples. The autolytic processes (i.e. post-mortem degeneration and necrosis) were active prior to the hyperthermic treatment, but we do not consider them to affect the thermal properties. On the other hand, the human esophagus has a greater amount of smooth muscle tissue than that used in our experiment, hence our results might not be totally comparable to the clinical stage. Moreover, the nature of the ex vivo samples implies that hyperthermic dosages were applied without blood flow in the esophagus. Under in vivo conditions, the blood flow could cool the tissue by thermal convection, and therefore may help to limit damage. However, since the esophagus has only small arteries and the aorta is a considerable distance from the esophagus from a thermal point of view, we think that in vivo and ex vivo conditions should not provide very different results.
Another important consideration involves the possibility of employing our fit equations for deriving information on hyperthermic treatments using some temperature and time combinations beyond those we have measured (i.e. >70°C and >90 s). In this respect, extrapolations would not be valid, as further morphological alterations due to thermal damage may be seen for temperatures greater than 70°C with exposure times equal to and greater than 90 s. In spite of this limitation, it should be emphasized that our study was planned to describe morphological alterations in the esophagus during RF ablation of the left atrium. The temperatures in the esophagus are normally below 70°C and application times are usually less than 120 s, hence our results can be said to be useful.
Also, as we chose the Masson Trichrome technique to stain the samples, future studies employing other staining techniques should be conducted in order to provide complementary information. For instance, the NADH technique makes it possible to assess cellular viability and has previously been validated in RF ablation studies Citation15,Citation16. Reticulin stain would provide a more accurate assessment of the lesion in the submucosa, since the reticulin fibers destroyed by heat do not stain after losing their quaternary structure. Nitro-BT stain has also shown excellent results in RF heating, and H&E stain makes the morphologic changes in stroma clearly visible. Finally, techniques for assessing the changes in birefringence of collagen could provide more objective information on thermal damage boundaries Citation8,Citation10,Citation17. However, they may not be valid for certain esophagus layers, and their validity should therefore be assessed in future studies. Additionally, the results could be processed by the CEM(43) parameter (cumulative equivalent minutes at 43°C) Citation18,Citation19 and then compared to those obtained by Equation 1.
Conclusions
The findings of this study suggest that: (1) hyperthermic treatment of the esophagus mainly affects the epithelium and deeper muscular layers; (2) the thermal damage threshold is around 50°C for treatments of 30–90 s; and (3) the proposed scoring system provides a good fit with the time and temperature parameters.
Acknowledgements
This work was partially supported by the ‘Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica del Ministerio de Educación y Ciencia’ of Spain, TEC2005–04199/TCM, and by R&D contract CSIC–20060633 between Edwards Lifescience Ltd. and the Spanish National Research Council (CSIC). We would like to thank the R&D&I Linguistic Assistance Office, Universidad Politécnica de Valencia (Spain), for granting financial support for the linguistic revision of this paper.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Nath S, Lynch C, III, Whayne JG, Haines DE. Cellular electrophysiological effects of hyperthermia on isolated guinea pig papillary muscle. Implications for catheter ablation. Circulation 1993; 88: 1826–1831
- Gillinov M, Pettersson G, Rice T. Esophageal injury during radiofrequency ablation of atrial fibrillation. J Thorac Cardiovasc Surg 2001; 122: 1239–1240
- Doll N, Borger M, Fabricius A, Sthepan S, Gummert J, Mohr FW, Hauus J, Kottkamp H, Hindricks G. Esophageal perforation during left atrial radiofrequency ablation: Is the risk too high?. J Thorac Cardiovasc Surg 2003; 125: 836–842
- Marrouche NF, Guenther J, Segerson NM, Daccarett M, Rittger H, Marschang H, Schibgilla V, Schmidt M, Ritscher G, Noelker G, et al. Randomized comparison between open irrigation technology and intracardiac-echo-guided energy delivery for pulmonary vein antrium isolation: Procedural parameters, outcomes, and the effect on esophageal injury. J Cardiovasc Electrophysiol 2007; 6: 583–588
- Aupperle H, Doll N, Walther T, Kornherr P, Ullmann C, Schoon HA, Mohr F. Ablation of atrial fibrillation and esophageal injury: Effects of energy source and ablation technique. J Thorac Cardiovasc Surg 2005; 130: 1549–1554
- Tsuchiya T, Ashikaga K, Nakagawa S, Hayashida K, Kugimiya H. Atrial fibrillation ablation with esophageal cooling with a cooled water-irrigated intraesophageal balloon: A pilot study. J Cardiovasc Electrophysiol 2007; 2: 145–150
- Lequerica JL, Berjano EJ, Herrero M, Melecio L, Hornero F. A cooled water-irrigated intraesophageal balloon to prevent thermal injury during cardiac ablation: Experimental study based on an agar phantom. Phys Med Biol 2008; 4: N25–N34
- Gehani AA, Rees MR. Can rotational atherectomy cause thermal tissue damage? A study of the potential heating and thermal tissue effects of a rotational atherectomy device. Cardiovasc Intervent Radiol 1998; 21: 481–486
- Anderson CD, Lin WC, Buttemere CR, Washington MK, Mahadevan-Jansen A, Pierce J, Nicoud IB, Pinson CW, Chari RS. Real-time spectroscopic assessment of thermal damage: Implications for radiofrequency ablation. J Gastrointest Surg 2004; 8: 660–669
- Thomsen S, Pearce JA, Cheong WF. Changes in birefringence as markers of thermal damage in tissues. IEEE Trans Biomed Eng 1989; 12: 1174–1179
- Lin WC, Buttemere C, Mahadevan-Jansen A. Effect of thermal damage on the in vitro optical fluorescence characteristics of liver tissue. IEEE J Select Topics Quantum Electron 2003; 9: 162–170
- Whittaker P, Zheng S, Patterson MJ, Kloner RA, Daly KE, Hartman RA. Histologic signatures of thermal injury: Applications in transmyocardial laser revascularization and radiofrequency ablation. Lasers Surg Med 2000; 4: 305–318
- Meeson S, Reeves JW, Birch MJ, Swain CP, Ikeda K, Feakins RM. Preliminary findings from tests of a microwave applicator designed to treat Barrett's oesophagus. Phys Med Biol 2005; 19: 4553–4566
- Berjano EJ. Theoretical modeling for radiofrequency ablation: State-of-the-art and challenges for the future. Biomed Eng Online 2006; 5: 24
- Scudamore CH, Lee SI, Patterson EJ, et al. Radiofrequency ablation followed by resection of malignant liver tumors. Am J Surg 1999; 177: 411–417
- Topp SA, McClurken M, Lipson D, Upadhya GA, Ritter JH, Linehan D, Strasberg SM. Saline-linked surface radiofrequency ablation. Factors affecting steam popping and depth of injury in the pig liver. Ann Surg 2004; 239: 518–527
- Pearce JA, Thomsen S. Kinetic models of laser-tissue fusion processes. Biomed Sci Instrum 1993; 29: 355–60
- Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia 2003; 19: 267–294
- Wright NT. On a relationship between the Arrhenius parameters from thermal damage studies. J Biomech Eng 2003; 125: 300–304