Abstract
Purpose: The goal of this study was to determine whether whole body hyperthermia (WBH) could reduce oxidative stress in the striatum produced by 3-nitropropionic acid (3-NP), a mitochondrial toxin that irreversibly inhibits succinate dehydrogenase (SDH), causing impairment of energy metabolism, oxidative stress and a selective degeneration of striatal cells.
Methods: Rats were subjected to WBH (42°C) or normothermia control conditions for 30 min and then treated with 3-NP. Striatum samples were processed and the levels of protein carbonyl groups, biogenic amines, Hsp72 and salicylate hydroxylation (to probe the hydroxyl radical (OH•) intervention) were determined.
Results: WBH significantly reduced oxidative stress in the striatum of animals treated with 3-NP, as judged by reductions in protein carbonyl and salicylate hydroxylation derivative levels, whereas striatal Hsp72 expression was significantly increased. The groups treated with 3-NP presented an increased in the dopamine (DA) derivatives 2,3-dihydroxyphenylacetic acid (DOPAC) and norepinephrine (NE) concentration, whereas the striatal relation DOPAC/DA concentration indicate a reduced dopamine turnover.
Conclusions: These studies show, for the first time, that a heat shock pretreatment can ameliorate the oxidative stress produced by a metabolic toxin (3-NP) capable of impairing energy supply and produce selective striatal degeneration. These data contribute to a better understanding of the potential for thermal stress to modulate the type of oxidative stress usually present in neurodegenerative disorders associated with metabolic defects.
Introduction
The brain, like other tissues, responds to stressful stimuli by activating a cascade of signaling events. Hyperthermia is a primordial stimulus and an inducer of the heat shock response. Heat shock preconditioning results in the overproduction of Hsp72 in some regions of the brain including the striatum, and this treatment confers protection against circulatory shock and striatal ischemic injury during heatstroke Citation1. Heat shock proteins (Hsps) protect cells against many chronically and acutely stressful conditions; subacute activation of Hsps results in stress tolerance and cytoprotection against otherwise lethal exposures to stress-induced molecular damage. Moreover, it has been demonstrated that heat shock pretreatment confers functional protection to synapses at the level of neurotransmission, and the time course of this protection was correlated with the temporal profile of Hsp72 Citation2,Citation3.
Thermotolerance is a biological response which enables organisms to survive high temperatures after the exposure to previous stress conditions including heating at sub-lethal temperatures Citation4. Thermotolerance reduces the consequences of heat stress induced hyperthermia as arterial hypotension, intracranial hypertension, brain hypoperfusion, decreased baroreceptor reflex sensitivity, cerebral ischemia and cerebral injury in the rat Citation4,Citation5. The mechanism underlying damage to the brain is thought to involve increased production of reactive oxygen and nitrogen species and probably decreased or deficient antioxidative defenses Citation6,Citation7. Indeed, circulatory shock and central nervous system ischemia have been associated with increased production of free radicals in the brain, including the striatum of heatstroke-affected animals; heat shock preconditioning significantly attenuated oxidative stress and levels of cytokines Citation1.
The mitochondrial toxin 3-nitropropionic acid (3-NP) has been used to generate an animal model of Huntington disease which has yielded evidence implicating oxidative stress in the damage produced in the striatum Citation8. 3-NP is a suicide inhibitor of SDH Citation9,Citation10, an enzyme that transfers electrons across the respiratory chain. SDH blockade causes a rapid depletion of intracellular ATP, leading to impairment of cation exchange pumps (Ca2+ATPase and Na+/K+ ATPase) and activation of glutamate receptors, particularly the N-methyl-D-aspartate (NMDA) subtype Citation11,Citation12.
Studies have shown that irreversible inhibition of SDH by acute or chronic administration of 3-NP leads to neurotoxicity especially in the striatum region of the brain Citation13,Citation14, and also in the hippocampus, thalamus, cerebral cortex and cerebellum Citation15,Citation16. Neuronal degeneration observed in 3-NP intoxications has been associated with decreased ATP synthesis, an increase of lactate Citation17 and the production of free radicals as well Citation18,Citation19. However, the mechanism of 3-NP toxicity is not yet thoroughly understood. Several reports indicate that DA contributes to the striatal damage induced by the impairment of energy metabolism. The removal of DA afferents reduces the severity of striatal lesions of the 3-NP Citation20. In addition, 3-NP toxicity was enhanced in animals administered amphetamines, a drug that stimulates dopamine release Citation21.
In the present study we found that a single exposure of animals to WBH provides protection against subsequent exposure of the brain to oxidative stress. In a metabolic-depletion model using 3-NP, we found that a heat shock pretreatment protects the striatum of rats given drug treatment, apparently by interfering with dopamine metabolism and the oxidative stress production. These results help to further understand 3-NP toxicity and the mechanism of by which damage may occur, as well as the protective effects that heat pretreatment could exert on the brain, threatened by an event of metabolic depletion.
