Abstract
Background: The use of electrocautery devices is associated with complications such as perforation or fistulisation when used near intestinal structures. This is likely due to its effect on vascularisation of the bowel wall. To test this hypothesis we established a murine model to quantify the effect of electrocautery injury on the intestinal microvascularisation.
Methods: Sprague-Dawley rats were subjected to five electrocautery injuries on the small bowel in coagulation mode (30 W intensity) and in cut mode (40 W, 80 W and 200 W intensities) for durations of 1, 2 and 5 s. 5 mg/kg of fluorescein was injected intravenously, the injured bowel segments harvested and the rat sacrificed. The segments were analysed to measure the fluorescence of injured bowel compared to adjacent unharmed tissue.
Results: A significant decrease in bowel wall microvascularisation occurred with increasing intensity (coag 30 W/cut 40 W versus cut 200 W 1 s: p < 0.05) and duration of electrocautery injury (cut 40 W 1/2 s versus 5 s: p < 0.05). There was a 40% perforation rate when decreased bowel wall microvascularisation was 25% or more. Despite similar electrocautery injury, a significantly greater microvascularisation decrease was observed in jejunum compared to ileum (p < 0.05).
Conclusion: We successfully established a murine model to quantify the decrease of bowel wall microvascularisation associated with electrocautery use. Unsurprisingly, the decrease in microvascularisation is greater with higher intensity and duration of electrocautery and is associated with more perforations in the experimental model. The jejunum seems more vulnerable to electrocautery injury than the ileum. These observations support caution when using electrocautery devices near intestinal structures.
Introduction
Electrosurgical devices are widely used in general surgery. Well-known complications are associated with its use, such as mechanical trauma, electrical burns or thermal injury, possibly leading to perforation or fistula formation [Citation1,Citation2].
Electrocautery devices are used near or on intestinal structures in cytoreductive surgery (CRS) combined with heated intraoperative intraperitoneal chemotherapy (HIPEC) for tumours with intraperitoneal dissemination. Historically complication rates in CRS and HIPEC were high, with morbidity ranging between 30 and 70% [Citation3] and mortality between 0 and 8% [Citation4], with recent mortality rates between 2 and 3% [Citation5,Citation6]. Up to 11% of patients require reoperation, 29% of these for gastrointestinal fistulas and 19% for anastomotic failure [Citation5]. The incidence of digestive fistulisation ranges between 3 and 6.5% [Citation7]. In certain series, up to 7% of patients had acute bowel perforation in the post-operative period [Citation3]. The use of the electrosurgical devices near intestinal structures could contribute to the high rate of fistulas or intestinal perforations observed after CRS by altering the bowel wall’s blood supply. Very few studies tried to establish a link between electrocautery use and complications. One study, aiming to understand accidental mechanical and thermal injuries in laparoscopy, discovered that short duration thermal injury to the bowel with electrocautery reduced bowel strength at 48 h post injury [Citation8].
The main objective of this study was to measure the intestinal microvascularisation after the use of electrocautery on the bowel wall in a murine model. The rationale for this model is to quantify the variation in bowel wall microvascularisation after electrocautery injury in order to better understand the origin of complications in gastrointestinal surgery. Our hypothesis is that the greater the electrocautery injury, the greater the reduction in bowel wall microvascularisation, which could induce complications in clinical situations. We also would like to correlate the reduction in bowel wall vascularisation to the temperature of the burn created by the cautery injury. The secondary goal was to establish a valid model that could be reproduced to test different experimental situations that could influence the impact of such cautery injuries on the bowel wall, such as hyperthermia and HIPEC.
Materials and methods
Animals
Forty-two male Sprague-Dawley rats, weighing between 350 and 650 g, were used in this study. Male rats were used for availability considerations. They were kept in optimal conditions according to the Canadian Council of Animal Care guidelines and cared for by the hospital’s animal facility.
