Abstract
Purpose: To investigate the potential of conductive interstitial thermal therapy (CITT) to inhibit recurrence and metastasis in a partially resected tumour model.
Method: Fifteen New Zealand white rabbits were implanted with VX2 tumour intramuscularly in the rear thigh. Once the tumour size reached 20–25 mm in diameter, three animals were randomly selected to serve as controls, while the remaining animals were designated as the study group and treated with CITT. In the CITT group, the partially resected tumour and margins were thermally ablated. In the control group the tumour was partially resected to simulate positive margins. The animals were monitored for up to 12 weeks. At the endpoint, the animals were sacrificed, and whole-body diagnostic necropsy was conducted immediately.
Results: Recurrences and metastatic lesions were observed in iliac and popliteal lymph nodes and abdomens of all control animals. In contrast, the observed rate of recurrence and metastatic lesion was 0% among CITT-treated animals, significantly less than the ≥50% null-hypothesis rate expected upon treatment failure (exact binomial P = 0.0002). Complete histopathological healing was obtained in 2 of 12 rabbits, and residual inflammation remained at the ablation site up to 12 weeks post-ablation in 10 of 12 rabbits. This pattern of necrosis and inflammatory response was not observed in any of the control rabbits.
Conclusions: The CITT device effectively ablated partially resected VX2 carcinoma in a rabbit model, and inhibited recurrence and metastasis in this model. CITT evoked an inflammatory response that may be linked to the mechanism involved in reduced metastatic spread.
Introduction
Surgical resection remains the gold-standard treatment for solid cancerous tumours. However, surgical resection often leaves microscopic ‘positive margins’, i.e. inadequate surgical margins in which viable cancer cells are left at the surgical site. Positive margins can lead to local and possibly distant recurrence, with consequent poor prognosis for the patient Citation[1–3]. In breast cancer studies, positive margins up to 56% have been reported following the initial lumpectomy Citation[1],Citation[2],Citation[4],Citation[5]. In prostate cancer patients, a recurrence rate of approximately 40% after radical prostatectomy was reported Citation[6]. Approximately 50% of patients had postoperative positive margin after radical prostatectomy Citation[7]. Furthermore, in head and neck cancer, even when the surgical margins were diagnosed as tumour-free by histopathological examination, local recurrence rates of up to 30% have been reported Citation[8].
Currently, there is no standardised sampling method to determine a surgical margin's pathological status. Most non-cytological methods assess only 10–15% of the surface of a relatively rounded lesion Citation[9]. Nor is there a standard definition of what constitutes a cancer-free region or ‘negative margin’ Citation[3]. De Mascarel et al. calculated that going from a 90% to a 99% probability of cancer detection required tripling the number of histological sections that needed to be examined, and an additional doubling in order to approach 100% probability Citation[10]. Carter reported that to fully evaluate the pathological status of a 2-cm biopsy specimen, 3,000 6 µm-thick sections would be needed, a formidable and impractical task Citation[9].
Recently, we have reported on the development of a pre-clinical conductive interstitial thermal therapy (CITT) device to obtain negative margins by thermal ablation at very high temperatures Citation[11],Citation[12]. During CITT ablation, the heat is delivered to the target tissue by conduction Citation[11],Citation[12]. Heat dissipation by conduction follows well-understood heat transfer principles, and the changes in heat-conduction coefficients in tissue as a function of temperature are linear and known Citation[13],Citation[14]. Thus, in CITT, we can accurately predict the extent of the ablation zone via mathematical simulations Citation[11],Citation[12]. The reliability, uniformity and controllability of the CITT device were previously demonstrated by ablating, in vivo, soft breast tissue in a swine model and VX2 carcinoma in the New Zealand White (NZW) rabbit model Citation[11],Citation[12]. In the rabbit VX2 carcinoma model, our CITT device effectively ablated partially resected VX2 carcinoma in live rabbits. A uniform ablation zone of 10 mm width was created via CITT ablation at maximum probe temperature of 120° to 150°C for 20 to 10 min, respectively, immediately following ablation. Based on this data, we hypothesised that CITT ablation may induce coagulation necrosis and local inflammation that may result in inhibition of local and possibly distant recurrence of VX2 tumours.
