Abstract
Purpose: The blood–retina barrier (BRB) is a biological barrier consisting of tightly interconnected endothelial cells inside the retinal vascular network that protects the neural tissue from harmful pathogens and neurotoxic molecules circulating in the bloodstream. Unfortunately, with regard to retinoblastoma, this barrier also prevents systemically administered therapeutics reaching the retinal tissue. In this study we introduce a novel technique to locally and transiently increase BRB permeability for drug delivery using hyperthermia of magnetic nanoparticles (MNPs).
Materials and methods: An alternating current (AC) magnetic field was used to induce hyperthermia of locally injected MNPs in the left ophthalmic artery of a rat model. To improve adherence on the surface of the endothelium, commercially available MNPs coated with human transferrin glycoproteins were used. After hyperthermia we assessed the extravasation of systemically injected sodium fluorescein (NaF) as well as Evans blue dye (EBD) into the retinal tissue.
Results: Spectrofluorometry and fluorescent microscopy image analysis show a significant increase of dye penetration in the retina where hyperthermia of MNPs was applied.
Conclusions: Our proposed new technique can allow both small and large dye molecules to cross the BRB. While the results are preliminary and thorough evaluation of the retinal tissue following hyperthermia is necessary, this technique has the potential to be an effective mean for the treatment of various diseases such as retinoblastoma.
Introduction
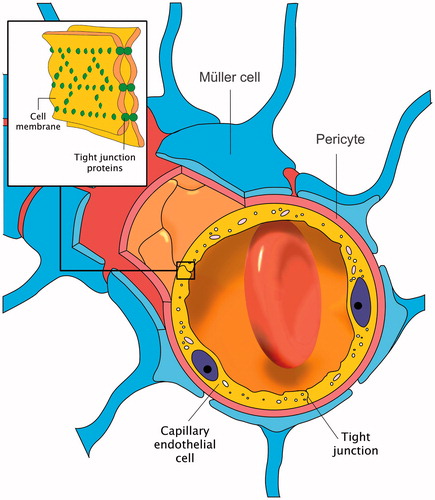
Similar to the brain microvasculature, the retinal capillaries in the central nervous system (CNS) consist of very complex inter-endothelial tight junctions that interconnect the endothelial cells (). The tight junctions seal the cell interspace and form a diffusion barrier that markedly controls the flow of molecules across blood vessel walls. This mainly constitutes the blood–retinal barrier (BRB), which limits the passage of the majority of therapeutic molecules into the retina of the eye [Citation1]. In this report we investigate a unique technique based on induction heating (hyperthermia) of magnetic nanoparticles (MNPs) to increase BRB permeability.
Current ocular drug delivery techniques can be classified into invasive and non-invasive approaches. The invasive approach consists of an intravitreal, intracameral, posterior juxtascleral, or retrobulbar injection of the medication, which can potentially lead to severe side effects and complete or partial loss of vision. The non-invasive drug delivery approaches include application of high drug dosage through topical, oral, systemic, or sub-Tenon’s administration [Citation2]. Despite encouraging results, in the case of ocular tumours such as retinoblastoma current approaches have been less promising and the results vary from only small increases in patients’ survival rate to increased normal tissue toxicity [Citation3–5].
The BRB is responsible for preserving the homeostasis of the retinal tissue and preventing toxic pathogens that may be circulating in the bloodstream from entering the eye [Citation1]. By extension, the BRB limits penetration of therapeutic molecules including most anti-cancer medications. Evidently, overcoming this barrier would greatly benefit drug delivery to the eye. Hyperthermia has reportedly been shown to increase the permeability of another physiologically similar barrier in the CNS, the blood–brain barrier [Citation6,Citation7].