Methods
These experiments were done in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH No. 80-23). All compounds used were from Sigma (Saint Louis, MO) and of the highest purity available.
Animals
12-week-old 350 ± 4.0 g male Sprague Dawley rats were used. The rats were housed in acrylic cages under 12 hours light–dark cycle and room temperature (20 ± 2°C) controlled conditions. Food (rodent laboratory chow) and water were provided ad libitum. Rats were randomly divided into 4 groups of 10 animals each: normothermic control group (CTRL); normothermic group treated with 3-nitropropionic acid (3NP); hyperthermic group without 3-NP treatment (HT); hyperthermic group treated with 3-nitropropionic acid (3NP + HT).
Induction of hyperthermia
The body temperature of rats was elevated by placing animals in a dry-air incubator set at 43°C, and the rectal temperatures monitored. Once the target temperature (42°C ± 0.2°C) was reached for the HT and 3NP + HT groups, they were maintained at this temperature for 30 min through all the experiments except for the movement evaluation experiments (see below, Evaluation of movement). Rats were then removed from the incubator and returned to their cages at standard conditions. Animals from the corresponding groups were treated with 3-NP or saline solution 0.9% 16 hrs after hyperthermic pulse.
Treatment with 3-nitropropionic acid (3-NP)
3-NP was dissolved in 0.9% saline solution and pH adjusted to 7.4. The rats in groups that received 3-nitropropionic acid (3NP and 3NP + HT), received a first dose of 20 mg/Kg of weight intraperitoneally 16 hours after hyperthermic treatment (corresponding to 0 hours) and then two additional identical doses corresponding to 12 hours and 24 hours after 3-NP administration. The groups CTRL and HT received saline solution at same intervals. Two hours after the last injection animals were decapitated. Their brains were rapidly dissected on ice, and the cerebellum, frontal cortex and bilateral striata were collected and immediately frozen at −80°C.
Evaluation of movement
To evaluate the motor abnormalities produced by 3-NP, and the duration of hyperthermia required for observable motor improvement, a quantitative neurological scale previously described Citation22 and adapted for this study was used. A neurological score was determined for each animal on day 3 from the first injection of 3-NP, in comparison to control animals. (Score = 0, normal behavior; 1, general slowness of displacement resulting from mild hind limb impairment; 2, in coordination and marked gait abnormalities; 3, hind limb paralysis; 4, inability to move resulting from forebrain and limb impairment; 5, recumbence.). A score level was accepted when 100% of the animals in a group (10/10) clearly presented motor abnormalities. Half point (0.5) in the score represented the combination of two subsequent motor abnormalities in a group (5/10) = 50%, (4/10) = 40% (0.4), etc. The same procedure was carried out for 6 groups (10 animals by group): control rats without 3-NP treatment neither heat (CTRL), rats treated with 3-NP without heat exposure (3-NP) and four more groups of rats treated with 3-NP and 15, 30, 45 and 60 min each of heat exposure (3NP + HT 15 min, 3NP + HT 30 min, 3NP + HT 45 min and 3NP + HT 60 min).
Measurement of carbonyls in proteins
To evaluate the oxidative stress resulting from 3-NP treatment, the protein carbonyl determination was carried out according to previous reports Citation23,Citation24 with slight modifications. Samples were sonicated in 0.1% ice-cold perchloric acid and centrifuged at 10,000 rpm for 10 min. From the supernatants, an equivalent of 2 mg were taken and added to 0.5 mL of 10 mM 2,4-dinitrophenilhydrazine (DNPH) in 2 N HCL. Tubes were left for 1 hour of incubation at room temperature in the dark; the tubes were vortexed every 15 min. The samples previously precipitated with trichloroacetic acid (20%) and re-suspended with a pulse of sonication were washed three times with 1 mL of ethanol-ethyl acetate (1 : 1; v/v) to remove the residual DNPH reagent. The final precipitates were dissolved in 6N guanidine hydrochloride solution (2 mL). The protein carbonyl content was determined by measuring the absorbance of the protein-2,4 dinitrophenylhydrazone derivative at 375 nm, using a molar absorption coefficient of 22,000 M−1 cm−1. The amount of protein was determined processing a parallel sample blank containing no DNPH for each sample. The carbonyl content was reported as nmol/mg of protein.
