Abstract
This work aimed to analyze the possibility and performance of the temperature controlled hyperthermia based on AC heating of magnetic nanoparticles with low Curie temperature. Temperature dependence of dynamic magnetic susceptibility has been studied experimentally on fine powders of La0.8Ag0.15MnO2.95 in the frequency range of 0.5–2.0 MHz. Critical drop of the AC magnetic losses was found in the vicinity of the Curie point, TC = 42°C. The obtained data was used in the numerical analysis of the bioheat equations under typical conditions of the hyperthermia treatment. The mathematical model includes a spherical tumor containing magnetic particles and surrounded by concentric healthy tissue, with account made for the blood perfusion. The calculations performed for various AC power, tumor sizes and doping geometries predict effective autostabilization of the temperature at T ≅ TC inside the tumor and steep temperature profile at the interface with the healthy tissue.
Introduction
Recently, fine magnetic particles are intensively studied as agents for local heating of living tissues, especially in cancer therapy Citation1–6. The particles are introduced into diseased tissue and then heated by application of sufficiently strong alternating magnetic field. Typical frequencies are in the range of 0.1–1.0 MHz, with the amplitudes H ∼ 10 kA/m; this ensures predominance of magnetic losses caused by magnetization reversal and magnetic relaxation, with negligible heating by inductive currents. Local hyperthermia using magnetic microparticles has the maximum potential for selective targeting and heating diseased regions without damaging the surrounding healthy tissue Citation4.
Magnetic fluids containing superparamagnetic or ferrimagnetic fine particles of magnetite (Fe3O4) and maghemite (γ-Fe2O3) were explored intensively Citation3,Citation6–8. These materials show sufficiently high specific absorption rate (SAR) which characterizes the AC magnetic losses per 1 g of the magnetic substance. A difficulty arises, however, when the precise temperature control is needed, which is of great importance in medical hyperthermia to protect the tissue from overheating and necrosis Citation2–5. Recently it was suggested overcoming this problem by the use of magnetic particles with low Curie temperature, TC ∼ 40–50°C Citation9–13. At temperatures T < TC, the material is ferro- (ferri-) magnetic with high AC losses, whereas the transition into paramagnetic phase ensures the drop of SAR and so the autostabilization of the temperature near TC. As prospective materials, the rare-earth manganites were proposed, such as La1-xSrxMnO3-δ (LSMO) Citation10–12 and La1-xAgyMnO3-δ (LAMO) Citation13, the proper TC values being provided by the choice of doping, stoichiometry and synthesis technology. For successful application, the sufficiently high value of SAR is needed, along with the narrow and steep phase transition at TC which is crucial for temperature stabilization. Of course, the standard biomedical requirements, such as chemical stability and biocompatibility, must be ensured as well. Currently, all these problems are under investigation in a number of laboratories Citation8–13.
To estimate the applicability of new materials in hyperthermia treatment it is useful to perform quantitative calculation of the temperature distribution in living tissues under typical conditions of magnetic hyperthermia. Usually, such calculations are based on various versions of the Pennes’ bio-heat transfer Citationequation 14. Many numerical simulations were carried out for a variety of hyperthermia modalities, biophysical parameters and specific tumor shapes, with account made for blood perfusion Citation15–33. Much work was performed using inductive currents and macroscopic magnetic implants (seeds) as heat sources Citation15–18,Citation25,Citation27,Citation28; in some cases, the calculations were supported experimentally Citation16,Citation34–36. In the present paper we deal with another branch of local hyperthermia which uses magnetic nanoparticles (magnetic fluids) Citation1–6. This techniques has its own specific features, just as in practical aspects (such as targeting, biocompatibility, intracell heating, etc.), so also in the heat transfer in tissues. The bioheat equation as applied to magnetic nanoparticles was analyzed recently in Citation31,Citation32; for the early publications, see the review article Citation26 and Citation19–24.
The main goal of these calculations was to find an optimal set of adjustable parameters, such as the concentration, SAR, and spatial distribution of the heat sources, as well as the amplitude and frequency of the alternating magnetic field, which provide the required, strictly limited temperature rise in the tumor (as a rule, acceptable temperatures must lie in the range of 42°–44°C Citation15) and minimum heating of the surrounding healthy tissue. The size of the absorbed power range that gives acceptable tissue temperature distributions is calculated and suggested as a deciding factor in successful hyperthermia treatment Citation15,Citation16. An inverse solution is developed to achieve constant therapeutic temperature within the tumor region Citation20,Citation21. In practice, however, the absorbed power can hardly be monitored and controlled with proper accuracy during the whole hyperthermia procedure. As a result, the risk of overheating may be a problem, both in the tumor and healthy tissue.
