Abstract
Purpose: To determine the efficacy and safety from our preliminary results of using high intensity focused ultrasound (HIFU) to treat liver metastasis from colon and stomach cancer.
Materials and methods: Ten patients with liver metastasis from colon cancer and three from stomach cancer underwent HIFU under general anesthesia. HIFU was performed using an extracorporeal, ultrasound-guided focused system. Complications during the study, extent of coagulative necrosis at two-week follow up, and evidence of tumor on further follow up were analyzed. Patients were divided into four categories: (I) complete ablation with no evidence of recurrence on follow up; (II) apparent complete ablation of target mass with new foci of disease in the target organ or distant malignancy and no local tumor progression; (III) local tumor progression after apparent complete ablation; (IV) partial ablation.
Results: Mean follow-up period was 22 weeks in the colon cancer group and 58 weeks in the stomach cancer group. The sum of total lesion size was between 1.8 cm and 21.4 cm (mean: 8.4 cm ± 6.7 cm) for the colon cancer group and between 1.7 and 16.3 cm (mean: 8.8 cm ± 7.3 cm) for the stomach cancer group. In the colon cancer group, one patient was categorized as category I, one as category II, three as category III, and the remaining five as category IV. The stomach cancer group showed two patients as category I, and one as category II.
Conclusion: For treating liver metastasis from colon and stomach cancer HIFU seems safe but its efficacy is questionable. Further research is warranted.
Introduction
The presence of liver metastasis is a common and poor prognostic factor for a variety of primary malignancies such as gastrointestinal cancer Citation1. Systemic chemotherapy alone has not been that successful in treating these patients. Surgical resection has shown improved survival, but is limited by morbidity and the small percentage of eligible patients. Other minimally invasive therapies, especially radiofrequency ablation (RFA) and transarterial chemoembolization (TACE) have shown significant benefits in unresectable disease Citation2, and in the case of RFA, the results are comparable to surgery Citation3. HIFU is a newer, non-invasive technology that may have potential benefits over RFA with very few preliminary studies to date.
High-intensity focused ultrasound (HIFU) ablation for liver tumors is an extracorporeal non-invasive treatment method using focused ultrasound beams that can cause complete coagulative necrosis of target lesions through intact skin without surgical exposure or instrumentation.
The concept of HIFU is not new and its biological effects were reported at the beginning of the last century Citation4, but early studies consisted mostly of only animal studies. With recent advances in imaging modality it is now gaining attention as a non-invasive surgical tool in humans Citation5, and numerous studies Citation6–8 including the sole randomized study Citation9 for any HIFU device have been published on the favorable use of HIFU for hepatocellular carcinoma (HCC).
This study represents a report of the author's experience of a case series of patients with liver metastasis from colon and stomach cancer treated with HIFU.
Materials and methods
Subjects
Between February 2006 and January 2007, HIFU treatment was performed in ten patients with liver metastasis from colon cancer and three patients with liver metastasis from stomach cancer. Through our electronic medical record (EMR) system we retrospectively reviewed their charts. In all patients, the primary lesions were surgically resected and the pathologies were of typical adenocarcinoma. Patients were not candidates for surgical resection of the liver or refused resection and specifically asked their physician for HIFU treatment. This study was approved from the institutional review board and informed consent was obtained in all patients. Ten patients were male and three female. The age range was between 35 and 82.
All patients also had combined chemotherapy treatment with HIFU treatment. In the colon cancer group, three patients had transarterial chemoembolization (TACE) before HIFU treatment, one patient had RFA, and one patient had had percutaneous ethanol injection (PEI). One of the patients with TACE treatment also had PEI performed after HIFU treatment, and one patient had TACE after HIFU treatment. In the stomach cancer group, one patient had PEI before HIFU, and two patients had TACE before HIFU.
The objective of treatment was complete ablation in nine patients and tumor debulking in one patient in the colon cancer group (). The objective was complete ablation in two patients and tumor debulking in one patient in the stomach cancer group ().