To test this hypothesis, we performed CITT ablation on partially resected VX2 carcinoma, and examined metastasis and recurrence rates up to 12 weeks post treatment, sufficient time to observe recurrences and pulmonary metastases of VX2 in live NZW rabbits Citation[15],Citation[16].
Materials and methods
CITT and animal model
The principles of CITT were described in detail by Shafirstein et al. in 2007 Citation[11],Citation[12]. Briefly, the CITT device consists of a thermal probe with deployable pins and a computer-controlled heater that allows delivery of high thermal doses to the target tissue via heat conduction.
All animal experiments in this study were approved by the UAMS Institutional Animal Care and Use Committee and were conducted according to the guidelines in the Guide for the Care and Use of Laboratory Animals, NIH Publication No. 86-23.
NZW rabbits weighing from 2 to 2.5 kg were obtained from Myrtle's Rabbitry Inc. (Thompsons Station, TN). The rabbits were maintained in cages in the animal care facility. The rabbits were subjected to alternating 12-hour periods of dark/light cycle, with access to standard rabbit chow (Harlan Teklad, Indianapolis, IN) and water ad libitum. At VX2 tumour implantation, a rabbit was randomly selected to be the donor, and anaesthetised by intramuscular injection of a mixture of ketamine (50 mg/kg, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (10 mg/kg, Fort Dodge Animal Health). A piece of cryopreserved VX2 tumour (2 × 2 × 2 mm, National Cancer Institute, Frederick Cancer Center, Frederick, MD) was transplanted into the thigh muscle of the donor rabbit. The rabbit was allowed to recover and returned to the cage until the tumour size reached 20–25 mm in diameter.
Study procedure
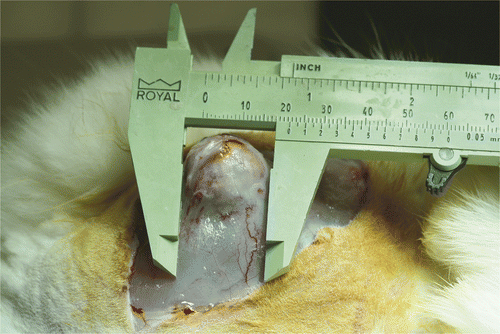
When the tumour on the donor rabbit reached 20–25 mm in diameter (), it was excised, diced to approximately 2 × 2 × 2 mm sections under sterile conditions, and then implanted into the thigh of experimental rabbits. Within 3–4 weeks the tumours reached 20–25 mm in diameter. Three experimental rabbits were randomly selected to serve as controls by undergoing partial resection only, to demonstrate that the VX2 tumour tissue retained virulence after cryopreservation and restoration in the donor animal. The remaining twelve rabbits underwent CITT.
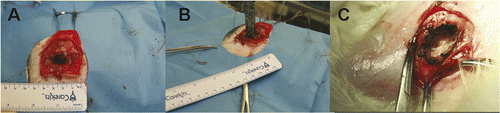
In the CITT group, the rabbits were anaesthetised with intramuscular injection of a mixture of 50 mg/kg ketamine hydrochloride and 10 mg/kg xylazine, followed by inhalation of 2% isoflurane with 2 L/min oxygen flow. A 30–40 mm subcutaneous incision was made; the tumour was partially resected, by removing about 5 × 5 × 5 mm cancerous tissue, to create a cavity that accommodated the CITT probe (). Prior to ablation, the cavity was drained of liquid and blood as much as possible. The sterilised CITT probe was then inserted into the cavity (). The temperature was increased at a constant rate of 0.5°C/sec, using a temperature controller, until maximal temperature was reached. Then the ablation was conducted for 10 minutes at the maximum probe temperature of 150°C, the preferred thermal dose suggested by the mathematical model to yield maximum ablation depth in minimum time Citation[11],Citation[12]. After the ablation phase was completed, the probe was removed from the cavity (). The wound was drained, and additional ablated tissue was excised near the skin surface, to improve wound healing. The incision was then aseptically closed with sutures. The rabbits were monitored every 2–3 days for a maximum time period of 12 weeks. The animals were euthanized at the end of the study or when significant pain and distress were observed. After euthanasia, the ablation site, lymph nodes, liver, and lungs were visually examined for the presence of local and distant metastatic lesions and submitted for histopathological examination.