While microwave, radio frequency, focused ultrasound, and induction heating using MNPs [Citation8–10] have shown promising complementary anti-cancer effects by opening the blood–brain barrier, they are not designed to increase barrier permeability for drug delivery to the eye. The thermal energy generated by these techniques is pervasive and it is dissipated over a wide range of tissue and cell types varying in thermal absorption rates, thresholds, and responses to the thermal dosage. In fact, studies show that elevation of temperature in the CNS could lead to acute and undesirable side effects such as neuronal damage and inflammation [Citation11]. To benefit from hyperthermia we have developed a technique in a rat model based on induction heating of MNPs to deliver the thermal energy specifically at the level of endothelial cells forming the blood–brain barrier [Citation12]. In the presence of an alternating current (AC) magnetic field the particles generate thermal energy which is then dissipated directly to the surface of the endothelium including the tight junctions. This technique allows us to increase barrier permeability locally, and it can be coupled with other potential applications of MNPs such as magnetic targeting [Citation13] and magnetic resonance navigation (MRN) [Citation14]. In addition, our theoretical findings suggest that the proposed technique minimises the risks affiliated with the undesirable heating of unrelated biological entities in the region. However, it is important to note that the effect of hyperthermia on the neuronal tissue is yet to be thoroughly investigated. Here we examine the feasibility of our technique for drug delivery to the retina.
Hyperthermia in the CNS
Hyperthermia above 43 °C can cause vascular damage and increase membrane permeability as well as cerebral blood flow. These, in turn, lead to the release of various neurochemicals and induce glutamate neurotoxicity [Citation15]. However, mild hyperthermia below 43 °C constitutes intense cellular stress and causes reversible disruption of the central nervous system (CNS) barrier [Citation16]. The permeability of adjacent endothelial cells (tight junctions) in the brain for instance, is known to increase in response to physiologically relevant temperature increases of 38.6–41.8 °C [Citation11]. We have previously reported a similar result in the brain of mice [Citation17]. Studies suggest that the morphological changes of individual endothelial cells in the monolayer lining of the microvessels cause the tight junctions between adjacent endothelial cells to loosen [Citation18]. This allows the transport of large molecules through the intercellular pathway. During local and mild hyperthermia, continuous blood flow and movement of the interstitial fluid in the microenvironment prevents healthy cells from overheating [Citation19]. In these conditions, hyperthermia redirects protein synthesis in the cells to produce heat-shock proteins, increases the membrane fluidity, and changes the vesicular and tubular components of microvascular endothelial cells [Citation20]. An interesting observation is that cells undergo a conditioning phenomenon that renders them capable of adapting to heat in order to survive [Citation21]. Heat shock or stress conditions experienced by cells and tissues alter cell structure and function in a homeoviscotic manner and render them more resistant to further stress. Therefore, cells can build tolerance to the damaging effects of temperature if they are gradually and repeatedly heated [Citation20,Citation22]. Clearly, the recovery rate from mild elevation of temperature depends on the amount of heat and exposure time [Citation23].
Induction heating and MNPs
Nanoparticles in general have successfully been used for diagnosis, drug and gene delivery [Citation24], and for the past decade or so there has been a significant interest in the use of MNPs in hyperthermia [Citation25]. The thermal effect of hyperthermia via induction is determined by the dielectric properties of the tissue and the magnetic properties of the medium. Since biological materials do not have significant magnetic properties, MNPs are used to increase the influence of the variation in the AC magnetic field. Clearly, biocompatible and biodegradable iron oxide MNPs have been given special attention in the medical community [Citation26].
The thermal energy generated by magnetic materials inside an AC magnetic field is mainly caused by three major mechanisms: hysteresis loss, and Néel or Brownian relaxations. Particle size, shape, composition, concentration and viscosity of the suspension medium as well as magnitude and frequency of the applied AC magnetic field determine the relative strength of each of these mechanisms. In small single-domain MNPs, such as those used in this study, the difference between the magnetic moment maximum and minimum potential energy per unit volume of the particle is much smaller than its thermal energy. The orientation of the magnetic moments of these particles therefore continuously changes due to thermal agitation [Citation27]. In other words, in the absence of an external magnetic field, the thermal energy causes magnetic moments to randomly fluctuate. Due to this behaviour, these particles lack hysteresis losses. By applying a moderate external AC magnetic field to MNPs, the energy from the field overcomes the thermal energy barrier and drives the magnetic moments to rotate and align with the magnetic field direction. Once the field is removed, magnetic moments do not relax immediately but rather take some time to randomise their orientations. The alignments and relaxations are responsible for the generation of heat [Citation27].