Surgical technique
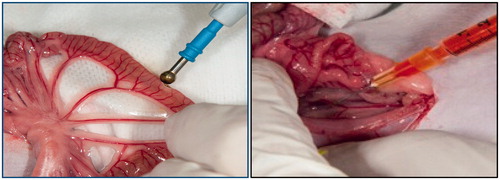
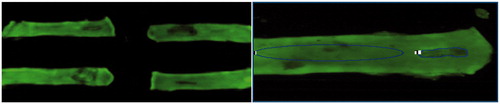
Under general anaesthesia with isoflurane, each animal was subjected to a 5-cm midline laparotomy, the abdominal viscera exposed and inspected to rule out any prior anomaly. The small bowel was exposed from the proximal jejunum to the ileo-caecal junction. Using a monopolar electrosurgical device with ballpoint tip, we performed five injuries on the anti-mesenteric border of the small bowel, each of 1 cm, and separated by at least 5 cm. Four injuries were inflicted on the jejunum and one on the ileum, 5 cm proximal to the ileo-caecal valve. The duration of the electrocautery injuries inflicted were 1 s, 2 s and 5 s with intensities of 40 W, 80 W, 200 W in the cut mode and 30 W in the coagulation mode. These variables were managed in order to obtain 10 measurements in each category (see ). We afterwards injected 5 mg/kg of 100 mg/cm3 solution of fluorescein diluted in normal saline solution for a final concentration of 10 mg/cm3 in the inferior vena cava (IVC). The dosage was based on paediatric suggestions for intravenous fluorescein of 7.7 mg/kg, as per Compendium of Pharmaceuticals and Specialties (CPS) recommendations. The use of fluorescein is a method used to detect bowel ischaemia in clinical settings [Citation9,Citation10]. One minute after injection, 5-cm bowel segments containing the injured and adjacent unharmed bowel were harvested and the rat sacrificed. The bowel sections were opened on their mesenteric border and exposed to a camera containing a fluorescent light filter to capture the fluorescence from the tissues. A numeric picture with 0.25 s exposure was taken with the ImageQuant 4000 (GE Life Sciences, Amersham, UK) camera (see ).
We analysed the resulting images with the AlphaEaseFC program (Alpha Innotech, San Leandro, CA). We measured the fluorescent light intensity of the injured section of the bowel tissue and compared it with an adjacent control tissue section to measure the impact of the electrocautery on the microvascularisation of the bowel tissue.
To complete our manipulations we measured the temperature in and adjacent (1 mm) to the electrocautery burn with a needle tip catheter 1 s after the injury to gather information regarding tissue temperature after cautery injury.
Statistical analysis
Data is presented as mean ± standard deviation. We used the Kolmogorov–Smirnov test on each group of data to confirm its normal distribution. Then we performed analysis of variance (ANOVA) for multiple group comparisons applying the Bonferroni correction to maintain an α value of 0.05. We considered differences were statistically significant if we could reject the null hypothesis with 95% confidence, as with a p-value below 0.05.
Results
A significant decrease in the murine bowel wall microvascularisation occurred with both increasing intensity (coag 30 W/cut 40 W versus cut 200 W 1 s: p < 0.05) and duration of electrocautery injury (cut 40 W 1/2 s versus 5 s: p < 0.05) on the jejunum. This decline in bowel wall microvascularisation was rapidly observed in the lower intensity and duration groups before reaching a less marked decrease with higher intensities and durations (see ).
Table 1. Variation in fluorescent intensities in each study group.
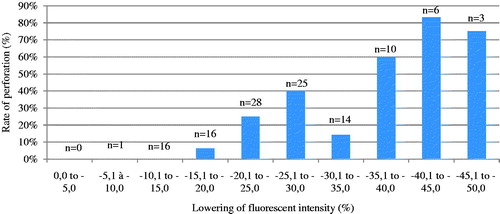
In the experimental model, bowel wall perforations were more frequent in higher duration and higher intensity injuries. We observed a cut-off point at 25% bowel wall vascularisation reduction beyond which macroperforation was observed in more than 40% of the specimens while we observed far fewer perforations when bowel wall vascularisation decrease was less than 25% (see ).
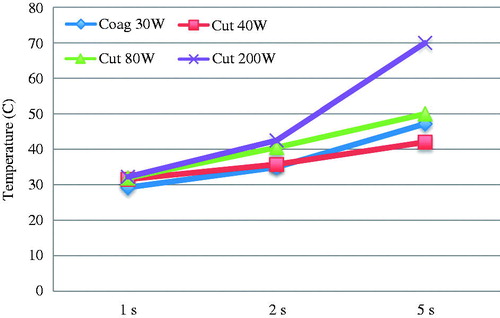
Afterwards, as stated above, we measured the tissue temperatures associated with the burns created by the electrocautery injuries. With a needle-tip catheter we measured the temperature in and adjacent to the burn to gather information about tissue temperature. The results are presented in , where we can observe tissue temperatures rising with intensity and most importantly duration of cautery injury (see ).