Figure 2. The cavity created for accommodating the CITT probe prior to placement of the device (A), after placing the CITT device (B) and at the end of the CITT ablation (C).
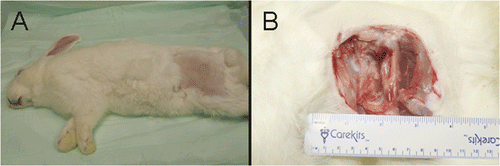
In the control group the rabbits were anaesthetised and prepared for surgery as described above. A 30–40 mm subcutaneous incision was made and the tumour was partially resected by removing cancerous tissue visible to the naked eye. The surgical site was aseptically drained and closed with sutures. The recovery and follow-up procedures were the same as described above for the CITT group.
Histopathology
The excised ablated region was sectioned into 4 × 10 × 4 mm slices. The tissue blocks were processed and embedded into paraffin and sectioned at 5 µm. Tissue slides were routinely stained with haematoxylin and eosin (H&E) and examined under a light microscope by a veterinary pathologist for presence of recurrent tumour and evaluation of healing in ablated regions. Representative sections of lymph nodes, liver and lungs and gross lesions were similarly examined.
Statistical analysis
The recurrence rate in the CITT group was estimated as the proportion of recurrences among the 12 animals. A one-sided upper 95% confidence limit on the recurrence rate was calculated using the binomial distribution. Finally, the one-sided exact binomial test of a single proportion was used at 5% alpha to compare recurrence in the CITT group against a null-hypothesis recurrence rate of 50%. The recurrence rate among control group was estimated as the proportion of recurrences among the three animals. The statistical analyses were conducted using Excel 2003 (Microsoft Corporation, Redmond, WA).
Results
Metastasis and recurrence
All tumours were treated at a similar size of about 10–15 mm. Over the study period, all the control rabbits (100%) developed histologically verified recurrences and metastatic lesions (), a result that is consistent with >50% chance of creating positive margins that tend to lead to recurrence.
Figure 3. A carcinoma mass developed after partial resection of the primary tumour in control group.
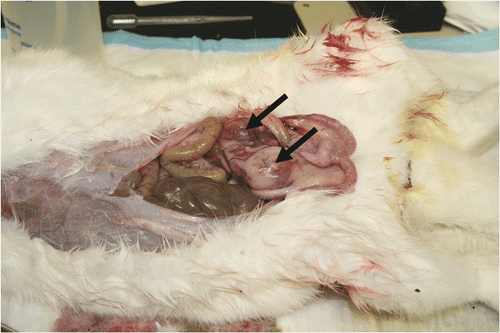
Enlarged lymph and carcinoma mass were seen in iliac and popliteal lymph nodes and the abdomens of these animals. The tumours recurred within 2 weeks, and the animals survived 2.5–5 weeks before being euthanized to minimise pain and distress. In contrast, none (0%) of the twelve CITT-treated rabbits developed metastasis or recurrence. This result was significantly lower than the null-hypothesis recurrence rate of 50% (P = 0.0002), and gives us 95% confidence that the CITT procedure yielded a true recurrence rate of 22.1% or less. The average follow-up time of the rabbits treated with CITT was 10 ± 1.5 weeks and was not associated with tumour recurrence or systemic toxicity. During the study, three animals (from the CITT group) exhibited distress at 6 weeks post therapy, and were euthanized at that time. All the animals undergoing the CITT procedure gained weight and had normal food intake. The ablation site healed within 3 weeks after treatment, and minimal scarring with minimal damage to muscle was observed at the end of the study ().