Table I. Summary of findings in colon cancer group (10 cases). All patients had chemotherapy treatment.
HIFU treatment
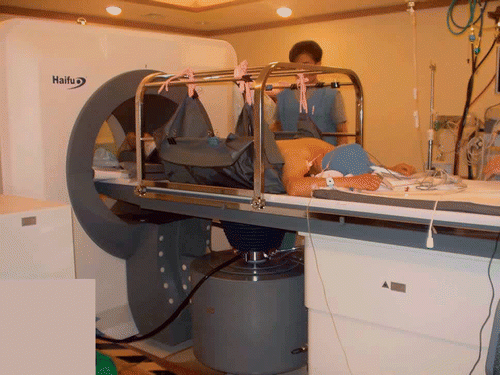
HIFU was performed using an extracorporeal, ultrasound-guided, focused Model-JC tumor therapy system (Haifu Technology Company, Chongqing, China) (). This system consists of three selectable therapeutic transducers and a real-time imaging transducer having overlapping beams to allow targeting. The transducers were mounted in a water reservoir with the beam axis directed upward and the patients were positioned above the transducers in a prone or right down decubitus position with the skin overlying the lesion inside the water. The therapeutic transducers focus ultrasound beams into small clinically relevant volumes which induce high temperatures locally at the focus. The area of destroyed cells is referred to as an ablation zone. Real-time ultrasound was done under the guidance of a 3.5–5.0 MHz diagnostic ultrasound transducer (Toshiba Medical System, Otawara, Japan). All patients underwent general anesthesia to insure immobilization during the lengthy procedure and to prevent superficial skin pain.
Figure 1. Picture of the HIFU device during an ablation procedure. The patient is under general anesthesia and in the prone position for treatment of metastatic tumor in the left lobe of the liver.
HIFU treatment can be largely divided into three stages. First of all, initial diagnosis and target assessment must be performed. In our study, initial diagnosis was done with MRI and CT, followed by assessment with diagnostic ultrasound to assure an adequate acoustic window and assure that the treatment boundaries could be clearly identified. For targeting and comparison with post-treatment images, all patients, except for one patient who had CT performed, had contrast enhanced MRI performed prior to ablation. During the second treatment stage, the correct ultrasound exposure for ablation must be determined, which in our study was done by setting the focal peak intensity and exposure time until the target ablation zones showed hyperechogenic changes during HIFU treatment. The last stage is post-treatment assessment, which in our case was done by two-week follow up contrast enhanced MRI, except for one patient who had CT performed who is the same patient that had only CT done prior to treatment. Further follow up varied between patients with MRI and in some cases additional PET-CT, CT being performed 1–4 times in the colon cancer group, with the last follow up performed 25–434 days (mean: 207 days) after ablation. Follow up was 6–9 times in the stomach cancer group, with the last follow up performed 273–534 days (mean: 403 days) after ablation.
Table II. Summary of findings in stomach cancer group. All patients had chemotherapy treatment.
During HIFU treatment, main parameters were configured as follows: therapy frequency 0.8 MHz, mean diameter of focal field 1.1 mm, length of focal field 9.8 mm, focus distance 135 mm, and therapy power 120–350 W. The target mass was ablated in multiple 5-mm sections until the whole mass was covered including at least a 5-mm ablation margin. HIFU ablation can be performed by a single exposure causing ellipsoidal focal ablation zones or as rectangular row ablation zones formed by combining multiple ellipsoidal ablation zones (). Since an ablation zone caused by a single ultrasonic exposure is very small, mostly multiple row ablation zones are applied until the whole target area is covered with only focal areas targeted using single ellipsoidal ablation zones when necessary. To prevent skin burns and HIFU-related side effects at non-target areas, the whole target area was not sequentially ablated, but ablated in a systematic fashion. For example, a 4-cm tumor with a 5-mm ablation margin can be divided into approximately 11 5-mm interval positions similar to slices on CT scans. If we number the interval positions from 1 to 11 from the left to right, we would ablate in the following order 1, 7, 2, 8, 3, 9, 4, 10, 5, 11, and 6.