Blood–retinal barrier and hyperthermia of MNPs
In this research project, emphasis is placed on the importance of applying hyperthermia exclusively to the BRB. The MNPs absorb the magnetic energy from the AC magnetic field and dissipate it in the form of thermal energy to the surrounding retinal endothelium. The MNPs must therefore be able to hold on to the surface of the endothelium for the duration of the time they are required to reach the target temperature. This is a difficult task for the infinitesimal particles facing the rapid blood flow and the vigorous reticuloendothelial system. On another note, introducing a precise dosage of MNPs can also be challenging. On the one hand administration of high doses of MNPs into the bloodstream may lead to capillary occlusion and result in neuronal damage; on the other hand, low particle dosage per volume of tissue leads to insufficient rise in temperature. In fact our in vitro studies suggest that among factors such as AC magnetic field amplitude and frequency, the concentration of MNPs is directly proportional to the elevation of temperature achieved [Citation28]. Therefore, while the surface coating of the MNPs should maximise particle affinity to the endothelium, an optimum dosage of the MNPs should also be used to enhance thermal delivery to the BRB, to increase efficiency of the barrier opening, and reduce possible side effects.
To address the concerns described above we empirically obtained an effective dosage for commercially available MNPs coated with human transferrin (Tf-MNPs) to target the BRB. Transferrin, a single chain of glycoproteins, has receptors on the endothelium [Citation29] specifically in the CNS [Citation30]. Studies indicate that high concentrations of transferrin receptors are also available in and around various tumours [Citation31]. In future studies, this property will allow us to target an intact BRB in the vicinity of a retinal tumour and increase barrier permeability for better drug penetration.
Fluorescent dye molecules
In order to evaluate barrier permeability during hyperthermia of MNPs, sodium fluorescein dye (NaF, 378 Da) and Evans blue dye (EBD, 68 kDa when bound to albumin) were systemically injected into the bloodstream of all animals. They are both highly charged lipophobic tracers that are commonly used to assess CNS barrier leakage. In this study NaF was detected by spectrofluorometry, and EBD was analysed by an algorithm using fluorescent images of the retinal tissue.
Material and methods
Transferrin-coated magnetic nanoparticles
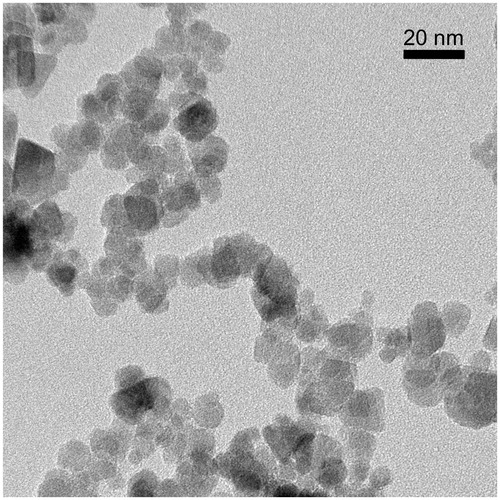
depicts an image taken by transmission electron microscopy (TEM) from the commercially available Tf-MNPs (Chemicell, Berlin, Germany). This image was acquired on a Jeol JEM-2100 field-emission gun (École Polytechnique de Montréal, Canada) and operated at 200 kV. The corresponding crystal diffraction patterns confirmed that they are indeed magnetite (Fe3O4) and have a relatively widespread size distribution ranging approximately from 8 to 20 nm in diameter. To prevent capillaries from blockage by high concentration of MNPs (visually seen under a microscope), preliminary in vivo experiments (data not shown) determined the appropriate particle dosage for injection in the animals to be 150 μL Tf-MNPs at a concentration of 25 mg/mL in phosphate buffer solution (PBS).
Figure 2. Transmission electron microscopy (TEM) image from commercially available Chemicell Tf-MNPs. This image was acquired on a Jeol JEM-2100 field-emission gun at École Polytechnique de Montréal, Canada. The magnetite particles have a relatively widespread size distribution ranging approximately from 8 to 20 nm in diameter.
Animal preparation
All animal procedures were performed according to the guidelines approved by the Ethics and Experimentation on Animal Committee (CDEA) of the University of Montreal. Thirty-seven pathogen-free male rats (350–450 g) were examined to confirm the efficacy of the proposed approach. During the experiments the body temperature of the animals was kept at 37 °C by means of a homeothermic plate. All animals received equal duration and dose of anaesthetic treatment. To assess the extravasation in the retina, the dyes were allowed to circulate in the vasculature for 30 min prior to any treatment.
