Abstract
Purpose: To evaluate the feasibility, safety and therapeutic effects of ultrasound (US)-guided percutaneous microwave (MW) ablation in the treatment of adrenal metastasis.
Materials and methods: From May 2006 to April 2008, five consecutive patients with pathologically proven unilateral adrenal metastases with a diameter of 2.3 to 4.5 cm were treated by US-guided percutaneous MW ablation. Four metastases were in the right side, one metastasis was in the left side. For each application, two cooled-shaft needle antennae were percutaneously inserted into the tumour under real-time US guidance. One thermocouple needle was inserted at the periphery of the tumour to monitor temperature in real-time during MW ablation. MW emission was ended when the entire tumour became hyperechoic and the temperature at the tumour border reached 54°C for at least 3 min. Technical success was defined as loss of tumour enhancement on contrast-enhanced imagings.
Results: All adrenal metastases were completely ablated after scheduled MW ablation sessions (mean, 1.2 sessions, range, 1 to 2 sessions). No major complications related to MW ablation occurred. In a median follow-up of 19 months (range 8 to 31 months), persistent absence of tumour enhancement was observed in the treated tumour in all patients.
Conclusions: US-guided percutaneous MW ablation appears to be a safe and effective therapy in selected adrenal metastasis.
Introduction
Adrenal metastases are frequently encountered in various malignancies, the most common primary sites include lung, stomach and liver Citation[1–3]. Due to the development of modern imaging techniques such as ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET), adrenal metastasis could be easily detected. Though metastasis to the adrenal gland is regarded as a late-stage manifestation of tumour progression, for metastasis confined to the adrenal gland, surgical resection, either via an open or laparoscopic approach, has been proven to be potentially curative and to offer survival benefits []. However, adrenalectomy may be technically challenging for some patients and may be associated with severe complications such as excessive haemorrhage. Therefore, less invasive techniques have been developed for treatment of adrenal neoplasms which include transcatheter arterial embolisation Citation[7], chemical ablation using ethanol or acetic acid Citation[8],Citation[9], and radiofrequency (RF) ablation Citation[10],Citation[11]. Microwave (MW) is one of the thermal ablation techniques which has become increasingly popular for treatment of both primary and metastatic liver cancers Citation[12–14]. Previous studies revealed that MW ablation can yield effective local tumour control and the long-term survival rate is comparable to that of hepatectomy for small hepatocellular carcinoma Citation[15]. The therapeutic effects, complication rates, and long-term survival of MW ablation are equivalent to that of RF ablation for treatment of liver cancer Citation[16]. The long-term survival rate of MW ablation is similar to that of ethanol injection for small solitary hepatocellular carcinoma Citation[17]. Recently, this technique had been used successfully for treatment of renal and lung cancer Citation[18],Citation[19]. To our knowledge, there are no reports on MW ablation of adrenal metastasis so far. Thus, the purpose of this study was to evaluate the feasibility and safety for ultrasound (US) -guided percutaneous MW ablation of isolated adrenal metastasis.
Methods
Patients
From May 2006 to April 2008, five consecutive patients with pathologically proven unilateral adrenal metastases underwent US-guided percutaneous MW ablation in our department. Institutional review board approval was obtained. All patients were treated as inpatients who signed informed consent at enrolment. The inclusion criteria were as follows: (1) unilateral adrenal lesion of 5 cm or smaller; (2) complete eradication of primary tumour; (3) no extra-adrenal metastases and tumour thrombus; (4) an appropriate MW antenna needle path is present on US; (5) prothrombin time of less than 25 seconds, prothrombin activity higher than 40%. For fear of post-ablation adrenal insufficiency, patients with bilateral adrenal lesions were excluded from this study. There were four men and one woman, aged 47–76 years. None of the patients were considered as surgical candidates because of old age, poor hepatic reserve or abdominal adhesion due to previous surgery. The blood pressures were normal or kept normal by medication before MW ablation in all patients. Four metastases were hepatocellular carcinoma, one metastasis was clear-cell renal carcinoma. The pathological diagnosis was obtained by US-guided biopsy in all tumours at the time immediately before MW ablation. The mean tumour size was 3.48 cm ± 0.96 [SD] (range, 2.3–4.5 cm). Four tumours were on the right side and one tumour was on the left side ().