Indirect quantitation of hydroxyl free radicals
To verify the possible intervention of the hydroxyl radical as an oxidative stress source, a high-pressure liquid chromatographic method with electrochemical detection was used Citation25,Citation26, with modifications. Two hours before the last administration of 3NP, an intraperitoneal injection of sodium salicylate (200 mg/Kg i.p.) was administered to the groups CTRL, 3NP, 3NP + HT and HT; an additional group received only saline solution 0.9% (N). The brains were dissected on ice and the bilateral striatum was collected and sonicated in 0.1% ice-cold perchloric acid. Before centrifugation at 10,000 rpm for 15 min, the supernatants were filtered through a Millipore (Bedford, MA) filter of 0.2 µm and 50 µL injected onto an Alltech C18 column, 3 µm, connected to a Waters liquid chromatography apparatus. The mobile phase was an isocratic mixture of sodium acetate-citrate buffer (100 mM, pH 7.4) and methanol (95:5) at a flow rate of 1 mL/min. Both, 2,3-DHBA and 2,5-DHBA were monitored by an electrochemical detector (Antec Leyden, the Netherlands) set at 450 mV versus a Hy-Ref reference electrode. Authentic DHBs were used as standards, and the data expressed as the ratio of the sum of areas of derivatives to mg of striatum tissue.
Determination of stress protein (Hsp72) expression
To evaluate the thermal induction of Hsp72 in the HT studied groups and the absence of induction from the 3-NP as a second stressor, a western blot analysis of the striatum proteins was carried out, using a monoclonal anti-heat shock protein 70 antibody (H5147, Sigma) Citation29. An additional monoclonal antibody specific for actin (AC-40, Sigma) was used to verify the protein concentration uniformity. The Hsp antibody used recognizes both the constitutive and inducible forms of Hsp70; only the inducible form modifies its expression on heat stress. The animals were killed by decapitation and the striata dissected from the brains on an ice cold plate. For protein extraction, the samples were weighed and sonicated with a buffer consisting of 20 mM Tris · HCl (pH 7.6), 100 mM KCl, 5 mM NaCl, 2 mM EDTA, 1 mM EGTA, 2 mM dithiothreitol, and 2mM phenylmethylsulfonyl fluoride at pH 7.2. After centrifugation at 10,000 g for 10 min at 4°C, a protein assay was carried out by the Bradford method. The samples (40 µg/lane) were incubated for 5 min at 95°C in Laemmli buffer, separated on 10% SDS-polyacrylamide discontinuous gel, and then electrophoretically transferred to a nitrocelulose membrane. The membrane was blocked for 1 h at room temperature with PBS containing 10% skim milk. Incubation with the primary antibodies (Hsp70 1 : 1, 000 dilution and actin 1 : 200) was carried out for 1 h at room temperature. The membrane was rinsed three times with TTBS and developed within 3 min by an alkaline phosphate conjugate at room temperature. For further quantitative analysis, gel and immunoblot images were obtained using a densitometer.
Evaluation of biogenic amines
To determine possible participation of DA and another potentially toxic metabolites in the oxidative stress generation, biogenic amines (DA, norepinephrine, serotonin and derivatives) were estimated by reverse-phase high pressure liquid chromatographic method with electrochemical detection, based on methods described previously Citation27,Citation28. Another two regions of the brain, cortex and cerebellum were studied for comparison of their dopamine levels to that of the striatum. Samples were sonicated in 0.1% ice-cold perchloric acid and centrifuged at 10,000 rpm for 10 min. 50 µL of supernatants was filtered through a Millipore (Bedford) filter of 0.2 µm and injected immediately. Analysis was carried out with the use of an Alltech-Catecholamine RP-18, 3 µm (250 mm × 4.6 µm) analytical column, using a Waters 517 and 717 pump and autosampler, electrochemical detector (Antec Leyden) and Waters Millenium 32 software. The mobile phase (1 mL/min flow) consisted of 0.1 M monochloracetic acid (pH 3.3) containing 2 mM Na2EDTA, 1 mM sodium octyl sulphate and 10% methanol. The compounds were oxidized with a glassy carbon electrode set at potential of +450 mV versus a Hy-Ref reference electrode. Authentic biogenic amines and derivatives were used as standards: DA, NE, DOPAC, Homovanilic acid (HVA), 5-Indolacetic acid (5-IAA) and 5-Hydroxytriptamine (5-HT), Sigma.
Statistical analyses
Data were analyzed with statistical software (Sigmaplot 10.0) using a one-way analysis of variance (ANOVA) with the Tukey's post-test. A level of statistical significance was set at p < 0.05.
Results
Whole body hyperthermia can improve the motor abnormalities produced by the 3-NP
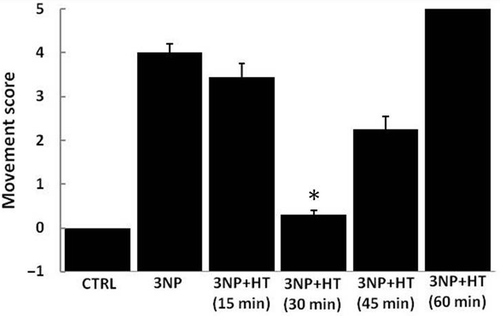
To test the effects of pretreatment with whole body hyperthermia on 3-NP-induced motor abnormalities, a simple neurological scale of movement was used. As shown in , the effect of the toxin is dependent upon the duration of hyperthermia used, showing a maximal positive effect at 30 and 45 min of heating, but showing no improvement (or even a worsening of movement) at 15 and 60 min of heating (, 3-NP + HT 60 min). In the case of 30 min of heating, the improvement was nearly the same as the CTRL score level. The period of time of 30 min (HT 30 min) was then used in subsequent experiments in which oxidative stress, biogenic amines and Hsp72 were evaluated.