As mentioned above, this problem can be overcome by using the low-TC magnetic materials which provide an abrupt fall of heat generation and, hence, the autostabilization of the temperature just near TC. Thus, the risk of overheating is eliminated automatically, without any worry about power control. This idea was firstly proposed by C.T. Cetas et al. for seed techniques Citation37 and revived recently for magnetic fluid modality Citation9–13.
From the computational point of view, the situation is as follows. In the majority of publications the heat generation was assumed to be independent of T; this approximation is well justified for the high-TC materials, such as magnetite and maghemite, where TC is much higher than the body temperature. In this case, the problem of the temperature autostabilization cannot be solved. Unlike that, the bioheat equation for the low-TC materials contains a heat generation term which critically depends on temperature near TC. The corresponding solution has not yet been analyzed for magnetic fluid modality. To investigate such solution and examine the possibility and real parameters of the temperature autostabilization is the main goal of the present communication. New experimental data on the AC magnetic losses in the recently synthesized La1-xAgyMnO3-δ particles with TC ∼ 42°C will be presented and used in the calculation. It will be shown that the temperature autostabilization near TC can be attained with typical parameters of diseased and healthy tissues, with account made for the blood perfusion. Besides, a good space distribution of the elevated temperature is predicted, ensuring proper heating of the tumor without harmful effect on the surrounding healthy tissue.
Magnetic material
Fine powders of La1-xAgyMnO3-δ were prepared and supplied by the Moscow University team Citation13. The proper TC and SAR values were obtained by the chemical homogenization technique at the composition of x = 0.2; y = 0.15; δ = 0.05. The preparation technology, basic chemical-physical properties, and the problem of biocompatibility are discussed in detail in Citation13.
To calculate the kinetics of heating and temperature profile under hyperthermia conditions, knowledge is needed on the temperature dependence of magnetic AC losses, especially in the vicinity of TC. To obtain these data, we have measured the imaginary part (χ″) of the dynamic magnetic susceptibility of the material under study. The χ″ value was determined from the change in the quality factor (Q) of the resonant circuit with and without the sample inside the coil. The data obtained on La0.8Ag0.15MnO2.95 at ω/2π = 1 MHz are shown in . It can be seen that the χ″ value changes only slightly in the ferromagnetic region, but drops steeply near TC ≅ 42°C and becomes negligible in the paramagnetic phase. The results were found to be almost independent on frequency in the range of 0.5–2.0 MHz. More detailed presentation, including thorough description of the experimental techniques and the data obtained on different samples, will be published separately.
Figure 1. Temperature dependence of the imaginary part of the dynamic magnetic susceptibility measured on the La0.8Ag0.15MnO2.95 powder at 1 MHz. Points are the experimental data; solid and dashed curves represent the complicated and simplified test functions, respectively.

The imaginary part of the AC susceptibility determines the absorption ability and can be related to the heat power (P) generated in 1 cm3 of the material subjected to AC magnetic field as follows Citation30:where μ0 is the permeability of vacuum and H is the amplitude of the AC magnetic field. Here P is expressed in W/cm3 and the rest in SI units. Further, SAR = P/ρ, where ρ is the material density (about 6.5 g/cm3 for the manganites). Note that we express the SAR value in W/g LAMO, not per gram of metal ions as adopted sometimes in literature Citation2. Using the experimental value of χ″ ≅ 0.5 at room temperature (), along with H = 10 kA/m and ω/2π = 0.8 MHz, one gets SAR = 22 W/g, in agreement with experimental data obtained recently on similar samples by AC heating of the LAMO aqueous suspensions Citation13. Thus our measurements of χ″ are consistent with independent experiments and can be employed in further calculations.