Animal protocol
Animals were randomly separated into two groups of NaF (n = 20) and EBD (n = 12). Each group was divided into four sub-groups as depicted in and described below.
Figure 3. Animal protocol. The experimental procedure for each group is schematically presented in this figure. Note that only the hyperthermia and recovery groups received elevation of temperature.
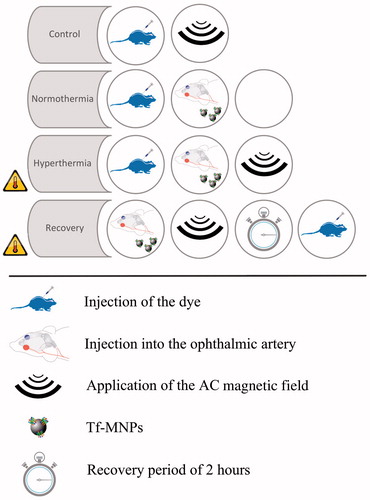
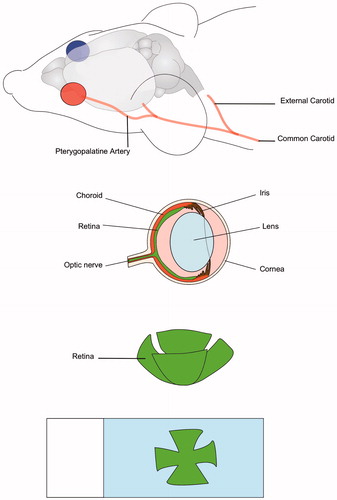
Normothermia (n = 8): While anaesthetised (2.5% isoflurane in oxygen), all animals in this group received an intravenous injection of either 60 mg/kg NaF dye (n = 5), or 40 mg/kg EBD (n = 3) through the tail vein. Each animal was then placed supinely on a heated platform. The neck of the animal was shaved and the skin was cut about 1 cm in the middle. The left common carotid artery (CCA), internal carotid artery (ICA), external carotid artery (ECA), and pterygopalatine artery were carefully isolated (see ). The distal portion of the ECA and the cerebral portion of the ICA were ligated with a 4-0 suture. A 32-gauge needle (Hamilton, Reno, NV, USA) was used to manually inject Tf-MNPs into the CCA. For trained personnel this procedure takes 30 min. After the injection occluded arteries were opened and the animal was then kept alive under anaesthesia for 30 min. Quickly after, the animal was sacrificed via intra-cardiac perfusion of 60 mL 0.9% sterile saline followed by 60 mL of 4% paraformaldehyde (PFA) at a rate of 10 mL/min to wash out the dye remaining in retinal capillaries. Once perfusion was completed the eyes of the animal were enucleated and conserved in 4% PFA overnight.
Figure 4. Experimental procedure. The Tf-MNPs reach the left retina through the pterygopalatine artery. Traces of Evans blue dye and sodium fluorescein are detected on extracted retinas for the hyperthermia group.
Hyperthermia (n = 8): Under anaesthesia all animals in this group received an intravenous injection of either 60 mg/kg NaF dye (n = 5), or 40 mg/kg EBD (n = 3) through the tail vein. The Tf-MNPs were then injected into the left CCA as described in normothermia. Each animal was then placed inside the AC magnetic field coils for 30 min. The animals were sacrificed after application of hyperthermia by an intra-cardiac perfusion as described for normothermia.
Recovery (n = 8): All animals in this group received an intra-arterial injection of Tf-MNPs in the left CCA as discussed before. They were then placed inside the AC magnetic field for 30 min and kept alive under anaesthesia for 2 h. After the 2 h recovery period, the animals received an intravenous injection of either 60 mg/kg NaF dye (n = 5), or 40 mg/kg EBD (n = 3) as described in previous groups. After 30 min, the animals were sacrificed via intra-cardiac perfusion.
Control and background (n =13): Animals in the control group (n = 8) received an intravenous injection of either 60 mg/kg NaF dye (n = 5), or 40 mg/kg EBD (n = 3). To allow equal circulation time of the dyes in all groups, animals in this group were allowed to rest under general anaesthesia for 30 min. Then they were placed inside the AC magnetic field for 30 mins and sacrificed as before. Animals in the background group (n = 5) were only sacrificed via an intra-cardiac perfusion.