Figure 2. Targets were ablated using mostly multiple HIFU row ablation zones with single ellipsoidal HIFU ablation zones when necessary. Deep portions were targeted first with shallower areas targeted subsequently.
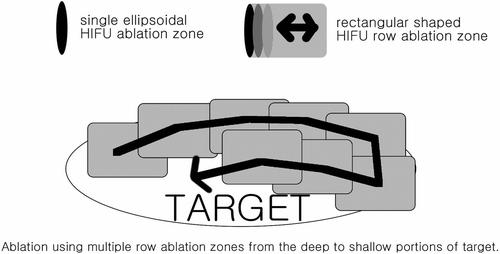
When performing HIFU ablation, we ablated the deep portions first because the attenuation coefficient is known to increase after ablation Citation10 due to increases in temperature and cavitation effects Citation11, which should theoretically provide a barrier to deeper structures when ablating the shallower areas. Also, although the beam is not focused in the shallower areas when ablating the deeper portions, the beam does pass through the shallower areas and if unwanted absorption causes heating, we would hope it would be in areas of our target.
Table III. Classification of results into four categories.
If the tumors to be ablated were located at the hepatic dome directly below the diaphragm, or adjacent to the air-filled lung, which due to its location near the lung had a poor sonographic window causing poor visualization on ultrasonography, artificial pleural effusion was created to separate the lung allowing complete visualization of the masses. After general anesthesia, the patient was positioned into the left down decubitus position, and then an initial small skin wound was made using a 17 gauge needle at or near the intercostal space between the sixth and seventh ribs. An aliquot of 500 to 1000 ml of normal saline was then injected to create an artificial pleural effusion. No patients had prior lung or pleural disease.
MRI protocol
We used two 1.5 T units (Magnetom Vision Plus, Siemens and Signa Excite, General Electric) with phased-array torso coils. The standard protocol included T2-weighted images (slice thickness 6 mm with 0.6 mm gap) with variable echo time, dual-echo in and out of phase spoiled gradient-echo T1-weighted images (slice thickness 7 mm with 1 mm gap), and dynamic 2D fast spoiled gradient-echo images (minimum TE, 150–200; flip angle, 80°; slice thickness, 5–8 mm; acquisition time, 24 seconds) at unenhanced, arterial (15-second delay), portal venous (45-second delay), late portal venous (90-second delay), and delayed (180-second delay) phases. Coronal and sagittal two-dimensional fast spoiled gradient-echo images were also obtained at delayed phase. Gadopentetate dimeglumine (Magnevist, Bayer Healthcare), 0.1 mmol/kg was injected via intravenous route by hand injection and followed by a saline flush.
CT protocol
The CT images were acquired using a multidetector scanner (Lightspeed VCT, General Electric). A total of 120 mL of non-ionic contrast material (iopromide, 300 mg I/mL (Ultravist 300, Bayer Healthcare) was administrated at a rate of 3 mL/s with an automatic power injector. Images were obtained before and at 25, 60–70, and 180 seconds after IV contrast material injection. Scanning of all phases was initiated at the dome of the right hemidiaphragm and continued caudally through the entire liver and on portal phase images the entire abdomen to the symphysis pubis was obtained. Slice thickness was 5 mm.
Image analysis
Two observers retrospectively evaluated the MR images and CT scans of the patients for the technical effectiveness by consensus. Before ablation all patients had the long axis diameter of lesions measured and the total sum was recorded ( and ).
Treatment evaluation
Technical success: was defined as when the planned targets were fully covered by HIFU Citation12. During HIFU treatment the change in echogenicity of the target was observed, and when the target area had hyperechoic changes it was determined that the area was optimally ablated. It should be noted that even when all areas of masses were covered some did not show echogenic changes. Even in these cases, if the targets were fully covered during HIFU treatment it was considered a technical success.
Technical effectiveness: Tumor response was evaluated based on criteria by Goldberg et al. Citation12, with further classification into four categories defined below to clarify results (). Complete ablation of masses was defined as when the mass showed complete coagulative necrosis on two-week follow up imaging ( and ) which except in one case (CT) was done by MRI.
