Abstract
Purpose: CD4+CD25+FoxP3+ regulatory T-cells (Treg) are responsible for immunoevasion mechanisms induced by cancer. Specific chemokines such as CCL22 are presumed to mediate active Treg trafficking into the tumour site. In this context, the effects of irradiation and hyperthermia of tumour cells on Treg migration and the CCL22 concentration in the tumour cell supernatants after treatment were studied. Moreover, the relationship between CCL22 concentration and Treg cell migration was also examined.
Materials and methods: Treg and CD4+CD25− T-cells were isolated from human peripheral blood. Supernatants were obtained from primary cell cultures derived from head and neck carcinoma patients. Tumour cell cultures were treated with a dose of 2 Gy and hyperthermia (41.5°C) or with hyperthermia or irradiation alone. Cancer cell culture supernatants were then used for a transmigration assay.
Results: Treg and CD4+CD25− T-cells showed an increased transmigration towards supernatants of hyperthermia-treated tumour cells. After combined application of hyperthermia and irradiation, Treg migration was similar to control levels, but CD4+CD25− migration was still enhanced. Irradiation caused a significantly decreased Treg influx, whereas the CD4+CD25− T-cell migration was not altered after the same treatment. Changes of Treg chemotaxis could be attributed to a treatment-associated escalation of the CCL22 in the tumour cell supernatants.
Conclusion: The combination of irradiation and hyperthermia is able to modify transmigration of tumour infiltrating lymphocytes beneficially and individually. In this in vitro system hyperthermia alone negatively impacts the immune response by selectively recruiting Treg, whereas hyperthermia with the addition of irradiation negates this effect.
Introduction
CD4+CD25+FoxP3+ regulatory T-cells (Treg cells) play an important role in maintaining immune homeostasis; however, it has also been suggested that they play a role in immunoevasion mechanisms induced by cancer cells. Active Treg cell recruitment by tumour cells suppresses the cytotoxic immune response by generating an immunoevasive environment. Immunodepression, especially mediated by Treg cells, is a characteristic feature of head and neck squamous cell carcinoma (HNSCC) Citation[1] and ovarian carcinoma Citation[2].
It has been shown that Treg cells are increased in the circulation of patients suffering from head and neck cancer with 10% ± 4.7% Treg PBMC−1 cells compared to a control group with 5.4 ± 2.7% Treg PBMC−1 cells Citation[3] and down-regulate functional CD8+ effector T-cells, which might inhibit antitumour immune response in this type of cancer. The number of peripheral Treg cells is inversely correlated with total CD8+ T-cell subset representing Tc1 and Tc2 cells, which are capable of lysing tumour cells Citation[4]. A significantly higher proportion of Treg cells was also found in the peripheral blood of patients with gastric and oesophageal cancer depending on tumour stage. After curative resection, Treg cell levels returned to normal. This observation suggested that tumour-related factors induce, expand and localize Treg cells Citation[5].
In this context it has been demonstrated that tumour cells and microenvironmental macrophages produce the chemokine CCL22, which enhances Treg cell infiltration into the tumour triggered by a specific CCR4 receptor-ligand interaction Citation[2]. In addition, higher levels of intratumoural CCL22 correlate with an elevated presence of Treg cells in gastric cancer Citation[6]. CCL22 was also identified to play an important role in trafficking Treg cells into head and neck tumours Citation[7], where they accumulate preferentially Citation[8]. Intratumoral accumulation of Treg cells could be associated with inferior survival rates of patients with ovarian cancer and served as a negative prognostic factor Citation[2],Citation[9]. Similarly, it was suggested that a high frequency of Treg cells at the tumour site of head and neck carcinoma patients is affiliated with a poorer prognosis, especially in advanced stages Citation[1].
As a consequence of these observations, several theoretical approaches to modify cancer immunotherapy have been considered, e.g. a humanized anti-CCR4 monoclonal antibody (defucosylated chimeric anti-CCR4 IgG1 mAb KM2760) to block receptor-ligand mediated routing of Treg cells into the tumour Citation[10] or intratumoral CD4+ depletion by means of anti-CD4 antibodies. In transplantable mouse tumour models, the administration of antibodies to CD4 (mAb GK1.5) or CD25 (mAb PC61), which effectively antagonizes Treg cell function, evoked impressive tumour regression and protection against subsequent tumour challenges Citation[11].