AC magnetic field
In this study the AC magnetic field was set to 7.4 kA/m at 150 kHz by a 2-kW HotShot induction-heating amplifier (Ameritherm, New York). To obtain this field, a cylindrical coil with a 7-cm diameter was designed. This diameter allowed for the head of the anaesthetised animal to be entirely and comfortably placed inside the coil. The AC magnetic field was applied to an area between the bregma and the maxillary bone (middle of the nasal bone).
Data analysis
In this investigation we used two different methods to measure the fluorescent reading from the extracted retinal tissue. While NaF was easily detectable by spectrofluorometry, we were required to use a fluorescence microscope for closer observation and evaluation of the EBD in the retina. Also, we developed an algorithm to quantify fluorescence intensity in the images. The data from spectrofluorometry and the algorithm was further analysed for statistical purposes by GraphPad Prism version 6 for Mac (GraphPad Software, La Jolla, CA).
Spectrofluorometry analysis
All retinas with NaF dye were carefully extracted and placed upward in a black 96-well plate filled with 100 μL PBS, and sent for spectrofluorometry reading (SpectraMax Gemini XS, Molecular Devices, Sunnyvale, CA) set to excitation wavelength of 480 nm and emission wavelength of 525 nm. The blank was a retina that did not receive the dye. For statistical analysis, NaF concentration was assessed across all groups using one-way ANOVA (95% confidence interval) followed by Tukey's multiple comparison test.
Fluorescent microscopy
All retinas with EBD were carefully extracted and placed on microscope glass slides. Sections were then mounted using a mounting medium and the EBD staining was directly imaged at the centre of each retina with a fluorescence microscope (Leica DM2000, Wetzlar, Germany). The EBD fluoresces with an excitation peak at 540 nm and an emission peak at 680 nm. The samples were imaged at 4× magnification in phase-contrast mode with a 20 ms exposure time. Images were exported for luminosity analysis in tagged image file format (TIFF). In this study the left eye was the target site and the contralateral right eye was used to normalise the data. The threshold was defined as the maximal intensity calculated for 95% of pixels in the right eye. The luminescence intensity thresholds for the contralateral images were computed and applied to their left counterparts. The ratio of the difference in the luminescence intensity between the two eyes with respect to the size of the retina was considered as the amount of fluorescence for each particular group. Here, the EBD concentration was assessed across all groups using one-way ANOVA followed by Tukey's multiple comparison test.
Results
Parenchymal NaF analysis
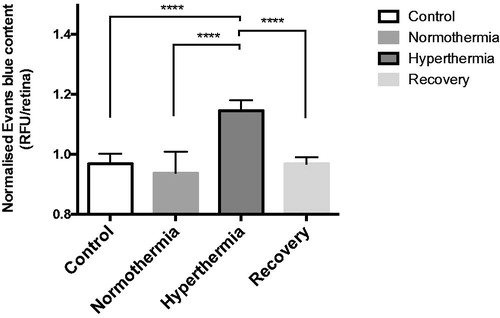
Spectrofluorometer readings are plotted in . These findings clearly indicate that hyperthermia of Tf-MNPs facilitates diffusion of small NaF dye molecules in the retinal tissue. Note that the readings for control, normothermia, and recovery groups are very similar and there is a significant increase in NaF detection when hyperthermia is applied (p < 0.0001 normothermia versus hyperthermia).
Figure 5. Sodium fluorescein (NaF) extravasation across the blood-retinal barrier after hyperthermia (n = 20, five in each group). This graph represents the relative fluorescence unit (RFU) due to penetration of NaF dye in the retinal tissue as measured with a spectrofluorometer. These values are normalised and computed using one-way ANOVA followed by Tukey’s multiple comparison test. As expected, the hyperthermia group (****p < 0.0001 vs. normothermia) has the highest RFU value. Hyperthermia is also significantly higher than the control (****p < 0.0001), and recovery groups (****p < 0.0001). Error bars represent standard error of mean (SEM).