Table 1. Summary of patients and tumours treated with MW ablation.
Equipment
The commercially available MW ablation system (KY2000, Kangyou Medical Instruments, Nanjing, China) consists of a MW generator, a flexible coaxial cable and a cooled-shaft antenna. The generator is capable of producing 1–100 Watts of power at 2450 MHz, which can drive two antennae simultaneously. The 15 G cooled-shaft antenna is coated with Teflon to prevent adhesion. Inside the antenna shaft there are dual channels through which distilled water is circulated by a peristaltic pump, continuously cooling the shaft to prevent the shaft overheating. The MW system is also equipped with 21 G thermocouple needles which are easily seen on US and can be percutaneously inserted at designated places under US guidance to monitor real-time temperature during MW ablation. The thermosensor embedded at the tip of the needle was made of iron-constant; the material is both accurate and sensitive for measuring temperature, it is also not influenced by the electromagnetic field of MW.
MW ablation procedures
Before treatment, all patients received contrast-enhanced CT/MRI and contrast-enhanced US examination. Contrast-enhanced US was performed on a Sequoia 512 unit (Siemens Ultrasound, Mountain View, CA) equipped with contrast pulse sequencing software. The sonographic contrast agent used in this study was sulphur hexafluoride (SonoVue, Bracco, Milan, Italy). Because all tumours could be clearly seen on US and we are more experienced with real-time US guidance, US instead of CT was chosen as the preferred method for guiding the proper placement of MW antennae; the appropriate MW antenna needle path was chosen on US. For right adrenal tumours, a trans-hepatic approach was adopted to access the adrenal tumour with the patient lying in left decubitus position. For the left adrenal tumour, a posterior MW antenna needle path was chosen carefully to evade the spleen with the patient lying in right decubitus position. After local anaesthesia with 1% lidocaine, MW antennae were percutaneously inserted into the tumour and placed at designated places under US guidance (). Based on our previous experience for MW ablation of liver cancer, for adrenal tumours measuring 2 cm or greater, two antennae were used with an inter-antenna distance of no more than 1.8 cm, which were activated simultaneously to obtain confluent ablation zones Citation[12]. In this study, two MW antennae were used for each application in all patients. After intravenous anaesthesia administered by a combination of propofol and ketamine via the peripheral vein, MWs were then emitted at a power output of 45 W. The power output was chosen according to the recommendations of the manufacturer and our experience. One thermocouple needle was percutaneously placed at the tumour border under US guidance before MW ablation. Temperature was monitored continuously during MW emission; the thermocouple needle was not moved around during MW ablation. All insertions were performed by either of the two experienced radiologists (LP and XLY), who had cooperated for more than 10 years in MW ablation for hepatocellular carcinoma.
Figure 1. Ultrasound-guided percutaneous MW ablation in a 53-year-old man with right adrenal metastasis (arrow) from hepatocellular carcinoma. (a) Greyscale ultrasound shows a hypoechoic tumour in the right adrenal gland before MW ablation. (b) The tumour (arrow) showed rich enhancement on contrast-enhanced ultrasound. (c) MW antenna (arrowhead) was percutaneously placed into the adrenal tumour (arrow) under real-time ultrasound guidance. (d) The tumour (arrow) became hyperechoic right after MW ablation.
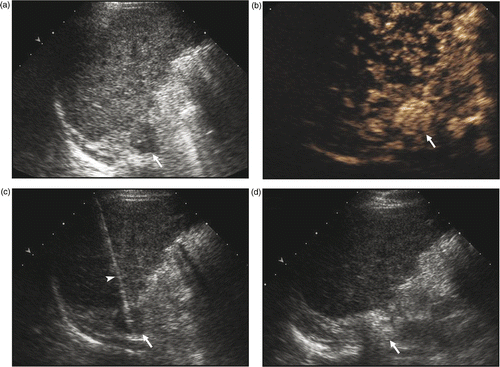
Blood pressure was also closely monitored during treatment. To prevent possible hypertension crisis, after consultation with the anaesthetists, we decided that if the systolic pressure exceeded 170 mmHg during MW ablation, MW emission should be suspended and anti-hypertensive drug (labetalol hydrochloride) be given intravenously, MW ablation could be resumed until the systolic pressure decreased to baseline level. Treatment was considered complete when the entire tumour became enveloped by hyperechoic microbubbles and the temperature at the tumour periphery reached 54°C for at least 3 min, or else MW emission was prolonged. After MW ablation of adrenal metastasis, the MW antenna needle path was routinely cauterised to avoid tumour seeding and bleeding. The antennae were gradually withdrawn after switching off the water peristaltic pump; MW emission was continued until the muscle along the needle path was cauterised by the active tip of the antenna. Then the MW emission was stopped and the MW antennae were pulled out.