Mathematical model
The most important effect to be studied is the auto-stabilization of temperature caused by the critical drop of magnetic losses near TC. To reveal this phenomenon with maximum clarity and avoid unnecessary complications, it is reasonable to restrict ourselves by spherical symmetry (that is, to use the one-dimensional bioheat equation). Thus, giving credit to detailed 3D analysis Citation28,Citation31, we will follow the approach used by Bagaria and Johnson Citation32 which may be considered as a prototype for our calculation. Similar to Citation32, the diseased tissue (‘tumor’) is represented by the inner sphere with a radius r1. The tumor (or some concentric region inside the tumor) contains uniformly distributed magnetic particles with volume concentration α ≪ 1. The mean distance between the particles is assumed to be small enough to consider the doped volume as a homogeneous effective medium with the averaged heat generation of αP W/cm3. Note that this assumption is well justified for typical parameters when working with microparticles, but cannot be applied to macroscopic seeds. Further, another sphere with the same center (r = 0) and radius r2 > r1 represents an ‘organ’, and the spherical layer between r1 and r2 is filled with healthy tissue without magnetic particles. The heat transfer in this model, with account made for blood perfusion, is described by the standard one-dimensional Citationequations 32:where the power density P may depend on r, accounting for various space distributions of the particles. The subscripts 1 and 2 correspond to the inner sphere (tumor) and outer spherical layer (healthy tissue), respectively, and the subscript b stands for blood. The following nomenclature is used: Ti, temperature; ρi, density; ci, specific heat capacity; ki, thermal conductivity; wb, blood perfusion. The boundary conditions include the finiteness of T(0, t) < ∞ in the centre and T(r2, t) = const. = T0 at the outer border, where T0 is the normal body temperature. At the interface of the tumor and the healthy tissue (r = r1), the temperature and the heat flow are continuous:
The initial conditions correspond to the normal body temperature in the whole volume, T(r, 0) = T0.
Equations 2 and 3 seem to be analogous to those studied in Citation32. However, in contrast to the previous analysis, we consider the case when the generated heat power P does depend on the temperature. As a consequence, the equations become non-linear. The most important for the temperature stabilization is the specific form of χ″(T) in the critical region, see . If the fall of χ″ were infinitely steep (rectangular, with discontinuity at TC), the analytical solution would be available both for spheric and cylindrical symmetries. In such an ‘ideal’ case, the strict stabilization (T = TC) is predicted inside an inner region (rstab < r1) which approaches the whole tumor volume at high values of the heat power. In reality, however, the drop of the AC magnetic losses is described by continuous function and so the heating temperature will depend somehow on AC power even in the critical region. Thus, the stabilization near TC is not perfect and can be estimated quantitatively by proper analysis of Equations 2 and 3 with account made for the real temperature dependence of P. In this case, the analytical solution cannot be obtained, and the problem should be solved numerically.
To make use of the experimental data we fitted the χ″(T) dependence by the test functionwhere a = 0.0165; b = 4.17°C; T1 = 43.35°C; T2 = 40.88°C; Δ1 = 1.21°C; Δ2 = 4.56°C, shown as the solid curve in . This function was used in all calculations presented in this paper. One should realize, however, that this complicated expression is not universal and may depend on specific sample. So we also tested the much simpler function
where c = 0.58; TC = 42°C; Δ = 1.5°C (dotted line in ) which fits the most important critial region well. We found that such simplification does not lead to significant changes in the solution and thus can be recommended for express analysis. To solve the problem numerically, the method of finite differences was used. Discrete approximation was made on non-uniform grid which was refined near the interface r = r1. Since the problem includes non-linearity (caused by the source αP), the solution can be obtained in iterative process. The implicit scheme with simple iteration process was employed, the non-linearity being included from the previous iteration. The iterations continued until the inequality
was fulfilled, where the subscript i stands for the grid node and superscripts s, s + 1 denote the iteration numbers. The value of ε = 10−3 was found to be sufficient for our objectives. After the iterations converged, the next temporal step was performed and so on, until the steady state.
In the calculation, the generally adopted values of the parameters Citation32 were accepted, namely: ρ1 = 1.1 g cm−3; ρ2 = 1.0 g cm−3; c1 = c2 = cb = 4.2 J g−1 K−1; k1 = 5.5 10−3 W cm−1 K−1; k2 = 5.0 10−3 W cm−1 K−1; Tb = T0 = 37°C. The radius of the tumor r1 was changed from 0.6 to 1 cm, with the outer radius of the organ fixed as r2 = 4 cm. The volume concentration of the magnetic particles in the inner sphere was fixed as α = 3·10−3 Citation32,Citation38. To study the effect of blood perfusion, the quantity wb1 = wb2 = wb was varied from 0.3 to 3 mg cm−3 s−1, the middle value of 1 mg cm−3 s−1 being typical Citation32.