Figure 1. Total movement score and the effect of thermal treatment in control rats (CTRL), rats treated with 3 NP (20 mg/Kg, i.p., 3 doses 0,12 and 24 h) and rats treated with 3-NP but subject to a heat pretreatment (WBH 15, 30, 45 and 60 minutes). Rats where subject to hyperthermia and injected with a first dose of 3-NP 16 hours later; two more doses of 3-NP were applied with an interval of 12 hours each. Groups were evaluated 6 hours after the last injection. Each column represents the mean ± SEM for five independent experiments (n = 10 animals by group). *p < 0.05 versus all the groups except control group (CTRL).
Whole body hyperthermia (HT 30 min) reduced the carbonyl stress produced in the striatum by the 3-NP toxicity
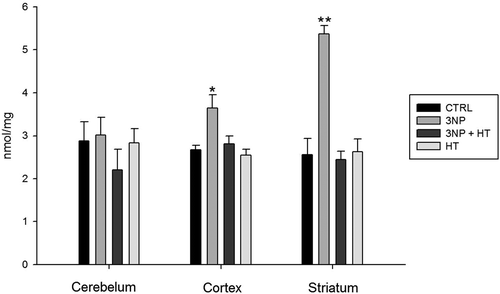
To evaluate the possible protective effect of WBH on the 3-NP-induced oxidative stress, the carbonyl level of striatum proteins was measured. As shown in , the carbonyl level in cortex and striatum is elevated only in the groups treated with 3-NP (3NP group). However, in the striatum it was possible to see the higher protein carbonyl level (even 100%), whereas the cerebellum did not show any evidence of carbonyl formation. WBH (HT 30 min) normalized the carbonyl level in cerebellum and striatum of the groups treated with 3-NP (3NP + HT). Hyperthermia itself did not show any ability to contribute to protein carbonyl formation (, HT group).
Figure 2. Carbonyl protein amount in cerebellum, cortex and striatum of rats treated with 3-NP (20 mg/Kg, i.p., 3 doses 0,12 and 24 h) and the effect of whole body hyperthermic pretreatment (WBH 42°C, 30 minutes). Groups: Control (CTRL); rats treated with 3-nitropropionic acid (3NP); rats treated with 3NP and previously subjected to whole body hyperthermia 30 minutes (3NP + HT); rats subject to whole body hyperthermia 30 minutes (HT). The protein amount was determined processing a parallel sample blank without 2,4-dinitrophenylhydrazine (DNPH) for each sample and constructing a standard curve with bovine serum albumin (BSA) dissolved in guanidine 6M. The carbonyl content was reported as nmol/mg of protein. Values are means ± SEM. n = 10. *p < 0.05 versus CTRL ** p < 0.01 versus CTRL and 3NP Cortex.
WBH (HT 30 min) reduces hydroxyl radical-dependent oxidative stress in the striatum produced by 3-NP treatment
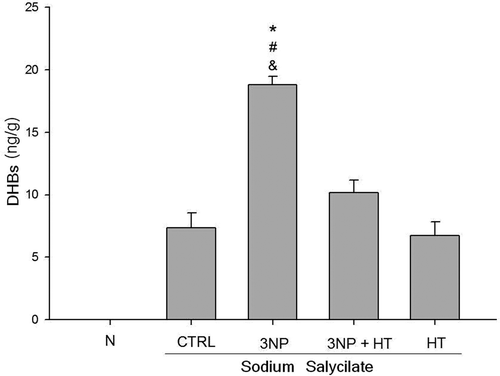
The possible intervention of the hydroxyl radical (OH•) in the striatum of rats treated with the 3-NP was evaluate using a (OH•) salicylate-trapping method and the formation of the metabolites 2, 3 and 2,5-Hidroxibenzoic acids (DHBs). As shown in , the formation of DHBs was increased in the striatum of the group of rats treated with 3-NP (3NP) more than twice the level of control group (CTRL), whereas 30 min of hyperthermia (3NP + HT group) reduced the DHBs level in 50% (p < 0.01). There was no evidence of salicylate metabolites increased concentration in the striatum of rat subject only to hyperthermia (HT group) compared with the control group (CTRL). No evidence of DHBs was observed in a group where only saline solution 0.9% was injected (N).