Histopathology evaluation
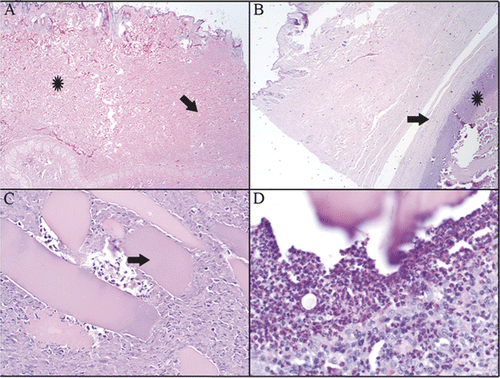
Complete histopathological healing was obtained in 2 of 12 rabbits. Complete healing is defined as presence of surgical scar with no evidence of ongoing inflammation or residual necrotic tissue (). In 10 of 12 rabbits, residual inflammation remained at the ablation site up to 12 weeks post-ablation. This inflammation was characterised by necrotic cellular debris surrounded by granulation tissue or mature fibrosis (). Often, the necrotic debris contained fragments of skeletal muscle fibres with well-preserved morphology (). These fragments were surrounded by intense eosinophilic and heterophilic cellular infiltrate (). Femoral lymph nodes in all ablated rabbits were mildly to moderately enlarged with varying degrees of lymphoid hyperplasia, oedema, and haemorrhage. Heterophilic and eosinophilic lymphadenitis was present in one animal, and necrosis with fibrosis was present in a second animal (data not shown) in the CITT group.
Figure 5. H&E staining of histological slides that were taken from tissue collected at the ablation site at 10 weeks post CITT treatment. Complete dermal healing (A). Dense mature fibrous tissue (arrow) is present in healed dermis. Adjacent dermis (star) is normal. Residual inflammation was present in 10/12 animals (B, C, D). (B) Cellular debris and inflammatory cells (star) are walled off by a capsule of fibrous connective tissue (arrow). (C) Fragments of skeletal myofibres (arrow) are surrounded by necrotic cellular debris and inflammatory cells. (D) Inflammatory infiltrate surrounding myofibre. H&E, magnification 20× (A), 40× (B), 200× (C) and 400× (D).
Discussion
The efficiency and predictability of the CITT was demonstrated by our group previously Citation[11],Citation[12]. In these studies, histopathology and viability staining were used to validate our mathematical simulations in predicting the size of the ablation site. Approximately 60 histopathological slides were required to test our ability to predict the ablation size. However, to determine that there are no viable cancer cells within 8000 mm3, i.e. within 20 mm3 of treated tumour and margins, approximately 3000 slides must be examined via histopathology Citation[9]. This is an extremely laborious and inefficient task to be completed. Therefore, in the present study, we elected to examine the rates of metastasis and recurrence for as long as 12 weeks following CITT, to determine the efficacy of the CITT in ablating partially resected cancerous tumour and creating negative margins in vivo. Spundzhiev et al. observed regional metastasis in 100% of the animals within 6 weeks following wide margin resection of an auricular VX2 tumour Citation[17]. In our study, it took about 2 weeks to observe local and distant recurrence and metastasis following partial resection of the VX2 tumour in our control group. We attribute the relatively short time of recurrence in this case to grossly positive margins following the partial resection of the VX2 tumour, which was intended in this study to simulate positive margins.
In the group of animals treated with the CITT, no local recurrence and no distant metastases were observed up to 12 weeks after CITT treatment. Since the same VX2 tumour source and type were used in the control and the CITT study group, we conclude that the CITT ablation inhibited local recurrence and distant metastasis in this animal model. In comparison to the 35% local recurrence rate seen in other studies with radiofrequency ablation (RFA) of the VX2 tumour Citation[16],Citation[18], the 0% outcome obtained in this study of observed recurrences (upper 95% confidence limit of 22.1%) suggests that CITT is more efficacious than RFA in ablating VX2 tumours. During RFA, temperature control within the tissue is obtained by thermocouples at the ends of the deployable electrodes or tines. This control can provide the desired temperature in the vicinity of the tines, but not the temperature of the tissue between the tines. Because most of the RF energy is preferentially absorbed by water and fat Citation[19], if the tissue near the deployable tines has higher water or fat content than the tissue between the tines, the temperature of the tissue between the tines could be significantly lower than the temperature measured by the thermocouples at the tines. Wren et al. found temperature deviations of 3–12°C during RFA of brain lesions Citation[20]. Chang stated that the errors in target temperatures during RFA would not depend on the power, but rather on the temperature at which RFA would operate Citation[21]. Therefore, it can be assumed that during RFA of the VX2 tumours, the target temperatures within tumour and margins were not achieved (i.e. positive margins) and viable cancer cells were able to migrate to undamaged nearby tissue to induce recurrence of the VX2 tumour.