Figure 3. A 63-year-old man with multiple hepatic metastasis from colon carcinoma. Initial contrast enhanced coronal T1-weighted MR image shows one (arrow) of three metastatic nodules to the right lobe of liver (a). Ultrasonographic images showing the target before (b, left image) and after HIFU ablation (b, right image) demonstrate the hyperechoic changes representing optimal ablation. Follow-up contrast-enhanced coronal T1-weighted image two weeks after HIFU shows complete coagulative necrosis of the lesion (arrow). Pericostal ablation is noted in the sonification port (arrowheads) (c). Further follow-up contrast-enhanced CT 114 days after HIFU shows interval increase of the tumor size (arrows), indicating recurrence (d).
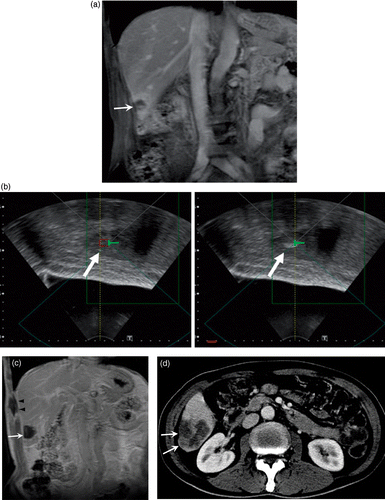
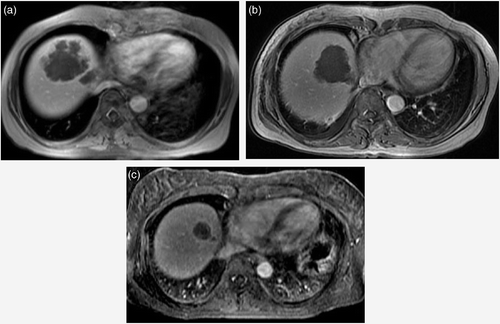
Figure 4. A 50-year-old woman with multiple hepatic metastasis from stomach carcinoma. Initial contrast-enhanced axial T1-weighted MR image shows one of multiple metastatic nodules in both lobes of the liver (a). Follow-up T1-weighted image two weeks after HIFU shows complete coagulative necrosis of the lesions (b). Although further follow up 107 days after HIFU shows progression of new lesions at non-ablated portions of the liver, no recurrence was seen at the ablated sites and decrease in size of the ablated sites are seen (c).
Patients were divided into four categories: (I) complete ablation with no evidence of recurrence on follow up; (II) apparent complete ablation of target mass with new foci of disease in the target organ or distant malignancy and no local tumor progression; (III) local tumor progression after apparent complete ablation; and (IV) partial ablation at two-week follow up.
The difference between category II and III being that the local progression was seen at the original site of the target mass in category III and that the hepatic lesions occurred elsewhere in category II patients. All the patients in the study had HIFU performed only once, and primary effectiveness rate was calculated by dividing category I and II patients by the total number of patients.
Complications: During and after the procedure, complications including side effects, pain, and post-ablation syndrome related to HIFU treatment, and complications related to artificial pleural effusions were classified according to the criteria by Goldberg et al. Citation12. Complications were classified as death, major complications such as an event that leads to substantial morbidity and disability, increasing the level of care, or results in hospital admission or substantially lengthened hospital stay, and all others being minor complications.
Patient pain before and after the procedure was recorded according to the National Cancer Institute Common Terminology Criteria for Adverse Events for reporting pain Citation13: grade 0, no pain; grade 1, mild pain that does not interfere with function; grade 2, moderate pain; pain or analgesics that interfere with function but not interfere with activities of daily living; grade 3, severe pain; pain or analgesics that severely interfere with activities of daily living; and grade 4, disabling pain.