Parenchymal EBD analysis
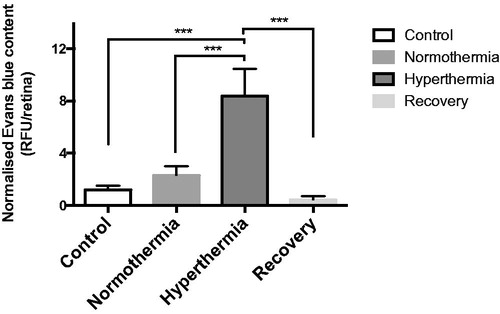
All retinas were imaged by a fluorescence microscope (Leica) and the images were processed and analysed by a computer algorithm. The results are plotted in where each column represents the relative fluorescent units (RFU) for EBD computed from all samples (n = 3) in that group. As expected for hyperthermia, the RFU for EBD is significantly higher than in any other group (p < 0.001 normothermia versus hyperthermia). Also, in the case of recovery, the amount of fluorescent units counted after 2 h of recovery period from hyperthermic disruption of the BRB is normalised.
Figure 6. Evans blue dye extravasation across the blood–retinal barrier after hyperthermia (n = 12, three in each group). In this study the left eye was the target site and the contralateral right eye was used to normalise the data. The ratio of the difference in the luminescence intensity between the two eyes with respect to the size of the retina was defined as the amount of fluorescence for each particular group. Statistical analysis was done using one-way ANOVA followed by Tukey's multiple comparison test. As expected, the hyperthermia group (***p < 0.001 vs. normothermia) had the highest RFU value. Hyperthermia was also significantly higher than the control (***p < 0.001), and recovery groups (***p < 0.001). Error bars represent SEM.
Discussion
The BRB is a highly organised complex consisting of endothelial cells that are tightly interconnected. This barrier markedly protects neurons by controlling the passage of molecules into the parenchymal tissue. By the same line of argument, therapeutic molecules are also exempt from reaching the retinal tissue. For instance, in the case of retinoblastoma, an aggressive tumour that occurs in the developing retina of children, the main reason for failure of systemic administration of chemotherapeutics and subsequent enucleation (surgical removal of the eye) is the recurrence of subretinal or vitreous seeds, lack of drug penetration, and high toxicity of the drugs on normal cells [Citation5]. Given the disadvantages of current remedies, a localised, safe, and reversible strategy is highly desirable. This strategy will reduce systemic toxicity thereby decreasing the need for hospitalisation. Furthermore, a localised therapy allows the administration of significantly higher doses of therapeutics directly to the tumour site, which enhances the biological effect, improves tumour growth control, and thus reduces the rate of recurrence.
In this investigation we raised barrier temperature by hyperthermia of MNPs. To examine the permeability of the barrier, EBD and NaF were systemically injected and allowed to circulate for 30 min prior to injection of the MNPs (normothermia, hyperthermia, and recovery) or PBS (control). This time interval has been reported suitable for the dyes to reach optimum concentration in the central tissue [Citation32]. The precise temperature and time at which hyperthermia causes the barrier to leak is not yet clear, therefore our strategy allows for the dye to readily leak into the retinal tissue as soon as the barrier opens. Future in-depth histological analysis will shine light on possible damage to the delicate retinal tissue after being exposed to hyperthermia for 30 min. However, it is important to note that after 2 h of recovery period, the BRB did not allow dye molecules to diffuse into the tissue. This may indeed be a simple indication that the proposed technique does not permanently damage the BRB.