In addition to these hypothetical molecular biologic methods, the influence of established therapeutic options like hyperthermia on the intratumoural immunological microenvironment was investigated. Experimental studies and clinical trials focused on the effects of hyperthermia on T-cell activation, differentiation and transmigration towards the tumour site. A redistribution of T-cell subpopulations as well as a change in the cytokine profile of patients suffering from solid tumours could be detected following hyperthermia Citation[12]. Moreover, local hyperthermia led to a modified CD4+/CD8+ ratio and an activation of NK cells Citation[13–15]. Several studies demonstrated that the presence of large numbers of tumour-infiltrating CD8+ T-cells or NK cells and a higher CD8+/Treg cell ratio were associated with a favourable prognosis in colorectal Citation[16–18], ovarian Citation[19],Citation[20], pancreatic Citation[21] and oesophageal carcinoma Citation[22],Citation[23].
Particularly the combination of hyperthermia and irradiation is able to potentiate antitumoural effects in vivo. A significantly better tumour control is obtained in carcinoma of the head and neck region by application of local hyperthermia combined with radiotherapy compared to radiotherapy alone Citation[24]. Furthermore, brachytherapy, hyperthermia and chemotherapy not only improve local tumour control when used in combination, but also reduce side effects such as osteoradionecrosis or oral mucositis Citation[25].
The aim of this study was to evaluate the ability of ionizing radiation, hyperthermia and the combination of both to modify the chemotactic capability of cancer cells. The chemotaxis of CD4+CD25+FoxP3+ regulatory T-cells and CD4+CD25− T-cells on supernatants from patient-derived primary tumour cells were studied. The association of the chemotactic activity with the CCL22 concentration in the supernatant was examined.
Materials and methods
Treg cell isolation
Peripheral blood mononuclear cells (PBMC) from healthy donors were isolated from heparinized venous blood by Biocoll (Biochrom, Berlin, Germany) density-gradient centrifugation. Treg and CD4+CD25− T-cells were then isolated from the PBMC pool using magnetically labelled antibodies (MACS® Micro Beads, Miltenyi Biotec, Bergisch-Gladbach, Germany).
Tumour cell cultures
Tumour biopsies were obtained from 20 patients with head and neck squamous cell carcinoma (Department of Oral and Maxillofacial Surgery, Friedrich-Alexander-University Erlangen-Nuremberg). The cohort included 16 male and 4 female subjects aged from 38 to 91 years. All patients had histologically proven HNSCC, with one cancer originating in the larynx, two in the oropharynx, three in the nasopharynx, four in the hypopharynx and ten in the oral cavity. Twelve out of 20 patients had T3 or T4 disease and nodal metastases ().
Table I. Clinicopathological features of patients with HNSCC included in the study.
Tumour cell separation was accomplished using a collagenase-based enzymatic digestion (ProteoExtract® Tissue Dissociation Buffer Kit, Calbiochem, Merck Chemicals, Darmstadt, Germany). Tumour cells, including stromal cells of the tumour, were then resuspended in Dulbecco's modified Eagle's medium (DMEM) (Biochrom, Berlin, Germany) supplemented with 10% foetal calf serum, 1% L-glutamine, 100 units cm−3 penicillin, 10 µg cm−3 streptomycin and cultured under standard conditions (37°C, 5% CO2). Cells were used to generate supernatants up to the fifth passage.