Figure 3. Hydroxyl radical-dependent oxidative stress in the striatum of rats treated with 3-NP (20 mg/Kg, i.p., 3 doses 0,12 and 24 h)-and the effect of whole body hyperthermic pretreatment (WBH 42°C, 30 minutes). Sodium salicylate (200 mg/Kg i.p.) was administered to all groups except saline solution (N) 2 h after the last 3-NP injection. The groups: control group (CTRL), group of rats treated with 3-nitropropionic acid (3NP), group of rats subject to heat pretreatment and then treated with 3-NP (3NP + HT) and the group of rats treated only with whole body hyperthermia 30 minutes (HT). Values are means ± SEM. n = 10. * p < 0.01 3NP versus CTRL; # versus 3NP + HT; & versus HT.
Whole body hyperthermia (30 min) increases the level of Hsp72 in the striatum
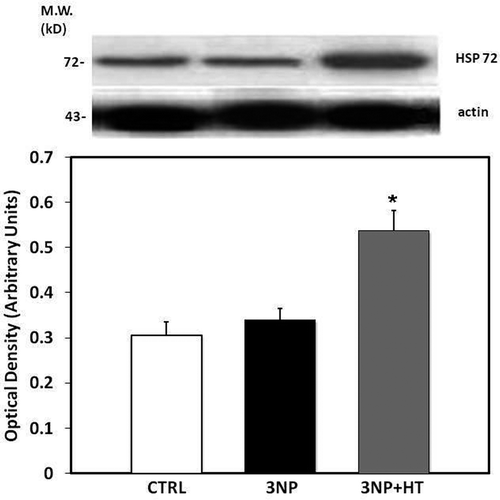
Using SDS-PAGE followed by electrophoresis and a western blot analysis, the level of Hsp72 was assessed in order to determined whether heat stress (30 min of hyperthermia) is able to result in an increase the expression of Hsp72 in the striatum of control rats or rats treated with 3-NP (). We observed that hyperthermia plus 3-NP but not nitropropionic acid alone increases the Hsp72 content in the striatum (16 hours after 30 min of WBH). In rats given 3-NP treatment alone, as with the control rats without heat pretreatment and drug administration, the level of expression of Hsp70 was similar, and corresponding to constitutive form of Hsp72 that can be detected by the antibody as well. It is possible to assume that heat treatment but not 3-NP increased Hsp72 expression (inducible form) such as was demonstrated in previous reports.
Figure 4. Representative Western blot of HSP 72 expression and densitometric analysis derived from striatum samples in the control, group (CTRL), in rats treated with 3-nitropropionic acid 20 mg/Kg, i.p, 3 doses, 0, 12 and 24 h (Group 3-NP) and in rats subject to heat pretreatment (WBH 42°C, 30 minutes) and then treated with 3-NP (Group 3-NP + HT). The Western blot of actin was carried out to assure constant amounts of protein in each lane. Means of three experiments ± SEM. *p < 0.05 versus CTRL.
Hyperthermia modifies the biogenic amines concentration and the dopamine recycling in the striatum
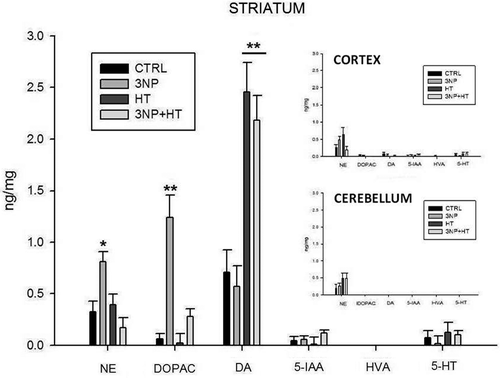
shows the effect of 30 min of whole body hyperthermia on the concentration of biogenic amines and metabolites in the striatum of rats evaluated using a liquid chromatographic method with electrochemical detection. 3-NP treated rats without prior hyperthermia showed a significant increase in DA metabolites, norepinephrine and 2,4-Dihydroxyphenylacetic acid concentration (p = 0.014 and p < 0.001 respectively). However, the concentration of DA showed no apparent changes. In contrast, in the group of rats subjected to hyperthermia and subsequently treated with 3-NP, the DA concentration appears elevated and is more than twice that seen in the control group (p < 0.001). Again, the DA metabolites (NE and DOPAC) remained unchanged with respect to the control group. The group of rats subjected only to hyperthermic treatment presents similar behavior, with high DA concentration and low DA and NE. In contrast to the striatum tissues, there were no significant changes in the biogenic amines level or metabolites in the cerebellum and cortex of groups in , inserted graphs; furthermore, no changes were detected in the levels of 5IAA, HVA or 5-HT in any of the studied groups.