Results
In the colon cancer group, five patients had treatment for a single nodule, two patients for two nodules, and three patients for three nodules (). The sum of total lesion size was between 1.8 cm and 21.4 cm (mean: 8.4 cm ± 6.7 cm). Artificial pleural effusion was created in all but one patient and the amount was between 300 and 1000 ml. In the stomach cancer group, two patients had treatment for one nodule and one patient for three nodules (), and the sum of total lesion size was between 1.7 and 16.3 cm (mean 8.8 cm ± 7.3 cm). Artificial pleural effusion was created in two patients and the amount was between 800 and 1200 ml.
Procedure information
The time under general anesthesia was between 150 and 650 minutes (mean: 448 minutes). Treatment time sonification, defined as the time actual HIFU was applied, was between 856 seconds and 14 344 seconds (mean: 7142 seconds). Time for procedure, defined as the time after anesthesia including the time to locate, target, and ablate lesions was between 95 minutes and 605 minutes (mean: 394 minutes). This included one patient who had a short anesthesia time, treatment time, and procedure time of 14 150, and 95 minutes due to problems during anesthesia. Mean follow up by MRI or CT was 243 days (range: 14–534 days). All patients had all metastatic lesions ablated except one patient in the stomach cancer group who had the larger lesions ablated but had multiple scattered smaller lesions treated with systemic chemotherapy.
Technical success was achieved in all patients except one patient in the colon cancer group, who due to the large size of lesions had only partial coverage of the target masses.
Outcomes
In the colon cancer group, one patient was categorized as category I, one as category II, three as category III, and the remaining five as category IV (). The stomach cancer group showed two patients as category I, and one patient as category II (). The category II patient in the stomach cancer group had only a few larger lesions targeted with HIFU, but non-targeted multiple small lesions showed decrease in size after systemic chemotherapy but increased in size on further follow up. Primary effectiveness rate was 20% in the colon cancer group and 100% in the stomach cancer group. In the category II patient in the colon cancer group, time to observation of new foci of tumor was 45 days. In the three category III patients in the colon cancer group, local tumor recurrence was between 50 to 127 days (mean: 96 days). Two patients categorized as III in the colon cancer group had surgical resection and pathological confirmation of the metastatic lesions 20 weeks after HIFU treatment.
Complications
In the colon cancer group, complications requiring treatment occurred in two patients. They both had periprocedural symptomatic pleural effusions after treatment requiring drainage or medical treatment, and of one of these patients later died due to biliary obstruction. Minor complications were skin redness and edema which was noted in all patients immediately after the procedure, but resolved in all cases without any special care. All patients had necrosis of the ribs along the main ultrasound beam path which did not require further treatment (c). In the stomach cancer group, one patient had a periprocedural abscess related to HIFU treatment with formation of a fistula to the subcutaneous layer, which was treated with drainage and antibiotics. This patient showed complete resolution of the abscess without evidence of tumor recurrence and was categorized as category I. Classifying the complications above, the colon cancer group had two patients (20%) with major complications which were periprocedural complications including one death (10%), and the stomach cancer group had one periprocedural major complication (33%). All patients had minor complications which were immediate complications.
Before ablation, two patients in the colon cancer group had grade 1 pain, one patient in the stomach cancer group had grade 1 pain, and all the others had grade 0 pain. Five days after ablation, six patients in the colon cancer group had grade 1 pain, one patient in the stomach cancer group had grade 1 pain, and all the others had grade 0 pain.
Discussion
Wang et al. Citation14,Citation15 proposed a concept of biological focal region (BFR) to describe the extent of coagulative necrosis. After long-term research, the principle of ablating a tumor mass from BRFs to rows, to slices, to mass, and finally removal of tumor was made. The row and slice lesions had clear margins with normal tissues and tissues within the target region became coagulative necrosis with no residual normal tissue. The method to move a BFR of small volume to cover a tumor mass instead of ablation by heat dissipation is the key difference between HIFU and modalities such as microwave, RFA, high-frequency current and argon and helium therapy. When the temperature in situ is greater than 50°C and coagulative necrosis is produced, the attenuation and absorption coefficients increase Citation16 leading to the volume of coagulative necrosis induced by sequential exposures to expand volume and increase tissue temperature, thus leading to real-time hyperechoic gray-scale changes Citation17. Unlike MR-guided focused ultrasound systems, US-guided systems cannot monitor target temperature directly. Thus, as in our method, when determining the ultrasound exposure for ablation by setting the focal peak intensity and exposure time until the target ablation zones show hyperechogenic changes during HIFU treatment, a greater than 50°C temperature can be expected at the target site which is sufficient to cause coagulative necrosis Citation18.