Tumour cell treatment and generation of supernatants
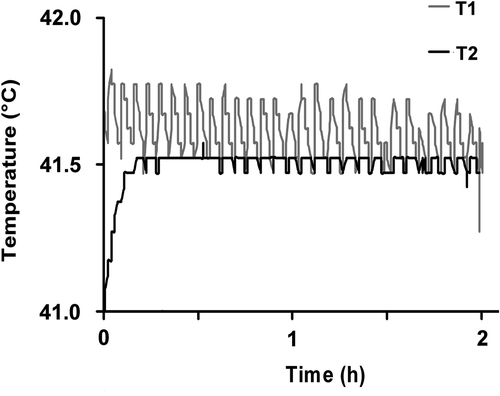
Tumour cells were transferred into 12-well plates at a density of 1 × 106 cells cm−3 and after 24 h treated sequentially with a dose of 2 Gy X-rays (120 kV, Isovolt Titan, General Electrics, Ahrensburg, Germany) and hyperthermia (41.5°C) for 2 h, or hyperthermia and irradiation alone, respectively. The dose of 2 Gy was chosen because of its clinical relevance in conventionally fractionated radiotherapy of head and neck squamous cell cancer. Additionally, the 2 Gy leads to a loss of clonogenicity in only a limited number of cells (range 20–30%) and therefore could actively secret cytokines. Continuous monitoring of the temperature directly within the wells during the application time verified that the incubator conditions produced a stable hyperthermic environment of 41.5°C ± 0.07° (). After an incubation time of 96 h and treatment under standard conditions, supernatants were used for a chemotaxis assay. Supernatants of untreated tumour cells served as control.
Chemotaxis assay
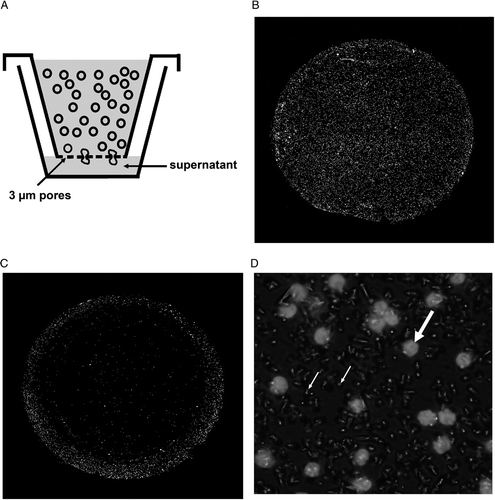
Chemotaxis 96-well assay plates (MultiScreen®-MIC Plate, Millipore, Schwalbach, Germany) with an inserted 3 µm pore size membrane were used for the T-cell transmigration towards tumour cell supernatants. Lymphocytes were added to the upper compartment (50 µL at a density of 1 × 106 cells cm−3) and tumour cell supernatants (200 µL) to the lower one. After a transmigration time of 2 h, cells were fixed on the membrane () for 15 min in 4% paraformaldehyde, permeabilized for 10 min in 0.1% Triton X-100 and DAPI stained (). The number of infiltrating T-cells was counted using fluorescence microscopy and a semi-automatic image analysis software (COUNT, Biomas, Erlangen, Germany) ().
Figure 2. T-cells migrating through the membrane in the chemotaxis assay (A): After a transmigration time of 2 h, migrated T-cells were fixed on the membrane with 3 µm pores and stained with DAPI. Membranes showing high (B) and low (C) T-cell transmigration at a magnification of 200×. (D) T-cells (bold arrow) transmigrate through 3 µm pores (small arrow) in the membrane (630×).
Enzyme-linked immunosorbent assay (ELISA)
The amount of CCL22 in the tumour cell culture supernatants was estimated with an ELISA kit (Human MDC/CCL22 Quantikine®, R&D Systems, Wiesbaden-Nordenstadt, Germany) performed according to the manufacturer's instructions.
Statistical methods
A t-test was used to specify statistical significance for the mean values of chemotaxis assay and ELISA data. Pearson correlation coefficient was applied to describe interdependence of CCL22 concentration and Treg cell migration. Mean differences were considered significant at the confidence level of p ≤ 0.05.