In what follows, the AC power is expressed through the amplitude H of AC magnetic field with the frequency ω/2π = 800 kHz, applied to the tissue containing magnetic particles with volume concentration α = 3 10−3. These values taken at H = 10 kA/m will be referred to as ‘standard’ ones. Note that the data presented below can be easily re-counted for other combinations of α, H, and ω for a given value of αP, see Equation 1.
Results and discussion
Some results of our calculations are shown in and listed in and . In , the kinetics of heating are presented at different AC field amplitudes, both for the centre (r = 0) and the interface between the diseased and healthy tissues (r = r1). In , the uniform space distribution of the magnetic particles is assumed in the whole tumor area, 0 ≤ r ≤ r1, for two different tumor radii: r1 = 1 cm () and r1 = 0.6 cm (). In , a more complicated shape is studied, namely, the particles are uniformly distributed in the spherical layer r′ ≤ r ≤ r1 of the tumor with the outer radius r1 = 1 cm. Such ‘shell’ geometry may be of particular interest in the case when the magnetic particles are delivered through the blood stream, and the tumor periphery is supplied with the blood more intensively than the central area.
Figure 2. Calculated temperature kinetics in the center, r = 0 (upper set, black) and at the boundary of the tumor, r = r1 (lower set red) for various amplitudes H of the AC magnetic field, from the bottom upwards: 7.1 kA/m (dotted); 10 kA/m (dash-dot); 14.1 kA/m (dashed); 17.3 kA/m (solid thin); 20 kA/m (solid thick). (A): r1 = 1 cm, uniform doping; (B) r1 = 0.6 cm, uniform doping; (C): r1 = 1 cm, shell doping at 0.6 < r < r1. Inset in (A) shows the kinetics at H = 10 kA/m for various values of the blood perfusion parameter, from the bottom upward: wb = 3; 1; and 0.3 mg cm−3 s−1.
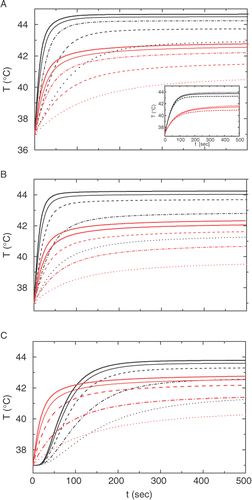
As it is seen from , the temperature inside the 1-cm tumor attains its steady state Tst ≅ TC during 200–300 sec at the standard conditions and considerably faster at higher AC levels. A bit higher power is needed to attain TC with smaller tumor radius (r1 = 0.6 cm), . This rather unexpected result suggests that the heating depends on the total amount of the magnetic adsorbent stronger than on its concentration.
Figure 3. Calculated dependencies of the steady state temperature at different distances from the tumor center (shown at the curves) on the amplitude H of the AC magnetic field. The A, B, and C symbols indicate the same geometries as in . The dashed and dash-dotted curves in (A) correspond to the blood perfusion magnitudes wb = 3 and 0.3 mg cm−3s−1, respectively; all solid curves are for wb = 1.0 mg cm−3s−1.
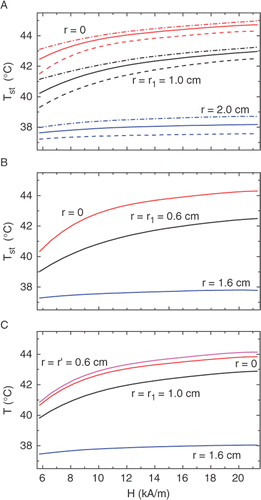
The most important feature is the temperature stabilization near TC. To show this effect clearer, the Tst values are plotted versus H in for two tumor sizes and different particle distributions. shows also the effect of blood perfusion, with the parameter wb changing from 0.3 to 3 mg cm−3 s−1. represents the main result of this work: it can be seen that, after approaching TC ≅ 42°C, the temperature inside the tumor depends only slightly on the AC power, thus protecting the tissue against overheating. In all cases shown, this result is achieved already at the ‘standard’ value of H = 10 kA/m. As a measure of stabilization, one can choose the temperature increment ΔTst caused by the two-fold increase of the AC power (that is, the increase in H) above the standard value. As can be seen from and , the value of ΔTst does not exceed 1°C both in the centre and at the border of tumor, in the whole range of the blood-perfusion rates considered. The latter shows that a moderate change in wb which may occur upon heating and depends on r Citation39 cannot affect substantially the temperature landscape.