Post-ablation observation and imaging follow-up
After MW ablation, patients were closely monitored for possible complications such as skin burn, bowel perforation, intraperitoneal bleeding and side effects such as pain, fever and pleural effusion. All patients received contrast-enhanced US examination 1–3 days after MW ablation to assess the treatment outcome. If residual tumour enhancement was found on contrast-enhanced US, a further MW ablation session under contrast-enhanced US guidance was planned, or else patients entered the follow-up protocol, which consisted of contrast-enhanced CT/MRI at 1 and 3 months after MW ablation and at a 4–6 month interval thereafter until December 2008.
Results
In this study, all adrenal tumours were satisfactorily visible by means of US, no patients referred to our department were excluded because the tumour was not clearly seen on US. MW ablation was well-tolerated in all patients. During MW ablation, the systolic blood pressure exceeded 170 mmHg 2 min after MW emission in one patient (185 mmHg in the patient with a 4.5 cm right adrenal metastasis of hepatocellular carcinoma). MW ablation was suspended for 3 min and the blood pressure decreased to baseline level, MW emission was then continued and the blood pressure did not exceed 170 mmHg again. The systolic blood pressure did not change significantly during MW ablation in the other four patients. In all patients, the temperature at the tumour border reached 54°C for at least 3 min, the total time of MW ablation ranged from 240 to 720 s.
After MW ablation, the tumour became hyperechoic due to the microbubbles generated during MW emission, which may impede accurate assessment of treatment outcome, therefore, contrast-enhanced US was not performed straight after the procedure, it was performed 1–3 days after MW ablation instead.
Residual tumour enhancement was found in one patient with a 4.4 cm left adrenal metastasis 3 days after MW ablation on contrast-enhanced US, which was treated by a second session of MW ablation. The contrast software of the US unit could display both the contrast-enhanced harmonic image and the greyscale image simultaneously; therefore the MW antenna could be clearly shown and accurately positioned by means of contrast-enhanced US. Two MW antennae were inserted into the area that showed contrast uptake on contrast-enhanced US to ablate the residual tumour. No major complications occurred, minimal to moderate pain was experienced, according to the reporting criteria for image-guided tumour ablation Citation[20], two were grade 1 pain requiring no medication, two were grade 2 pain requiring oral analgesic of ibuprofen tablets. Minimal asymptomatic pleural effusion was found in one patient, which resolved spontaneously in one week. All patients were discharged 3 to 4 days after scheduled MW sessions and consulted our department regularly as outpatients.
During a median follow-up of 19 months (range 8–31 months), persistent absence of contrast enhancement was observed in treated tumours on contrast-enhanced imaging examinations in all patients ( and ). The patient who had a left adrenal metastasis of liver cancer developed new intrahepatic nodules of hepatocellular carcinoma and pathological fracture of the right femoral neck due to bone metastasis, the new liver cancer was successfully treated by MW ablation, the bone metastasis was treated by total hip replacement. No local recurrence and extra-adrenal metastasis was found in other patients.
Figure 2. MW ablation in a 76-year-old woman who had a right adrenal metastasis from clear-cell renal carcinoma two years after right nephrectomy. (a) Before treatment the tumour was slightly enhanced on contrast-enhanced MRI. No enhancement was observed on contrast-enhanced MRI at three months (b), one year (c) and two years (d) after MW ablation, the ablated tumour gradually shrank over time.