Results
Treg cell migration
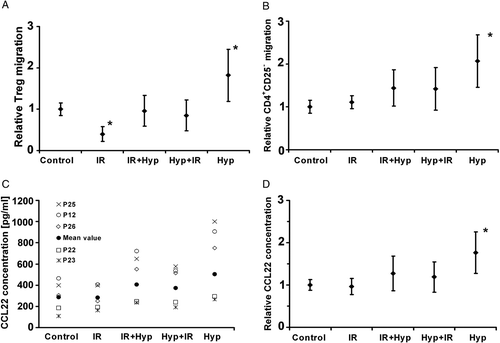
The migration of Treg cells after treatment with supernatants from tumour cells was studied first. The supernatant is the medium derived from primary tumour, which was treated with radiation or hyperthermia, or the combination of both, and then cultured. After 96 h the medium was removed from the cells, centrifuged to remove cells and debris, and used in the chemotaxis assay. T-cells with an average diameter of 5–10 µm had to transmigrate a membrane with 3 µm diameter pores attracted by the chemokines of the supernatant (). Due to the relatively wide inter-individual variation of the absolute values, the data were normalized to the controls. Irradiation alone reduced Treg cell migration by 60% compared to the untreated control (p = 0.03). Combined application of hyperthermia and ionizing radiation did not intensify Treg cell migration significantly (p = 0.55). In contrast, the supernatants of hyperthermia-treated tumour cells induced an increased Treg cell transmigration by an average of 81% (p = 0.001) ().
Figure 3. Relative changes of Treg (A) and CD4+CD25− cell (B) migration towards tumour cell supernatants. Changes of absolute CCL22 concentration in tumour cell culture supernatants exemplified for five patient cell lines (C) and relative changes of CCL22 concentration (D). The untreated control was compared to tumour cell treatment with ionizing radiation (IR), sequential application of irradiation and hyperthermia (IR + Hyp) or in reversed order (Hyp + IR) and hyperthermia alone (Hyp). Each point represents the average ± standard deviation of 3 independent experiments (n = 20). Values marked with an asterisk are significantly different (p ≤ 0.05) from the untreated control.
CD4+ T-cell migration
Additionally, the migration of CD4+CD25− T-cells under the same conditions as the Treg cell migration was studied. Ionizing radiation did not alter the chemotaxis of CD4+ cells. However, the combined treatment of radiation and hyperthermia increased transmigration of this T-cell subpopulation by 43%, independently of the sequence of application (p = 0.001; p = 0.006). Similarly to the effects shown for Treg cell infiltration, hyperthermia increased CD4+CD25− T-cell migration by 107% (p = 0.001) ().
CCL22 concentration
CCL22 concentration varied widely between patients due to interindividual variation, but tended to be more uniform after treatment (). Ionizing radiation did not lead to an increased level of CCL22 in the tumour microenvironment compared to the control (p = 0.27). Radiation followed by hyperthermia caused a mean increase of 26% (p = 0.01), and 19% (p = 0.02) for the sequential application in reversed order. Hyperthermia induced a prominent level of CCL22 into the supernatant, demonstrating an increase of 76% relative to the control (p = 0.001) ().
Correlation of Treg cell chemotaxis with CCL22 concentration
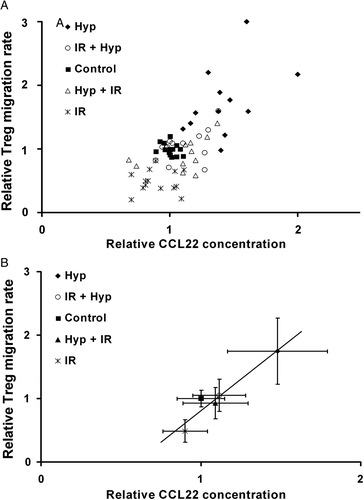
It became evident that there is a positive correlation between the altered CCL22 concentration in the supernatants of tumour cells after treatment and the corresponding changes of Treg cell migration towards the tumour cells (r = 0.74; p = 0.02). The enrichment of CCL22 in supernatants produced by hyperthermia-treated tumour cells was associated with a significantly stronger Treg cell infiltration (). The combination therapy including irradiation reduced CCL22 concentration as well as Treg cell migration. Interestingly, sole application of irradiation decreased CCL22 concentration and Treg cell migration below control level.