Figure 4. Temperature profiles calculated for H = 14.1 kA/m (upper set, red) and 10 kA/m (lower set, black) at various durations of the AC heating, from the bottom upward: 50 s (dash-dot), 100 s (dashed) and steady state (solid curves). The A, B, and C symbols indicate the same geometries as in . Thick borders show the doped region.
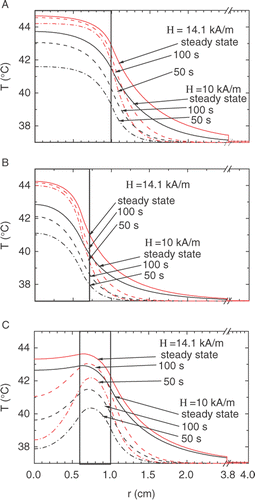
Another essential feature to be investigated is the temperature profile inside and outside the tumor. The strong temperature contrast between the diseased and healthy tissues is quite desirable since it provides minor damage of the living organ. The comparison of the temperature kinetics at r = 0 and r = r1 is presented in , whereas shows the steady-state regimes at r = 0, r′, r1 and r1 + 1 cm (the latter being in the healthy region). These results are also shown in . In , the calculated temperature profiles are shown at various points of time during the heating.
Table I. Characterization of temperature stabilization under AC heating: steady state temperatures Tst at various distances r from the centre and the temperature increment ΔTst caused by 2-fold raising of AC power above standard level (H = 10 kA/m; ω/2π = 0.8 MHz).
To characterize the steepness of the temperature profile in the interface between the diseased and healthy tissues, we use the value of ∂T/∂r (r = r1). The data for the three geometries and various durations of the heating are presented in . It can be seen that the temperature gradient attains its maximum at t ≅ 100 sec, and then decreases a bit when approaching the steady state. To take advantage of this feature, one may suggest some sort of pulse hyperthermia, with the AC source working periodically with the pulse length of about 100 sec. This suggestion is, however, unsuitable in the shell geometry (), since in this case the temperature dip in the center disappears only in the steady state.
Table II. Characterization of the temperature profile: the calculated temperatures T inside and outside of the tumor and temperature gradient ∂T/∂r at the interface at various heating durations. Assumed: H = 10 kA/m; ω/2π = 0.8 MHz; α = 0.003; wb = 1.0 mg cm−3 s−1.
Anyway, the profile at the boundary is sufficiently steep in all geometries, providing slight enough heating outside the tumor, where the temperature does not exceed the harmless level of 37°–39°C. The steep profile is the natural consequence of the temperature constraint in the inner region (similar effect is widely used in radio engineering for forming the rectangular pulses).
Conclusion
As a conclusion, the temperature dependence of the AC magnetic losses χ″(T) has been studied experimentally on fine powder of the specially synthesized low-TC manganite, La0.8Ag0.15MnO2.95. Steep decreasing of χ″ is recorded in the vicinity of the Curie point, TC ≅ 42°C, in the frequency range of 0.5–2 MHz. The data are fitted by the test function which was then used in the numerical analysis of the bioheat equations under typical conditions of the hyperthermia treatment. The calculations were performed for spherical tumors of different sizes, both for uniform and shell distributions of magnetic particles, and at typical values of bioheat parameters, including the effect of blood perfusion. The main result of the work is the prediction of good temperature autostabilization near TC inside the tumor and steep temperature profile at the interface of the diseased and healthy tissues. These features are shown to be attainable at the ‘standard’ level of the AC power (H = 10 kA/m; ω/2π = 0.8 MHz) and particle concentration (α = 0.003). The effect of autostabilization becomes still stronger at higher power, but in practice the values of H, ω, and α may be restricted both by technical and biocompatibility reasons.