The high efficacy of the CITT in inhibiting local recurrence and distant metastasis of the partially resected VX2 tumour might be attributed to the ability of the CITT to seal the margins by thermal coagulation. With CITT, heat is delivered to the tissue via conduction from the probe's surface, which is heated to as high as 150°C. Upon heating, the tissue sears immediately Citation[11],Citation[12]. These high temperatures allowed rapid treatment (10 minutes) of the partially resected tumour and margins while adjacent tissues remain intact, as we demonstrated previously Citation[11],Citation[12]. The 0% local recurrence observed in this study demonstrated that no viable cells were left within the partially resected tumour and margins treated with CITT device. These results also suggest the possibility that the ablation was able to disrupt the transit of tumour cells from the primary tumour to other parts of the body, the bone marrow, and back again to the site of the primary, leading to metastatic and recurrent lesions. We have no definitive evidence for this, yet many other groups have reported intriguing new data to support the idea that cancer may be a disease of self-seeding Citation[22–27]. Therefore, we hypothesise that thermal fixation of the margin and the long-term inflammatory response to ablation may disrupt a cyclic transit of tumour cells from the primary site and back again.
Lymphoid hyperplasia was observed in local lymph nodes of all CITT-ablated rabbits, suggesting on-going immune stimulation even at 12 weeks post-ablation. The presence of active eosinophilic and heterophilic inflammation in ablated regions, surrounding fragments of skeletal muscle fibres, suggests that skeletal muscle subjected to the high temperatures of CITT may release immunomodulators including chemoattractants. The pattern of this inflammation is reminiscent of eosinophilic myositis, a condition involving intense eosinophilic infiltrate into skeletal muscle tissue with resulting muscle necrosis. This pattern of necrosis and inflammatory response was not observed in any of the control rabbits, and is therefore interpreted to be the result of CITT treatment rather than tumour-related necrosis of skeletal muscle. A few studies suggested that thermal ablation induces anti-tumour immunity, in about 6 weeks following ablation Citation[28],Citation[29]. Others have found that radiofrequency ablation (RFA) generated local necrosis and inflammation resulted in infiltration of T cells Citation[30]. However, it was also demonstrated that anti-tumour immune response induced by RFA is not sufficient to cause complete tumour regression Citation[29],Citation[30]. In this study we observed complete tumour regression after a single treatment. This observation can be explained by the level of thermal injury induced during CITT. In comparison to RFA, the higher temperature during CITT ablation may result in substantially more intense, but local, thermal trauma to the tumour and adjacent tissue (margins). This local thermal trauma can amplify the immune response. It has been suggested that massive cell death and disruption of tumour architecture may further stimulate the immune response by amplifying the ‘danger signal’, which will significantly increase the generation of cytotoxic T cells Citation[31]. Thus, we can speculate that the thermal damage caused by CITT amplified the anti-tumour immune response. The mechanisms that dictate thermal-induced immune response are not well understood and are still being investigated by several groups. At this time, it is unclear if, how, and to what degree the CITT-induced inflammatory response we observed may contribute to the inhibition of tumour recurrences and metastasis. Further research is underway to determine the mechanisms by which the CITT inhibits recurrence and metastasis of solid tumours.