Table 2. Tissue temperature after electrocautery injury.
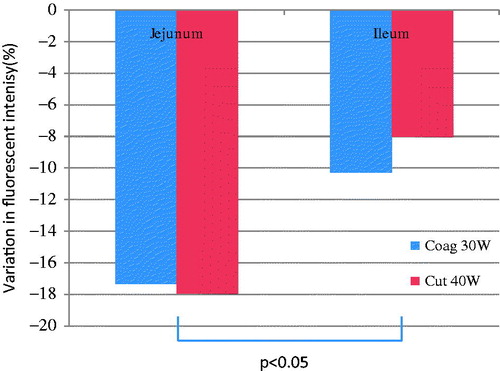
Finally, we compared the impact of electrosurgical injuries on jejunum and ileum in similar experimental conditions. Despite similar electrocautery injury, a significantly greater bowel wall microvascularisation decrease was observed in the jejunum compared to the ileum (p < 0.05). These observations were based upon limited experimental variations, considering ileum injuries were only inflicted for durations of 1 s with coag 30 W and cut 40 W intensities to be compared to similar jejunal injuries (see ).
Discussion
Our observations are consistent with what was expected of this experiment and support our primary hypothesis. Our results show a decrease in bowel wall microvascularisation with increasing duration and intensity injuries. It is logical to believe that lower intensity injuries have a lesser impact on the bowel wall microvascularisation while higher intensity and duration injuries have a greater impact, although the relation between the variables is not linear. The observed plateau of injuries at 200 W and 5 s could be explained in part by limitations in our measuring system and by the high rate, over 40%, of experimental perforation observed with such injuries which limits our ability to measure fluorescent intensity in our harvested bowel segments. Technical issues such as reproducibility of injuries, depth of injury in the bowel wall and manipulation of the harvested bowel segments also limit our ability to generalise our observations. Nevertheless, the tendency of our observations supports the validity of our experimental model.
We did not observe any significant difference between the coagulation and the cutting modes on the electrosurgical device used except in one scenario: the injury caused by low intensity coagulation mode for 5 s was significantly less severe than in the cutting mode. This could be explained by the greater coagulum crust formed by the coagulation mode on the bowel wall, which could protect the underlying vessels from prolonged injury.
Higher perforation rates were noted in groups with a greater decrease in bowel wall vascularisation. We observed that a bowel wall microvascularisation decrease of 25% or more indicated a greater risk of perforation in the experimental conditions. This does not take into consideration the secondary risk of perforation observed with electrocautery injuries that can occur from hours to days after the initial injury.
Also as expected, we observed a higher tissue temperature with increased cautery intensity and injury duration. The duration of the injury seems to have a greater impact on the tissue temperature, although we cannot at this time provide statistical proof of such concept. It is nevertheless interesting to see that short duration injuries could be equivalent independent on the cautery intensity, especially in CRS and HIPEC surgery where the cautery is often used to burn small tumour nodules on the bowel wall surface. More detailed work is needed in this area to better understand the relation between tissue temperature, vascularisation and fragility.
An interesting observation was the greater decrease in jejunal bowel wall microvascularisation compared to the ileum subgroup for similar injuries. The abundant ileal vascularisation could explain the greater resistance of the tissue to injuries, and greater caution should perhaps be used when working near the jejunum with the electrocautery device.
Having a seemingly valid experimental murine model could give us the opportunity to add different variables important to current surgical practice. The rationale for using a murine model is its accessibility and to use an animal our research centre has used in the past with a HIPEC model [Citation11]. Our idea for future work would be to combine both murine models to better understand cautery injuries after hyperthermia and HIPEC and to evaluate whether certain drugs or levels of hyperthermia are more harmful than others in regards to electrocautery injuries. We could also transpose to a rabbit or a pig model.
Temperature can have a significant impact on microvascularisation and tissue resistance to heat in the acute or chronic setting [Citation12,Citation13]. The animals used in this study were housed at 20.5 °C and the experiment was conducted at room temperature between 21° and 23 °C. These variables can have an impact in the pattern of protein expression in the animal subject, which we may have to take into consideration when hyperthermia is introduced [Citation12]. We decided to conduct this experiment at room temperature comparable to an operating room to validate the model before adding the hyperthermia variable. It has been stated that hyperthermia can have a significant impact on cell response, which could influence the results of our experiment. In addition, different levels of hyperthermia have a different impact on molecular pathways [Citation13], which could have created an unnecessary bias in our observations. In our experiment the small bowel was exteriorised as soon as entry to the peritoneal cavity was achieved, rendering temperature quite uniform in all animals. The size distribution of the animals would also not influence heat distribution from the cautery injuries as the small bowel was outside the abdominal cavity at the moment of injury. Our observations also show that bowel diameter is quite comparable between animals regardless of weight. This lowered the impact of temperature variations and heat dissipation issues that could be raised [Citation14,Citation15].