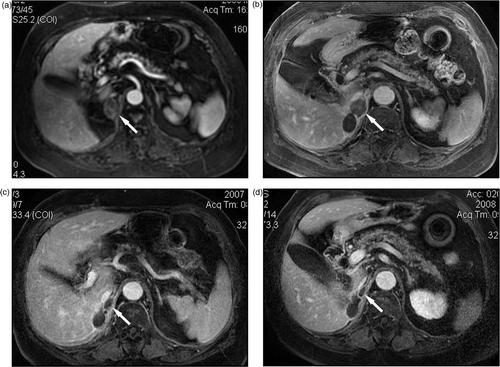
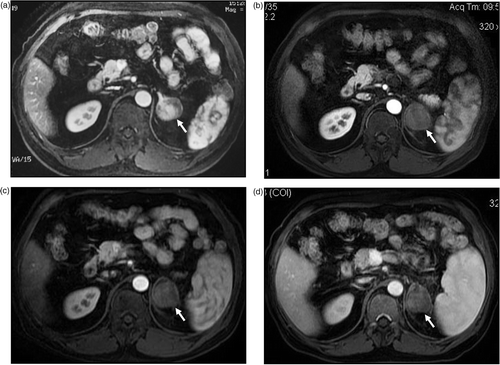
Figure 3. MW ablation in a 51-year-old man who had a left adrenal metastasis from hepatocellular carcinoma. (a) Before treatment the tumour was enhanced on contrast-enhanced MRI. No enhancement was observed on contrast-enhanced MRI at three months (b), six months (c) and one year (d) after MW ablation.
Discussion
In this pilot study, US-guided percutaneous MW ablation was used for the first time as a minimally invasive therapy for treatment of adrenal metastasis. There was minimal morbidity associated with the procedure and the recovery was speedy. All adrenal metastases were successfully ablated without major complications. Four metastases were completely ablated in one session, one metastasis required a repeat MW ablation. Contrast-enhanced imaging follow-ups indicated that persistent absence of enhancement was achieved in treated adrenal metastases after scheduled MW ablation sessions. The results showed that MW ablation could yield a large enough ablation zone to envelope small isolated adrenal tumours if the antennae were properly placed. The outcome favours MW ablation as a minimally invasive therapy for small isolated adrenal metastasis.
MW ablation, which is the newer technique, is technically similar to RF ablation. RF ablation had been proven to be safe and effective for local control of adrenal cancers, particularly those smaller than 5 cm in diameter Citation[10],Citation[11]. Compared with RF, MW has a much broader zone of active heating and its transmission is not limited by tissue desiccation and charring. Therefore, intratumoural temperatures can be driven consistently high, which may theoretically contribute to larger ablation zones, less treatment time and more complete tumour kill Citation[21]. Current MW antennae can yield ablation zones as large as that of RF electrodes. Therefore, as suggested in this study, MW ablation could also be effective for treatment of adrenal neoplasms. Further randomised study is needed to compare the treatment efficacy between the two techniques.
Percutaneous chemical ablation can also be employed to treat adrenal metastasis, however, it requires multiple sessions of ethanol or acetic acid injection. Chemical ablation is most effective for tumours of less than 3 cm in diameter and considered a poor alternative for tumours larger than 5 cm Citation[9]. As MW ablation can induce larger ablation zones with fewer treatment sessions, it may be more appropriate for management of adrenal metastasis.
Hypertensive crisis, although rare, is a major concern for adrenal neoplasm ablation, pheochromocytoma is predisposed to the life-threatening complication, whereas hypertensive crisis may also occur in treating liver cancers adjacent to the adrenal gland Citation[22] and adrenal metastatic lesions Citation[23]. To prevent excessive catecholamine release due to heat stimulation, according to the suggestion of the anaesthetists, MW emission was suspended when the systolic pressure exceeded 170 mmHg and anti-hypertensive drug was given, no hypertensive crisis was encountered by taking the precautions.
This study has some limitations. First, it was limited by the small sample size, the relatively short follow-up period and limited pathological tumour type included. Therefore, though the preliminary results were encouraging, the exact efficacy of MW ablation and proper patient inclusion criteria need to be defined in future clinical studies. More patients need to be recruited and long-term follow-up is mandatory. Second, in this study, US was used as the imaging modality for guiding MW antenna placement. For some adrenal tumours, especially small left ones, it may be difficult to find a safe MW antenna needle path on US, placement under CT guidance may be more appropriate instead. Third, the treatment efficacy was assessed by serial contrast-enhanced imaging examinations. As absence of tumour enhancement on contrast imaging may not always ensure complete eradication of tumours, an ‘ablate and resect’ protocol may be more accurate to determine whether viable tumour is still present after treatment.