Figure 5. Determination of biogenic amines in the striatum, cerebellum and cortex of rats treated with 3-NP (20 mg/Kg, i.p., 3 doses, 0,12 and 24 h) and the effect of whole body hyperthermia pretreatment (WBH 42°C, 30 minutes). NE, norepinephrine; DOPAC, 3,4-dihydroxyphenylacetic acid; DA, dopamine; 5-HIAA, 5-hydroxyindole-3-acetic acid; HVA, homovanillic acid; 5-HT, 5-hydroxytryptamine. Groups: Control (CTRL); rats treated with 3-nitropropionic acid (3NP); rats treated with 3NP plus whole body hyperthermia 30 minutes (3NP + HT) and rats treated only with whole body hyperthennia 30 minutes (HT). Values are means ± SEM. *p = 0.014 versus CTRL. ** p < 0.001 versus CTRL.
Discussion
In a previous study, it was demonstrated that pretreatment with heat shock can exert protective effects against subsequent cerebral ischemic damage and death from heatstroke Citation1. These experiments demonstrated the positive effect that the heat can exert on striatal ischemic parameters; however, the basis by which this positive effect achieved is still not clear. The results presented in the current work demonstrate that a hyperthermic pretreatment (30 min whole body heating) reduces the oxidative stress associated with protein oxidation in the striatum (and less extensively in the cortex), produced by the metabolic toxin 3-nitropropionic acid (). Furthermore, the results also provide evidence of hydroxyl radical intervention and its reduction by hyperthermia ().
In brain tissue that has been preconditioned by thermal or chemical injury, there is increased synthesis of Hsp72 in the striatum. This induction has been frequently associated with attenuation of heatstroke-induced cerebral ischemia, but is difficult to show a cause-and-effect relationship between Hsp overexpression and thermal tolerance since is not possible to avoid the cellular and physiological changes associated with hyperthermic treatment Citation1,Citation2. In the present work, we noted a time-dependent effect of hyperthermia (): 15 min appears to be an insufficient duration for sufficient induction of Hsp72 while a much longer period of time (60 min) may result in cerebral ischemia produced by heat. However, while a treatment midway between these durations (30 min) appears to reduce the effects of 3-NP toxicity, it does not explain how Hsp70 by itself can reduce oxidative stress and improve movement.
When transgenic mice were used to examine the role of Hsp72 in experimental heatstroke, the results demonstrated that the Hsp72 in the striatum was correlated with improved survival during heatstroke, reduced circulatory shock, and cerebral ischemia and damage in mice. Again however, the results do not clearly explain how Hsp72 overexpression confers protection by reducing oxidative stress and energy depletion Citation3. Our hypothesis based upon these previous results is that there are other factors in addition to Hsp70 overexpression that results in the protection. Although the results presented here show a similar overexpression of Hsp72 () this does not explain entirely the reduction of protein oxidative stress derived from 3-NP toxicity by thermal treatment.
Some explanation may come from results presented here regarding biogenic amines (, ) and specifically dopamine and derivatives. Previous studies showed that when the striatal DA system was destroyed by 6-hydroxydopamine (6-OHDA), DA depletion protected striatal neurons from heatstroke-induced ischemia and cell death Citation30,Citation31. In other studies the evidence showed that removal of nigrostriatal dopaminergic fibers with the use of 6-OHDA attenuated 3-NP-induced striatal neurotoxicity Citation20. DA itself can be neurotoxic and this could explain the preferential vulnerability of the striatum to bioenergetic impairment Citation32, but the production of reactive oxygen species (ROS) following DA oxidation and not DA itself seem to be the mechanism of striatum damage Citation33. Indeed, DA has the property to generate ROS when it is metabolized by the enzyme monoamine oxidase (MAO) to DOPAC, forming the derivative 3,4-dihydroxyphenylacetaldehyde (DOPAL). The conversion of DA to DOPAC causes the concomitant production of H2O2, from which hydroxyl radicals can be generated through the Fenton reaction; however DOPAL can be a powerful oxidant by itself Citation33–35.
Table I. Concentrations of dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), and DA turnover (DOPAC/DA) in the striatum of rats treated with 3-nitropropionic acid, with and without whole body hyperthermia (WBH). (WBH 30 min and 3-NP 20 mg/Kg i.p. 3 doses 0, 12 and 24 h.)
Although further study is necessary to completely understand the mechanism which underlies the ability of thermal treatment to protect from oxidative stress in the striatum, the evidence indicates that in addition to Hsp72 overexpression, at least part of the effect may involve the DA metabolism () and mobilization. Indeed, from data presented in , it is possible to suggest that hyperthermia may interfere with DA turnover, which then reduces oxidative stress in the striatum by avoiding the formation of DA derivatives.