Figure 4. Correlation between CCL22 concentration and Treg cell transmigration after tumour cell treatment (A) and the mean values of each group (B) after treatment with ionizing radiation n = 14 (IR), sequential application of irradiation and hyperthermia n = 14 (IR + Hyp) or in reversed order n = 12 (Hyp + IR) and hyperthermia alone n = 12 (Hyp). Each point represents the value derived from the supernatant or cells of a single patient. For each point the average and standard deviation of three independent experiments are indicated as error bars.
Discussion
Hyperthermia combined with radio- and/or chemotherapy is a method to increase tumour control with minimal increase in toxicity. Therefore, hyperthermia is used in radical treatment schedules for many tumours, including head and neck squamous cell cancer Citation[26]. Due to the combined therapy, improved local tumour control can be achieved without toxic doses. Furthermore, improved outcome is probably supported by a modified immune response against the tumour Citation[12],Citation[13].
The importance of tumour-infiltrating lymphocytes (TIL) was underlined by recent studies on anal squamous cell carcinoma following chemoradiation. Tumour-infiltrating lymphocytes were also identified as a negative prognostic indicator in this type of cancer with granzyme B+ cytotoxic cells indicating a significant association with poorer outcome (p = 0.008). This was explained by the selection of therapy-resistant tumour cell clones. Moreover, high numbers of CD3+ and CD4+ T-cells correlated with an inferior 3-year survival in this study Citation[27], however, no prognostic influence of Treg cells was found. Lymphocyte homeostasis was possibly affected by the chemoradiation protocol in this case.
Head and neck squamous cell carcinoma patients with active disease had significantly lower CD3+, CD4+ and CD8+ T-cell counts in the peripheral blood than healthy donors Citation[28]. This observation affirms that patients with HNSCC have an altered lymphocyte homeostasis. Knowledge of local immune responses is important for the development of immunotherapeutic strategies. Using hyperthermia and radiation, especially in combined treatment schedules, T-cell infiltration and interaction seems to be modified beneficially.
In this in vitro study, we showed that hyperthermia-treated head and neck tumour cells induced an increase in Treg cell migration by 84%, while radiation alone reduced Treg cell chemotaxis by 60% compared to the untreated control. After combined treatment of hyperthermia and irradiation, Treg cell migration was in the range of control levels.
It is likely that altered chemotaxis is mainly associated with a concomitant escalation of CCL22 concentration in the tumour cell culture. Our results demonstrate that 2 h hyperthermia increased the CCL22 concentration in head and neck squamous cells of HNSCC patients by 76% compared to the untreated control. In contrast, irradiation alone did not change the level of CCL22 significantly.
It has to be taken into consideration that Treg cell migration is not only an effect of the CCL22 production of cancer cells. CCL17 and CCL22 were both identified to specifically recruit Treg cells into the cerebrospinal fluid in lymphomatous and carcinomatous meningitis Citation[29]. Additionally, CCL17 and CCL22 levels within the tumour microenvironment are related to accumulation of Treg cells in gastric cancer Citation[6]. Analysis of the CCL22 and CCL2 concentrations in glioblastoma multiforme supernatants indicated that CCL2 may be the principal chemokine for Treg cell migration in this type of tumour Citation[30]. The increase of the CCL22 concentration in the tumour microenvironment of the head and neck tumour cells in our in vitro studies was sufficient to recruit Treg cells. These results suggest that CCL22 in head and neck tumour cell microenvironment may be the main cause of Treg cell attraction in HNSCC patients. Validation of these results in clinical in vivo studies is now necessary.
The clinical significance of CD4+CD25− T-cells inside tumours is controversial. A higher number of tumour infiltrating CD4+ T-cells was found to be a favourable prognostic factor in pancreatic adenocarcinomas and oesophageal squamous cell carcinomas Citation[31],Citation[32]. This observation is explained by an improved interaction between CD4+ and CD8+ T-cells and a consecutive activation of antitumoural cytotoxic mechanisms as well as an overcoming of a so-called CD8+ lethargy in the presence of CD4+ Citation[33]. On the other hand, it was reported that an increased level of tumour-infiltrating CD4+ T-cells leads to inferior outcome in renal cell carcinoma Citation[34],Citation[35]. This controversial issue probably results from the fact that these two studies do not differentiate between CD4+CD25− and CD4+CD25+ regulatory T-cells, which means that prognostically unfavourable Treg cells are incorporated into the CD4 pool.