There are numerous reports on the use of HIFU for HCC, but we know of only one that describes its use for liver metastasis. Li et al. Citation6 published a study with their results on HIFU treatment in 100 patients with liver cancer, including 38 metastatic liver cancers with favorable results, but their study did not mention the results for metastatic cancer separately and only short-term follow up for up to two weeks after HIFU treatment were included. The study also did not have details on technical success, effectiveness, complete ablation, and incomplete ablation.
The principle mechanism of HIFU treatment is delivering sufficient energy to increase tissue temperature at the target lesion to cytotoxic levels sufficiently fast that the cooling by tissue vasculature does not have a significant effect on the extent of cell killing. Also HIFU can lead to vessel wall disruption and vascular occlusion Citation5. The study by Wu et al. showed that combined TACE with HIFU was superior to TACE alone for treating stage IVa HCC, the rationale being that HIFU is superior to repeated TACE for destroying residual tumor blood supply and that the reduction of blood supply after TACE allows HIFU ablation with lower levels of ultrasound energy, shortened treatment time, and reduction of potential side effects Citation9. Although difficult to assess the usefulness due to the low number of cases, in some patients a combination of TACE and PEI was performed before and after HIFU treatment. It should be noted that in the sole category I patient in the colon cancer group, the patient only had systemic chemotherapy and HIFU treatment.
The time under general anesthesia was between 150 and 650 minutes, relatively long compared to many other ablation therapies. In contrast, the treatment time sonification, defined as the time actual HIFU was applied, was between 856 seconds and 14 344 seconds. This large difference is due mostly to the lengthy process of moving the treatment transducer to target areas that are apart from each other to allow the skin to cool down and prevent procedure related burns, and returning again to ablate the adjacent areas.
HIFU treatment in patients was considered effective if patients were evaluated as category I or II. Compared to the gastric cancer group, the colon cancer group had a relatively high incomplete ablation and local tumor progression rate.
Two patients had complications related to artificial pleural effusion creation, and were treated with drainage or medical treatment, and of these one eventually died due to biliary obstruction. These complications were thought to be related to inflammatory changes following.
Livraghi et al. reported a rate of 1.7–6.0% for major complication, 4.7% for minor complications, and 0.3% deaths in a multicenter study involving 2320 patients for treating focal liver tumors with RFA Citation19. In contrast, our study had a major complication rate of 20% and a mortality rate of 10% for the colon cancer group and 33% for the stomach cancer group, with 100% minor complication rate for both groups, but it has little meaning to compare these results directly due to the low number of cases and difference in lesions. It should be also noted that many of the lesions in our study were at locations difficult to treat with RFA, and the average size was 8.4 cm ± 6.7 cm in the colon cancer group and 8.8 cm ± 7.3 cm in the stomach cancer group, much larger than the average size of 3.1 cm ± 1.1 cm mentioned by Livraghi et al. Citation19. Even with the numbers above, in our clinical experience we still consider HIFU ablation to be a safe procedure without significant complications for the treatment of liver metastasis from both the colon and stomach.
Our study is limited in its retrospective nature, multiple therapies being highly potential confounding factors, and low number of cases. In the ten colon cancer patients in our study, the short-term and long-term effectiveness of HIFU for liver metastasis appears overall questionable. On the other hand, in the three cases from gastric cancer, short-term and long-term results appear more promising, but due to the limited number of cases, this is a preliminary study and no definite conclusion can be reached, and further study is necessary to confirm these findings and to also assess whether HIFU treatment has an effect on survival.