Clinical relevance
The 1990 Consensus Conference on Breast Cancer from the NIH stated that breast conservation surgery followed by radiation is the preferred method of treatment for stages I and II breast cancer Citation[32]. Since 2002, completed studies on 20- and 25-year follow-ups of randomised trials comparing mastectomy with lumpectomy followed by radiation concluded that survival rates were similar for both therapeutic modalities for women with stage I or II breast cancer when the tumour was <4 cm and the resection margins of the specimen were negative Citation[33–35]. Furthermore, a recent meta-analysis of 78 clinical trials involving 42,000 women with breast cancer concluded that, over a 15-year time period, local recurrence accounts for 25% of cancer-related deaths Citation[36]. A direct link between positive margins and recurrence was suggested in several retrospective studies Citation[1],Citation[2]. In a recent clinical study that was conducted at our institute, Klimberg et al. reported on the use of radiofrequency ablation to assist surgery in assuring negative margin Citation[37]. In that study, 24 of 41 patients did not have postoperative radiation therapy, and no in-site local recurrences occurred during a median follow-up of 24 months. The results of this small pilot study suggest that margin ablation could benefit patients with stage I and II breast cancer.
In this study we demonstrate that CITT can create negative margins that inhibit local recurrence and metastasis in animal model. Although the VX2 carcinoma have a different response to heat treatment than human tumours in various ways, the NZW rabbit with VX2 carcinoma is an established model for use in translating thermal ablation techniques into the clinic Citation[11],Citation[38–45].
Thus, CITT has the potential to reduce recurrence rates following surgical resection of the primary tumour. The mechanisms by which CITT disrupts metastatic and recurrent lesions remain to be further elucidated. However, the results of the current study have already suggested some possible mechanisms that include inhibiting tumour-cell transit, and stimulating host immune responses.
Conclusion
The CITT device can effectively ablate partially resected VX2 carcinoma in a rabbit model, and inhibited recurrence and metastasis of VX2 tumour under the conditions of our study. In addition, CITT tumour ablation evoked an inflammatory response which might be linked to the reduced metastatic spread observed. Following surgical resection of the primary tumour, the CITT procedure could be used to reduce the chance of recurrence and metastasis, by inducing coagulation necrosis and local inflammation at the margins of the surgical cavity. Further studies are being planned to translate use of the CITT device into clinical settings.
Acknowledgements
Funding for this work was provided by the National Cancer Institute NIH/NCI Grant No. CA108678 to GS. PN, EGM and RG effort was partially funded by the Central Arkansas Radiation Therapy Institute (CARTI). RG was supported by CA 44114.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. Dr. Gal Shafirstein and Scott Ferguson have significant financial and proprietary interests related to the CITT technology. Dr. Robert Griffin and Petr Novak have minor financial interests related to the CITT technology.
References
- Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg 2002; 184: 383–393
- Mullenix PS, Cuadrado DG, Steele SR, Martin MJ, See CS, Beitler AL, Carter PL. Secondary operations are frequently required to complete the surgical phase of therapy in the era of breast conservation and sentinel lymph node biopsy. Am J Surg 2004; 187: 643–646
- Henry-Tillman R, Johnson AT, Smith LF, Klimberg VS. Intraoperative ultrasound and other techniques to achieve negative margins. Semin Surg Oncol 2001; 20: 206–213