Questions may be raised concerning the state of the harvested bowel and the validity of our method of quantification for microvascular injury. To control for variation in tissue optics during our measurements we decided to control each individual cautery injury with normal adjacent bowel within the same animal, at the same moment after fluorescein injection and harvested in the same conditions. We thereafter measured the vascularisation difference as a ratio between the normal bowel and the injured bowel expressed as a percentage of reduction of bowel wall microvascularisation. Variations are therefore low, reducing our margin of error.
To conclude, caution remains key when using electrosurgical devices near gastrointestinal structures. Acquiring a greater knowledge of the physiology and response to injury of the bowel wall could be important in reducing morbidity and improving patient outcomes after this procedure.
Disclosure statement
This research was funded by the Maisonneuve-Rosemont Research Centre. The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article. The authors alone are responsible for the content and writing of the paper.
References
- Tou S, Malik A, Wexner SD, Nelson RL. Energy source instruments for laparoscopic colectomy. Cochrane Database Syst Rev 2011;5: CD007886.
- Wu MP, Ou CS, Chen SL, Yen EY, Rowbotham R. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg 2000;179:67–73.
- Saxena A, Yan TD, Chua TC, Morris DL. Critical assessment of risk factors for complications after cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol 2009;17:1291–301.
- Glockzin G, Schlitt HJ, Piso P. Peritoneal carcinomatosis: patients selection, perioperative complications and quality of life related to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol 2009 Jan 8;7:5.
- Sugarbaker PH, Alderman R, Edwards G, Marquardt CE, Gushchin V, Esquivel J, et al. Prospective morbidity and mortality assessment of cytoreductive surgery plus perioperative intraperitoneal chemotherapy to treat peritoneal dissemination of appendiceal mucinous malignancy. Ann Surg Oncol 2006;13:635–44.
- Youssef H, Newman C, Chandrakumaran K, Mohamed F, Cecil TD, Moran BJ. Operative findings, early complications, and long-term survival in 456 patients with pseudomyxoma peritonei syndrome of appendiceal origin. Dis Colon Rectum 2011;54:293–9.
- Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg 2006;243:212–22.
- Tsigris C, Tsechpenakis A, Nikiteas N, Tzogios I, Vlachos IS, Diamantis T, et al. Laparoscopic bowel injury: role of the site and the instrument type: study with an animal model. Surg Endosc 2008;22:2164–7.
- Urbanavičius L, Pattyn P, de Putte DV, Venskutonis D. How to assess intestinal viability during surgery: a review of techniques. World J Gastrointest Surg 2011;3:59–69.
- Bulkley GB, Zuidema GD, Hamilton SR, O'Mara CS, Klacsmann PG, Horn SD. Intraoperative determination of small intestinal viability following ischemic injury. Ann Surg 1981;193:628–37.
- Piché N, Leblond FA, Sidéris L, Pichette V, Drolet P, Fortier LP, et al. Rationale for heating oxaliplatin for the intraperitoneal treatment of peritoneal carcinomatosis: a study of the effect of heat on intraperitoneal oxaliplatin using a murine model. Ann Surg 2011;254:138–44.
- Eng JW, Reed CB, Kokolus KM, Repasky EA. Housing temperature influences the pattern of heat shock protein induction in mice following mild whole body hyperthermia. Int J Hyperthermia 2014;30:540–6.
- Glory A, Bettaieb A, Averill-Bates DA. Mild thermotolerance induced at 40 °C protects cells against hyperthermia-induced pro-apoptotic changes in Bcl-2 family proteins. Int J Hyperthermia 2014; 30:502–12.
- Karakaş BR, Sırcan-Küçüksayan A, Elpek OE, Canpolat M. Investigating viability of intestine using spectroscopy: a pilot study. J Surg Res 2014;191:91–8.
- Yarmolenko P, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti B, et al. Thresholds for thermal damage to normal tissues: an update. Int J Hyperthermia 2011;27:320–43.