Conclusion
Our preliminary study consisted of a total of only 13 patients, and further study is necessary before reaching a more definite conclusion. In the limited number of patients in our study, HIFU treatment for liver metastasis from stomach and colon cancer seems to be a safe procedure, but the results for colon cancer are questionable. However, although there were only three patients in the stomach cancer group, and no definite conclusion can be offered, the results seem more positive and further research is warranted.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Kakeji Y, Maehara Y, Tomoda M, Kabashima A, Ohmori M, Oda S, Ohno S, Sugimachi K. Long-term survival of patients with stage IV gastric carcinoma. Cancer 1998; 82: 2307–2311
- Evans J. Ablative and catheter-delivered therapies for colorectal liver metastases (CRLM). Eur J Surg Oncol 2007; 33: S64–S75
- Majeed AW. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases (Br J Surg 2003;90:1240–1243). Br J Surg 2003; 90: 1611
- Wood RWLA. The physical and biological effects of high-frequency sound waves of great intensity. London Edinburgh Dublin Phil Mag J Sci 1927; 4: 20
- Kennedy JE, Ter Haar GR, Cranston D. High intensity focused ultrasound: Surgery of the future?. Br J Radiol 2003; 76: 590–599
- Li CX, Xu GL, Jiang ZY, Li JJ, Luo GY, Shan HB, Zhang R, Li Y. Analysis of clinical effect of high-intensity focused ultrasound on liver cancer. World J Gastroenterol 2004; 10: 2201–2204
- Wu F, Wang ZB, Chen WZ, Wang W, Gui Y, Zhang M, Zheng G, Zhou Y, Xu G, Li M, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: An overview. Ultrason Sonochem 2004; 11: 149–154
- Illing RO, Kennedy JE, Wu F, ter Haar GR, Protheroe AS, Friend PJ, Gleeson FV, Cranston DW, Phillips RR, Middleton MR. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a western population. Br J Cancer 2005; 93: 890–895
- Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, Li KQ, Jin CB, Xie FL, Su HB. Advanced hepatocellular carcinoma: Treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology 2005; 235: 659–667
- Zderic V, Keshavarzi A, Andrew MA, Vaezy S, Martin RW. Attenuation of porcine tissues in vivo after high-intensity ultrasound treatment. Ultrasound Med Biol 2004; 30: 61–66
- Clarke RL, Bush NL, Ter Haar GR. The changes in acoustic attenuation due to in vitro heating. Ultrasound Med Biol 2003; 29: 127–135
- Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, III, Dupuy DE, Gervais D, Gillams AR, Kane RA, Lee FT, Jr, et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria. Radiology 2005; 235: 728–739
- Cancer Therapy Evaluation Program: Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS March 31, 2003 (http://ctep.cancer.gov), Publish Date: August 9, 2006
- Wang ZB, Wu F, Wang ZL, Zhang Z, Zou JZ, Liu C, Liu YG, Cheng G, Du YH, He ZC, et al. Targeted damage effects of high intensity focused ultrasound (HIFU) on liver tissues of Guizhou province miniswine. Ultrason Sonochem 1997; 4: 181–182
- Wang ZB, Wu F, Wang ZL, Liu C. Concept of BFR and its importance in tissue. Resection with high intensity focused ultrasound. J Acoust Soc Am 1998; 103: 2869
- Bush NL, Rivens I, ter Haar GR, Bamber JC. Acoustic properties of lesions generated with an ultrasound therapy system. Ultrasound Med Biol 1993; 19: 789–801
- Wang Z, Bai J, Li F, Du Y, Wen S, Hu K, Xu G, Ma P, Yin N, Chen W, et al. Study of a 'biological focal region' of high-intensity focused ultrasound. Ultrasound Med Biol 2003; 29: 749–754
- Haemmerich D, Laeseke PF. Thermal tumour ablation: Devices, clinical applications and future directions. Int J Hyperthermia 2005; 21: 755–760
- Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: Complications encountered in a multicenter study. Radiology 2003; 226: 441–451