Thus, the fine particles of La0.8Ag0.15MnO2.95 can be considered as a promising material for the temperature-controlled hyperthermia with good temperature autostabilization in the narrow range of 42°–44°C. Further advance, including still stronger stabilization at different critical temperatures within the range of interest (40°–50°C), is seemed to be quite realistic; to this end, other compositions of the same manganite family should be examined. Problems of targeting and biocompatibility are also very important for practical applications. Preliminary data obtained on the same La-Ag manganites are encouraging Citation13, but can be considered as the first step only. Further experimental work with living objects is necessary.
Acknowledgements
The authors are grateful to A.A. Generalov, V.V. Demidov, and B.M. Odintsov for their help and useful discussions. The work was supported by RFBR (grant numbers 08-02-00040, 07-03-01019a, 07-02-91567-NNIO-a), Russian Academy of Sciences (Programs P03-2-24 and ‘Spintronics’), and HFSP (Grant RGP-0047/2007-C).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Gilchrist RK, Medal R, Shorey WD, Hanselman RC, Parrott JC, Taylor CB. Selective inductive heating of lymph. Ann Surg 1957; 146: 596–606
- Andrä W. Magnetic hyperthermia. Magnetism in medicine, W Andrä, H Nowak. Wiley, Berlin 1998; 450–470
- Jordan A, Scholz R, Wust P, Fahling H, Felix R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J Magn Magn Mater 1999; 201: 413–419
- Moroz P, Jones SK, Gray BN. Magnetically mediated hyperthermia: Current status and further directions. Int J Hyperthermia 2002; 18: 267–284
- Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J Phys D: Appl Phys 2003; 36: 167–181
- Hergt R, Hiergeist R, Hilger I, Kaiser WA, Lapatnikov Y, Margel S, Richter U. Maghemite nanoparticles with very high AC-losses for application in RF-magnetic hyperthermia. J Magn Magn Mater 2004; 270: 345–357
- Kawashita M, Tanaka M, Kokubo T, Inoue Y, Yao T, Hamada S, Shinjo T. Preparation of ferrimagnetic magnetite microspheres for in situ hyperthermic treatment of cancer. Biomaterials 2005; 26: 2231–2238
- Pradhan P, Giri J, Samanta G, Sarma HD, Misra KP, Bellare J, Banerjee R, Bahadur D. Comparative evaluation of heating ability and biocompatibility of different ferrite-based magnetic fluids for hyperthermia application. J Biomed Mater Res, Part B: Appl Biomater 2007; 81B: 12–22
- Giri J, Ray A, Dasgupta S, Datta D, Bahadur D. Investigation on TC tuned nano particles of magnetic oxides for hyperthermia applications. Biomed Mater Eng 2003; 13: 387–399
- Kuznetsov AA, Shlyakhtin OA, Brusentsov NA, Kuznetsov OA. ‘Smart’ mediators for self-controlled inductive heating. European Cells and Materials 2002; 3: 75s–77s
- Pollert E, Knizek K, Marysko M, Kaspar P, Vasseur S, Duguet E. New TC-tuned magnetic nanoparticles for self-controlled hyperthermia. J Magn Magn Mater 2007; 316: 122–125
- Prasad NK, Rathinasamy K, Panda D, Bahadur D. J Biomed Mater Res Part B: Applied Biomaterials 2008; 85B: 409–416
- Melnikov OV, Gorbenko OYu, Popova MN, Kaul AR, Atsarkin VA, Demidov VV, Roy EJ, Soto C, Odintsov BM. Ag-doped Manganite Nanoparticles: New Materials for Temperature-Controlled Medical Hyperthermia. J Biomed Mater Res, Part A, in press. DOI: 10.1002/jbm.a.32177
- Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol 1948; 1: 93–122
- Halac S, Roemer RB, Oleson JR, Cetas TC. Magnetic induction heating of tissue: Numerical evaluation of tumor temperature distributions. Int J Radiat Oncol Biol Phys 1983; 9: 881–91
- Roemer RB, Cetas TC, Oleson JR, Halac S, Matloubieh AY. Comparative evaluation of hyperthermia heating modalities. I. Numerical analysis of thermal dosimetry bracketing cases. Radiat Res 1984; 100: 450–472