DA in the cytosol (where it is metabolized) arrives there via principally by recycling; DA that is released during an exocytotic event is moved into the cytosol via the dopamine transporter (DAT). When neuronal firing is inhibited, the amount of cytosolic DA decreases as there is no recycling Citation36. Both possibilities, either a direct effect of hyperthermia on the DAT activity or the inhibition of the neuronal firing, could cause DA turnover reduction and depletion of DA derivatives. These effects do not exclude the possibility of finding elevated DA concentration in the striatum under these circumstances, because it could come from vesicles and/or from previous exocytotic events (presynaptic terminal). In particular, we founded elevated concentrations of DA but not metabolites in the group of animals treated only with hyperthermia (). A time-dependent effect of hyperthermia on the DAT activity and the inhibition of the neuronal firing could account for the data presented in . In this case, as in the case of a possible induction of Hsp72, a longer period of time will result in cerebral ischemia and a deleterious effect.
Another possibility exists that may be a primary mechanism of action of hyperthermia and its ability to improve antioxidative defenses. In some cells such as CHO and L929 cells, hyperthermia (42°C) increases basal activity of the pentose phosphate cycle, resulting in an increased production of NADPH Citation37,Citation38. Reduced glutathione (GSH) can be regenerated from oxidized glutathione (GSSG) by glutathione reductase, enzyme, which uses NADPH as an electron donor. Glutathione is the substrate for the enzyme glutathione peroxidase (which counteracts the damaging effects of peroxides) and is the most important endogenous low molecular weight antioxidant. Thus, hyperthermia could improve antioxidative defenses increasing the reductive capacity (NADPH) necessary in the GSH regeneration and recycling; however additional studies are needed to clarify the importance of this mechanism of action of hyperthermia on striatum cells.
In conclusion, the present study provides additional evidence of protection of oxidative damage in the striatum by prior heat pretreatment and at the same time supports the notion that oxidative stress derived from dopamine mobilization and metabolism is related to a greater vulnerability of this brain region to 3-NP.
Acknowledgements
This research was supported by a grant from the Consejo Nacional de Ciencia y Tecnología, (CONACYT) 52853, México 2006.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Wang JL, Ke DS, Lin MT. Heat shock pretreatment may protect against heatstroke-induced circulatory shock and cerebral ischemia by reducing oxidative stress and energy depletion. Shock 2005; 23(2)161–167
- Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444: 860–867
- Lee WC, Wen HC, Chang CP, Chen MY, Lin MT. Heat shock protein 72 overexpression protects against hyperthermia, circulatory shock, and cerebral ischemia during heatstroke. J Appl Physiol 2006; 100: 2073–2082
- Field SB, Anderson RL. Thermotolerance: A review of observations and possible mechanisms. Natl Cancer Inst Monogr 1982; 61: 193–201
- Yang YL, Lin MT. Heat shock protein expression protects against cerebral ischemia and monoamine overload in rat heatstroke. Am J Physiol 1999; 276: H1961–H1967
- Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem 1992; 59: 1609–1623
- Knight JA. Reactive oxygen species and the neurodegenerative disorders. Ann Clin Lab Sci 1997; 27: 11–25
- Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: Central role for amyloid beta-peptide. Trends Mol Med 2001; 7: 548–554
- Alston TA, Mela L, Bright HJ. 3-Nitropropionate, the toxic substance of Indigofera, is a suicide inactivator of succinate dehydrogenase. Proc Natl Acad Sci USA 1977; 74: 3767–3771
- Coles CJ, Edmondson DE, Singer TP. Inactivation of succinate dehydrogenase by 3-nitropropionate. J Biol Chem 1979; 254: 5161–5167
- Novelli A, Reilly JA, Lysko PG, Henneberry RC. Glutamate becomes neurotoxic via the N-methyl-D-aspartate receptor when intracellular energy levels are reduced. Brain Res 1988; 451: 205–212
- Calabresi P, Gubellini P, Picconi B, Centonze D, Pisani A, Bonsi P, Greengard P, Hipskind R, Borrelli E, Bernardi G. Inhibition of mitochondrial complex II induces a long-term potentiation of NMDA-mediated synaptic excitation in the striatum requiring endogenous dopamine. J Neurosci 2001; 21: 5110–5120
- Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, Miller JM, Storey E, Srivastava R, Rosen BR, Hyman BT. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci 1993; 13: 4181–4192