Our in vitro studies demonstrate that in contrast to the Treg cells migration behaviour, CD4+CD25− T-cell influx was not only increased after 2 h thermal exposure of tumour cells, but also remained heightened after exposure to combined treatment. Ionizing radiation alone did not reduce the migration of CD4+CD25− T-cells compared to the untreated control.
The CD4+CD25−/Treg cell migration ratio was introduced in order to compare the opposed effects of treatment protocols on the chemotactic profiles of these two different T-cell subtypes. For hyperthermia alone, migration ratio is 1.1, which indicates that thermal exposure of tumour cells similarly enhances CD4+CD25− and Treg cell migration. A migration ratio of 1.5 and 1.7, results from combined application of hyperthermia and irradiation, respectively, reflecting that Treg cell infiltration lags behind an increased CD4+CD25− attraction. Supernatants of irradiated tumour cells caused a migration ratio of 2.8, which means that elevated CD4+CD25− transmigration exceeds the effect of Treg cell invasion.
Conclusion
Our results indicate that the migration behaviour of tumour infiltrating lymphocytes can be influenced by the use of individual or combined treatment schedules of hyperthermia and irradiation. We showed that the target orientated application is able to selectively affect prognostically important immune cells. Moreover, it became evident that the cytokine profile of head and neck tumour cells is altered after thermal exposure and irradiation. The modification of specific chemokine levels are directly associated with varied lymphocyte attraction. We conclude from this study that the antitumoural immune response, represented by the lymphocyte transmigration and its interdependency with neoplastic cytokine expression can be changed in a beneficial way by employing the wide array of current therapeutical approaches like hyperthermia and irradiation. We point out that unfavourable Treg cell infiltration is increased by employing hyperthermia alone, but reset to control levels when combined with irradiation. This finding supports the current concept that local or regional hyperthermia must not be used alone, but rather, in combination with established antineoplastic treatments like radiotherapy, chemoradiation, and chemotherapy in the clinical setting of cancer therapy.
Acknowledgements
Grant support: Interdisciplinary Centre for Clinical Research of the Friedrich Alexander University of Erlangen-Nuremberg, Germany (Project D4).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res 2007; 13: 4345–4354
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10: 942–949
- Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, Whiteside TL. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer 2005; 92: 913–920
- Chikamatsu K, Sakakura K, Whiteside TL, Furuya N. Relationships between regulatory T cells and CD8+ effector populations in patients with squamous cell carcinoma of the head and neck. Head Neck 2007; 29: 120–127
- Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, Omata H, Fujii H. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother 2006; 55: 1064–1071
- Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer 2008; 122: 2286–2293
- Schaefer C, Albers A, Whiteside TL. Regulatory T cells Attracted by chemokines are enriched in head and neck cancer. Otolaryngol–Head Neck Surgery 2005; 133: 142
- Schwarz S, Butz M, Morsczeck C, Reichert TE, Driemel O. Increased number of CD25(+) FoxP3(+) regulatory T cells in oral squamous cell carcinomas detected by chromogenic immunohistochemical double staining. J Oral Pathol Med 2008; 37: 485–489
- Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res 2005; 11: 8326–8331
- Ishida T, Ueda R. CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci 2006; 97: 1139–1146
- Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, Fu YX. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med 2005; 201: 779–791