Conclusion
In summary, our preliminary results showed US-guided percutaneous MW ablation is safe and effective for local control of selected adrenal metastases. Further studies are warranted to observe its long-term treatment efficacy and compare the results with those of other treatment options.
Acknowledgement
We thank the National Scientific Foundation Committee of China for financial support (06J016).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bullock WK, Hirst AE, Jr. Metastatic carcinoma of the adrenal. Am J Med Sci 1953; 226: 521–524
- Lam KY, Lo CY. Metastatic tumours of the adrenal glands: A 30-year experience in a teaching hospital. Clin Endocrinol 2002; 56: 95–101
- Katyal S, Oliver JH, Peterson MS, et al. Extrahepatic metastases of hepatocellular carcinoma. Radiology 2000; 216: 698–703
- Paul CA, Virgo KS, Wade TP, Audisio RA, Johnson FE. Adrenalectomy for isolated adrenal metastases from non-adrenal cancer. Int J Oncol 2000; 17: 181–187
- Kim SH, Brennan MF, Russo P, Burt ME, Coit DG. The role of surgery in the treatment of clinically isolated adrenal metastasis. Cancer 1998; 82: 389–394
- Sarela AI, Murphy I, Coit DG, Conlon KC. Metastasis to the adrenal gland: The emerging role of laparoscopic surgery. Ann Surg Oncol 2003; 10: 1191–1196
- Hokotate H, Inoue H, Baba Y, Tsuchimochi S, Nakajo M. Aldosteronomas: Experience with superselective adrenal arterial embolization in 33 cases. Radiology 2003; 227: 401–406
- Shibata T, Maetani Y, Ametani F, Itoh K, Konishi J. Percutaneous ethanol injection for treatment of adrenal metastasis from hepatocellular carcinoma. Am J Roentgenol 2000; 174: 333–335
- Xiao YY, Tian JL, Li JK, Yang L, Zhang JS. CT-guided percutaneous chemical ablation of adrenal neoplasms. Am J Roentgenol 2008; 190: 105–110
- Wood BJ, Abraham J, Hvizda JL, Alexander HR, Fojo T. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer 2003; 97: 554–560
- Mayo-Smith WW, Dupuy DE. Adrenal neoplasms: CT-guided radiofrequency ablation – Preliminary results. Radiology 2004; 231: 225–230
- Dong BW, Liang P, Yu XL, et al. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: Results in 234 patients. Am J Roentgenol 2003; 180: 1547–1555
- Kuang M, Lu MD, Xie XY, et al. Liver cancer: Increased microwave delivery to ablation zone with cooled-shaft antenna – Experimental and clinical studies. Radiology 2007; 242: 914–924
- Iannitti DA, Martin RC, Simon CJ, et al. Hepatic tumor ablation with clustered microwave antennae: The US Phase II Trial. HPB (Oxford) 2007; 9: 120–124
- Liang P, Dong BW, Yu XL, et al. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology 2005; 235: 299–307
- Lu MD, Xu HX, Xie XY, et al. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: A retrospective comparative study. J Gastroenterol 2005; 40: 1054–1060
- Seki T, Wakabayashi M, Nakagawa T, et al. Percutaneous microwave coagulation therapy for patients with small hepatocellular carcinoma: Comparison with percutaneous ethanol injection therapy. Cancer 1999; 85: 1694–1702
- Liang P, Wang Y. Ultrasound guided percutaneous microwave ablation for small renal cancer: Initial experience. J Urol 2008; 180: 844–848
- He W, Hu XD, Wu DF, et al. Ultrasonography-guided percutaneous microwave ablation of peripheral lung cancer. Clin Imaging 2006; 30: 234–241
- Goldberg SN, Charboneau JW, Dodd GD, III, et al. Image-guided tumor ablation: Proposal for standardization of terms and reporting criteria. Radiology 2003; 228: 335–345
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: Principles and applications. RadioGraphics 2005; 25: S69–S83
- Onik G, Onik C, Medary I, et al. Life-threatening hypertensive crises in two patients undergoing hepatic radiofrequency ablation. Am J Roentgenol 2003; 181: 495–497
- Chini EN, Brown MJ, Farrell MA, Charboneau JW. Hypertensive crisis in a patient undergoing percutaneous radiofrequency ablation of an adrenal mass under general anesthesia. Anesth Analg 2004; 99: 1867–1869