- Cellini C, Hollenbeck ST, Christos P, Martins D, Carson J, Kemper S, Lavigne E, Chan E, Simmons R. Factors associated with residual breast cancer after re-excision for close or positive margins. Ann Surg Oncol 2004; 11: 915–920
- Schnitt SJ. Risk factors for local recurrence in patients with invasive breast cancer and negative surgical margins of excision. Where are we and where are we going?. Am J Clin Pathol 2003; 120: 485–488
- Scattoni V, Montorsi F, Picchio M, Roscigno M, Salonia A, Rigatti P, Fazio F. Diagnosis of local recurrence after radical prostatectomy. BJU Int 2004; 93: 680–688
- Milecki P, Kwias Z, Skowronek J, Stachowski T. [Adjuvant and salvage radiotherapy after radical prostatectomy]. Pol Merkur Lekarski 2004; 16: 495–499
- van Houten VM, Leemans CR, Kummer JA, Dijkstra J, Kuik DJ, van den Brekel MW, Snow GB, Brakenhoff RH. Molecular diagnosis of surgical margins and local recurrence in head and neck cancer patients: A prospective study. Clin Cancer Res 2004; 10: 3614–3620
- Carter D. Margins of ‘Lumpectomy’ for breast cancer. Human Pathology 1986; 17(4)330–332
- De Mascarel I, Trojani M, Bonichon F, Coindre JM. Histological examination of 2859 breast biopsies. Analysis of adequate sampling. Pathology Annual 1993; 28: 1–13
- Shafirstein G, Hennings L, Kaufmann Y, Novak P, Moros EG, Ferguson S, Myhill J, Swaney M, Spring P. Conductive interstitial thermal therapy (CITT) device evaluation in VX2 rabbit model. Technol Cancer Res Treat 2007; 6: 235–246
- Shafirstein G, Novak P, Moros EG, Siegel E, Hennings L, Kaufmann Y, Ferguson S, Myhill J, Swaney M, Spring P. Conductive interstitial thermal therapy device for surgical margin ablation: In vivo verification of a theoretical model. Int J Hyperthermia 2007; 23: 477–492
- Valvano JW. Tissue thermal properties and perfusion. Optical-thermal response of laser-irradiated tissue, AJ Welch, MJCv Gemert. Plenum Press, New York 1995; 445–489
- Shafirstein G, Baumler W, Lapidoth M, Ferguson S, North PE, Waner M. A new mathematical approach to the diffusion approximation theory for selective photothermolysis modeling and its implication in laser treatment of port-wine stains. Lasers Surg Med 2004; 34: 335–347
- van Es RJ, Dullens HF, van der Bilt A, Koole R, Slootweg PJ. Evaluation of the VX2 rabbit auricle carcinoma as a model for head and neck cancer in humans. Journal of Cranio-Maxillo-Facial Surgery 2000; 28: 300–307
- Lee JM, Jin GY, Li CA, Chung GH, Lee SY, Han YM, Chung MJ, Kim CS. Percutaneous radiofrequency thermal ablation of lung VX2 tumors in a rabbit model using a cooled tip-electrode: Feasibility, safety, and effectiveness. Invest Radiol 2003; 38: 129–139
- Sapundzhiev N, Dunne AA, Ramaswamy A, Werner JA. The auricular VX2 carcinoma: Feasibility of complete tumor resection. Anticancer Res 2005; 25: 4209–4214
- Weinberg BD, Patel RB, Wu H, Blanco E, Barnett CC, Exner AA, Saidel GM, Gao J. Model simulation and experimental validation of intratumoral chemotherapy using multiple polymer implants. Med Biol Eng Comput 2008; 46: 1039–1049
- Johnson CC, Guy AW. Nonionizing electromagnetic wave effects in biological materials and systems. Proc IEEE 1972; 60: 692–718
- Wren J, Eriksson O, Wardell K, Loyd D. Analysis of temperature measurement for monitoring radio-frequency brain lesioning. Med Biol Eng Comput 2001; 39: 255–262
- Chang I. Finite element analysis of hepatic radiofrequency ablation probes using temperature-dependent electrical conductivity. Biomed Eng Online 2003; 2: 12
- Gupta GP, Massague J. Cancer metastasis: Building a framework. Cell 2006; 127: 679–695
- Gupta GP, Perk J, Acharyya S, de Candia P, Mittal V, Todorova-Manova K, Gerald WL, Brogi E, Benezra R, Massague J. ID genes mediate tumor reinitiation during breast cancer lung metastasis. Proc Natl Acad Sci USA 2007; 104: 19506–19511
- Norton L, Massague J. Is cancer a disease of self-seeding?. Nat Med 2006; 12: 875–878
- Alix-Panabieres C, Riethdorf S, Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res 2008; 14: 5013–5021