- Roemer RB, Cetas TC, Oleson JR, Halac S, Matloubieh AY. Comparative evaluation of hyperthermia heating modalities. II. Application of the acceptable power range technique. Radiat Res 1984; 100: 473–486
- Matloubieh AY, Roemer RB, Cetas TC. Numerical simulation of magnetic induction heating of tumors with ferromagnetic seed implants. IEEE Trans Biomed Eng 1984; 31: 227–234
- Osteltree KB, Frizzell LA. Determination of power deposition patterns for localized hyperthermia: A steady state analysis. Int J Hyperthermia 1987; 3: 269–279
- Osteltree KB, Frizzell LA. Determination of power deposition patterns for localized hyperthermia: A transient analysis. Int J Hyperthermia 1988; 4: 281–296
- Durkee JW, Antich PP, Lee CE. Exact solution to the multiregion time-dependent bioheat equation I: Solution development. Phys Med Biol 1990; 35: 847–867
- Durkee JW, Antich PP. Exact solution to the multiregion time-dependent bioheat equation II: Numerical evaluation of the solution. Phys Med Biol 1990; 35: 869–889
- Durkee JW, Antich PP. Exact solution to the multi-region time-dependent bioheat equation with transient heat sources and boundary conditions. Phys Med Biol 1991; 36: 345–368
- Durkee JW, Antich PP. Characterization of bioheat transport using an exact solution to the cylindrical geometry, multi-region, time-dependent bioheat equation. Phys Med Biol 1991; 36: 1377–1406
- Haider SA, Cetas TC, Roemer RB. Temperature distribution in tissues from a regular array of hot source implants: An analytical approximation. IEEE Trans Biomed Eng 1993; 40: 408–417
- Arkin H, Xu LX, Holmes KR. Recent developments in modelling heat transfer in blood perfused tissues. IEEE Trans Biomed Eng 1994; 41: 97–107
- Kotte ANTJ, van Wieringen N, Lagendijk JJW. Modelling tissue heating with ferromagnetic seeds. Phys Med Biol 1998; 43: 105–120
- van Wieringen N, Kotte ANTJ, van Leeuwen GMJ, Lagendijk JJW, van Dijk JDP, Nieuwenhuys GJ. Dose uniformity of ferromagnetic seed implants in tissue with discrete vasculature: A numerical study on the impact of seed characteristics and implantation techniques. Phys Med Biol 1998; 43: 121–138
- Andrä W, d’Ambly CG, Hergt R, Hilger I, Kaiser WA. Temperature distribution as function of time around a small spherical heat source of local magnetic hyperthermia. J Magn Magn Mat 1999; 194: 197–203
- Rosensweig RE. Heating magnetic fluid with alternating magnetic field. J Magn Magn Mater 2002; 252: 370–374
- Lv YG, Deng ZS, Liu J. 3-D numerical study on the induced heating effects of embedded micro/nanoparticles on human body subject to external medical electromagnetic field. IEEE Trans Nano Bioscience 2005; 4: 284–294
- Bagaria HG, Johnson DT. Transient solution to the bioheat equation and optimization for magnetic fluid hyperthermia treatment. Int J Hyperthermia 2005; 21: 57–75
- Stańczyk M. Numerical model of heat exchange in perfused tissues. Acta Bioeng Biomech 2007; 9: 25–34
- Stauffer PR, Cetas TC, Fletcher AM, DeYoung DW, Dewhirst MW, Oleson JR, Roemer RB. Observations on the use of ferromagnetic implants for inducing hyperthermia. IEEE Trans Biomed Eng 1984; 3: 76–90
- Roemer RB, Fletcher AM, Cetas TC. Obtaining local SAR and blood perfusion data from temperature measurements: steady state and transient techniques compared. Int J Radiat Oncol Biol Phys 1985; 11: 1539–1550
- Cetas TC, Gross EJ, Contractor Y. A ferrite core/metallic sheath thermoseed for interstitial thermal therapies. IEEE Trans Biomed Eng 1998; 45: 68–77
- Cetas TC, Gross EJ, Contractor Y. A ferrite core/metallic sheath thermoseed for interstitial thermal therapies. IEEE Trans Biomed Eng 1998; 45: 68–77
- Hilger I, Hiergeist R, Hergt R, Winnefeld K, Schubert H, Kaiser WA. Thermal ablation of tumors using magnetic nanoparticles. Invest Radiol 2002; 37: 580–586
- Toglia A, Kittelson JM, Roemer RB, Hodak JA, Carter LP. Cerebral bloodflow in and around spontaneous malignant gliomas. Int J Hyperthermia 1996; 12: 461–476