- Atanackovic D, Pollok K, Faltz C, Boeters I, Jung R, Nierhaus A, Braumann KM, Hossfeld DK, Hegewisch-Becker S. Patients with solid tumors treated with high-temperature whole body hyperthermia show a redistribution of naive/memory T-cell subtypes. Am J Physiol Regul Integr Comp Physiol 2006; 290: R585–594
- Ostapenko VV, Tanaka H, Miyano M, Nishide T, Ueda H, Nishide I, Tanaka Y, Mune M, Yukawa S. Immune-related effects of local hyperthermia in patients with primary liver cancer. Hepatogastroenterology 2005; 52: 1502–1506
- Szmigielski S, Sobczynski J, Sokolska G, Stawarz B, Zielinski H, Petrovich Z. Effects of local prostatic hyperthermia on human NK and T cell function. Int J Hyperthermia 1991; 7: 869–880
- Dayanc BE, Beachy SH, Ostberg JR, Repasky EA. Dissecting the role of hyperthermia in natural killer cell mediated anti-tumor responses. Int J Hyperthermia 2008; 24: 41–56
- Menon AG, Janssen-van Rhijn CM, Morreau H, Putter H, Tollenaar RA, van de Velde CJ, Fleuren GJ, Kuppen PJ. Immune system and prognosis in colorectal cancer: A detailed immunohistochemical analysis. Lab Invest 2004; 84: 493–501
- Prall F, Duhrkop T, Weirich V, Ostwald C, Lenz P, Nizze H, Barten M. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol 2004; 35: 808–816
- Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, Imamura M. Increased intratumor Valpha24-positive natural killer T cells: A prognostic factor for primary colorectal carcinomas. Clin Cancer Res 2005; 11: 7322–7327
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 2005; 102: 18538–18543
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348: 203–213
- Ryschich E, Notzel T, Hinz U, Autschbach F, Ferguson J, Simon I, Weitz J, Frohlich B, Klar E, Buchler MW, et al. Control of T-cell-mediated immune response by HLA class I in human pancreatic carcinoma. Clin Cancer Res 2005; 11: 498–504
- Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res 2001; 61: 3932–3936
- Cho MY, Kim TH, Yi SY, Jung WH, Park KH. Relationship between Epstein-Barr virus-encoded RNA expression, apoptosis and lymphocytic infiltration in gastric carcinoma with lymphoid-rich stroma. Med Princ Pract 2004; 13: 353–360
- Datta NR, Bose AK, Kapoor HK, Gupta S. Head and neck cancers: Results of thermoradiotherapy versus radiotherapy. Int J Hyperthermia 1990; 6: 479–486
- Geiger M, Strnad V, Lotter M, Sauer R. Pulsed-dose rate brachytherapy with concomitant chemotherapy and interstitial hyperthermia in patients with recurrent head-and-neck cancer. Brachytherapy 2002; 1: 149–153
- Gabriele P, Amichetti M, Orecchia R, Valdagni R. Hyperthermia and radiation therapy for inoperable or recurrent parotid carcinoma. A phase I/II study. Cancer 1995; 75: 908–913
- Grabenbauer GG, Lahmer G, Distel L, Niedobitek G. Tumor-infiltrating cytotoxic T cells but not regulatory T cells predict outcome in anal squamous cell carcinoma. Clin Cancer Res 2006; 12: 3355–3360
- Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL. Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clin Cancer Res 2004; 10: 3755–3762
- Haas J, Schopp L, Storch-Hagenlocher B, Fritzsching B, Jacobi C, Milkova L, Fritz B, Schwarz A, Suri-Payer E, Hensel M, et al. Specific recruitment of regulatory T cells into the CSF in lymphomatous and carcinomatous meningitis. Blood 2008; 111: 761–766
- Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother 2008; 57: 123–131
- Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 2004; 28: e26–31
- Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y, Hida Y, Oshikiri T, Kurokawa T, Suzuoki M, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res 2003; 63: 1555–1559
- Bourgeois C, Veiga-Fernandes H, Joret AM, Rocha B, Tanchot C. CD8 lethargy in the absence of CD4 help. Eur J Immunol 2002; 32: 2199–2207
- Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: Clinicopathologic demonstration of antitumor immunity. Cancer Res 2001; 61: 5132–5136
- Bromwich EJ, McArdle PA, Canna K, McMillan DC, McNicol AM, Brown M, Aitchison M. The relationship between T-lymphocyte infiltration, stage, tumour grade and survival in patients undergoing curative surgery for renal cell cancer. Br J Cancer 2003; 89: 1906–1908