- Slade MJ, Payne R, Riethdorf S, Ward B, Zaidi SA, Stebbing J, Palmieri C, Sinnett HD, Kulinskaya E, Pitfield T, et al. Comparison of bone marrow, disseminated tumour cells and blood-circulating tumour cells in breast cancer patients after primary treatment. Br J Cancer 2009; 100: 160–166
- Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res 2008; 14: 2519–2526
- Saji H, Song W, Nakamura H, Saijo T, Hosaka M, Hagiwara M, Ogata A, Kawasaki N, Engleman EG, Kato H. [A possibility of overcoming local tumour immune tolerance by radiofrequency ablation in combination with intratumoural injection of naive dendritic cell]. Gan To Kagaku Ryoho 2006; 33: 1736–1738
- den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, Boerman OC, Figdor CG, Ruers TJ, Adema GJ. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer 2006; 95: 896–905
- Habibi M, Kmieciak M, Graham L, Morales JK, Bear HD, Manjili MH. Radiofrequency thermal ablation of breast tumors combined with intralesional administration of IL-7 and IL-15 augments anti-tumor immune responses and inhibits tumor development and metastasis. Breast Cancer Res Treat 2009; 114: 423–431
- Sabel MS. Cryo-immunology: A review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology 2009; 58: 1–11
- NIH consensus conference. Treatment of early-stage breast cancer. JAMA 1991; 265: 391–395
- Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347: 1233–1241
- Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 2002; 347: 567–575
- Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, Aguilar M, Marubini E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002; 347: 1227–1232
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James s, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005; 366: 2087–2106
- Klimberg VS, Kepple J, Shafirstein G, Adkins L, Henry-Tillman R, Youssef E, Brito J, Talley L, Korourian S. eRFA: Excision followed by RFA–A new technique to improve local control in breast cancer. Ann Surg Oncol 2006; 13: 1422–1433
- Hazle JD, Stafford RJ, Price RE. Magnetic resonance imaging-guided focused ultrasound thermal therapy in experimental animal models: Correlation of ablation volumes with pathology in rabbit muscle and VX2 tumors. J Magn Reson Imaging 2002; 15: 185–194
- Boehm T, Malich A, Reichenbach JR, Fleck M, Kaiser WA. Percutaneous radiofrequency (RF) thermal ablation of rabbit tumors embedded in fat: A model for RF ablation of breast tumors. Invest Radiol 2001; 36: 480–486
- Boehm T, Malich A, Goldberg SN, Reichenbach JR, Hilger I, Hauff P, Reinhardt M, Fleck M, Kaiser WA. Radio-frequency tumor ablation: Internally cooled electrode versus saline-enhanced technique in an aggressive rabbit tumor model. Radiology 2002; 222: 805–813
- Boehm T, Malich A, Goldberg SN, Hauff P, Reinhardt M, Reichenbach JR, Muller W, Fleck M, Seifert B, Kaiser WA. Radio-frequency ablation of VX2 rabbit tumors: Assessment of completeness of treatment by using contrast-enhanced harmonic power Doppler US. Radiology 2002; 225: 815–821
- Tsuda N, Tsuji T, Kato N. Interstitial magnetic resonance lymphography using gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid in rabbits with lymph node metastasis. Invest Radiol 2005; 40: 306–312
- Palussiere J, Salomir R, Le Bail B, Fawaz R, Quesson B, Grenier N, Moonen CT. Feasibility of MR-guided focused ultrasound with real-time temperature mapping and continuous sonication for ablation of VX2 carcinoma in rabbit thigh. Magn Reson Med 2003; 49: 89–98
- Shah SS, Jacobs DL, Krasinkas AM, Furth EE, Itkin M, Clark TW. Percutaneous ablation of VX2 carcinoma-induced liver tumors with use of ethanol versus acetic acid: Pilot study in a rabbit model. J Vasc Interv Radiol 2004; 15: 63–67
- Lee JM, Kim SW, Chung GH, Lee SY, Han YM, Kim CS. Open radio-frequency thermal ablation of renal VX2 tumors in a rabbit model using a cooled-tip electrode: Feasibility, safety, and effectiveness. Eur Radiol 2003; 13: 1324–1332