- Wullner U, Young AB, Penney JB, Beal MF. 3-Nitropropionic acid toxicity in the striatum. J Neurochem 1994; 63: 1772–1781
- Hamilton BF, Gould DH. Nature and distribution of brain lesions in rats intoxicated with 3-nitropropionic acid: A type of hypoxic (energy deficient) brain damage. Acta Neuropathol 1987; 72: 286–297
- Binienda Z, Simmons C, Hussain S, Slikker W, Jr, Ali SF. Effect of acute exposure to 3-nitropropionic acid on activities of endogenous antioxidants in the rat brain. Neurosci Lett 1998; 251: 173–176
- Brouillet E, Jenkins BG, Hyman BT, Ferrante RJ, Kowall NW, Srivastava R, Roy DS, Rosen BR, Beal MF. Age-dependent vulnerability of the striatum to the mitochondrial toxin 3-nitropropionic acid. J Neurochem 1993; 60: 356–359
- Beal MF, Ferrante RJ, Henshaw R, Matthews RT, Chan PH, Kowall NW, Epstein CJ, Schulz J. B.3-Nitropropionic acid neurotoxicity is attenuated in copper/zinc superoxide dismutase transgenic mice. J Neurochem 1995; 65: 919–922
- Schulz JB, Matthews RT, Jenkins BG, Ferrante RJ, Siwek D, Henshaw DR, Cipolloni PB, Mecocci P, Kowall NW, Rosen BR, et al. Blockade of neuronal nitric oxide synthase protects against excitotoxicity in vivo. J Neurosci 1995; 15: 8419–8429
- Maragos WF, Jakel RJ, Pang Z, Geddes JW. 6-Hydroxydopamine injections into the nigrostriatal pathway attenuate striatal malonate and 3-nitropropionic acid lesions. Exp Neurol 1998; 154: 637–644
- Bowyer JF, Clausing P, Schmued L, Davies DL, Binienda Z, Newport GD, Scallet AC, Slikker W, Jr. Parenterally administered 3-nitropropionic acid and amphetamine can combine to produce damage to terminals and cell bodies in the striatum. Brain Res 1996; 712: 221–229
- Ahuja M, Bishnoi M, Chopra K. Protective effect of minocycline, a semi-synthetic second-generation tetracycline against 3-nitropropionic acid (3-NP)-induced neurotoxicity. Toxicology 2008; 244: 111–122
- Reznick AZ, Packer L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol 1994; 233: 357–363
- Levine RL. Carbonyl modified proteins in cellular regulation, aging, and disease. Free Radic Biol Med 2002; 32: 790–796
- Halliwell B, Grootveld M, Gutteridge JM. Methods for the measurement of hydroxyl radicals in biomedical systems: Deoxyribose degradation and aromatic hydroxylation. Methods Biochem Anal 1988; 33: 59–90
- Ghiselli A, Laurenti O, De Mattia G, Maiani G, Ferro-Luzzi A. Salicylate hydroxylation as an early marker of in vivo oxidative stress in diabetic patients. Free Radic Biol Med 1992; 13: 621–626
- Loullis CC, Felten DL, Shea PA. HPLC determination of biogenic amines in discrete brain areas in food deprived rats. Pharmacol Biochem Behav 1979; 11: 89–93
- Ase AR, Reader TA, Hen R, Riad M, Descarries L. Altered serotonin and dopamine metabolism in the CNS of serotonin 5-HT(1A) or 5-HT(1B) receptor knockout mice. J Neurochem 2000; 75: 2415–2426
- Belay HT, Brown IR. Spatial analysis of cell death and Hsp70 induction in brain, thymus, and bone marrow of the hyperthermic rat. Cell Stress Chaperones 2003; 8: 395–404
- Cheng N, Maeda T, Kume T, Kaneko S, Kochiyama H, Akaike A, Goshima Y, Misu Y. Differential neurotoxicity induced by L-DOPA and dopamine in cultured striatal neurons. Brain Res 1996; 743: 278–283
- Hattori A, Luo Y, Umegaki H, Munoz J, Roth GS. Intrastriatal injection of dopamine results in DNA damage and apoptosis in rats. Neuroreport 1998; 9: 2569–2572
- Reynolds DS, Carter RJ, Morton AJ. Dopamine modulates the susceptibility of striatal neurons to 3-nitropropionic acid in the rat model of Huntington's disease. J Neurosci 1998; 18: 10116–10127
- Johnson JR, Robinson BL, Ali SF, Binienda Z. Dopamine toxicity following long term exposure to low doses of 3-nitropropionic acid (3-NPA) in rats. Toxicol Lett 2000; 116: 113–118
- Burke WJ. 3,4-dihydroxyphenylacetaldehyde: A potential target for neuroprotective therapy in Parkinson's disease. Curr Drug Targets CNS Neurol Disord 2003; 2: 143–148
- Wallace LJ. A small dopamine permeability of storage vesicle membranes and end product inhibition of tyrosine hydroxylase are sufficient to explain changes occurring in dopamine synthesis and storage after inhibition of neuron firing. Synapse 2007; 61: 715–723
- Burke WJ, Li SW, Williams EA, Nonneman R, Zahm DS. 3,4-Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: Implications for Parkinson's disease pathogenesis. Brain Res 2003; 989: 205–213
- Lord-Fontaine S, Averill-bates D. Heat shock inactivates cellular antioxidant defenses against hydrogen peroxide: Protection by glucose. Free Radic Biol 2002; 32: 752–765
- Gao JP, Friedman S, Lanks KW. The role of reduced nicotinamide adenine dinucleotide phosphate in glucose and temperature-dependent doxorubicin cytotoxicity. Cancer Chemother Pharmacol 1993; 33: 191–196