While it is important to further evaluate the safety of this technique, the preliminary results compare quite favourably with our recent in vivo efforts to locally increase the permeability of a similar barrier in the brain [Citation12]. They suggest that the permeability of the barrier could increase without activation of the glia cells by immunohistochemical analysis of CD68 marker in the brain. In fact, the proposed approach in this investigation is designed to minimise damage to the complex and sensitive environment of the retina by exclusively targeting the barrier. This is achieved when circulating MNPs in the retinal capillaries adhere to the target endothelial cells. Specific surface coating of the MNPs can increase particle affinity to the endothelium and thus enhance thermal delivery and efficiency. Transferrin (Tf), which has receptors that are widely expressed on the surface of the endothelium, is among proteins that are often used in drug targeting techniques. Once the Tf-MNPs are positioned on the target endothelium, they are exposed to hyperthermia by placing the subject inside an AC magnetic field. The field causes each particle to act as a miniaturised heat source where a moderate thermal energy is dissipated from its surface to the surrounding endothelium. Evidence shows that hyperthermia of MNPs for this purpose operates at relatively low focal temperatures (no more than 1–2 °C) [Citation17]. The thermal behaviour of a single particle inside the AC magnetic field for 30 min is simulated in . This simulation uses an algorithm based on time-dependent thermodynamic heat transfer equations in a biological setting [Citation33]. The diameter of each particle is set to 14 nm (averaged diameter of Tf-MNPs), and using the equations given in Ma et al. [Citation34], the specific absorption rate (SAR) was calculated to be 21.9 × 103 W/m3. According to , the elevation of temperature is low and fades away in less than 1 μm from the surface of the particle. As is evident from our results, the low focal temperatures also help accelerate the recovery process and the return to the status quo for the endothelial cells and tight junctions. In fact, in contrast to other available techniques, this highlights an important advantage for hyperthermia of MNPs; the dissipated heat from the particles does not travel far from the monolayer lining of the microvessels. At least theoretically, this advocates that the neurovascular unit (e.g. neurons, Müller cells) in the retinal tissue remains unaffected. In addition, the blood flow and movement of the interstitial fluid in the retinal microenvironment will further help prevent other sensitive structures from overheating. We speculate that the rate at which the temperature of an aggregate of the MNPs increases ultimately becomes higher than the thermoregulation mechanism of the endothelial cells. This provides sufficient time for the cells to effectively receive the thermal energy and subsequently increase BRB permeability. Needless to say future in-depth investigation about the effect of hyperthermia by the proposed technique on the neurovascular unit is necessary to further support the theoretical claim.
Figure 7. Temperature increase as function of distance. This illustration reveals that elevation of temperature for 30 min from a single particle (R = 14 nm) is extremely local and it is close to zero after 1 μm away from the particle. This software simulation is based on the thermal conductivity of the bilayer lipid [Citation42] and the thermodynamics of the thermal release from a single particle during hyperthermia [Citation33]. The specific absorption rate is set to 21.9 kW/m3.
![Figure 7. Temperature increase as function of distance. This illustration reveals that elevation of temperature for 30 min from a single particle (R = 14 nm) is extremely local and it is close to zero after 1 μm away from the particle. This software simulation is based on the thermal conductivity of the bilayer lipid [Citation42] and the thermodynamics of the thermal release from a single particle during hyperthermia [Citation33]. The specific absorption rate is set to 21.9 kW/m3.](/cms/asset/1e88650e-b990-46e4-ae85-289743e098fe/ihyt_a_1193903_f0007_c.jpg)
It is evident that once the BRB is breached, the retina is exposed to blood-derived factors from which it is normally isolated. While there are other examples where treatment may promote health risks, often the benefit of having much needed therapeutics crossing the BRB outweighs the risks. The unfortunate sequelae of chemotherapy-induced immunosuppression that seriously increases susceptibility of the patient to harmful pathogens is one of many examples. In order to minimise the risks, patients may be quarantined for a few days with prescribed antibiotics to clear unwanted pathogens from their circulatory system. Also, the extent of BRB opening can be engineered to exact molecular weight and size of the drug of interest. Adjusting the AC magnetic field parameters, the exposure time to the field, as well as surface modification of MNPs, concentration, and volume could potentially enhance our control of BRB permeability and minimise possible pathological risks.
Magnetic properties suitable for hyperthermia
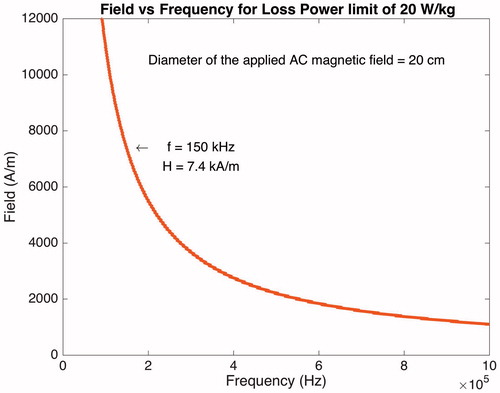
Magnetic properties of the MNPs play a critical role in their ability to be heated by an AC magnetic field. Superparamagnetic magnetite (Fe3O4) nanoparticles have shown great potential in this regard. Magnetic properties suitable for hyperthermia are not only governed by the magnetic parameters of the particles, but also by the physiological limitations that are set to protect living organisms against high magnetic fields. By optimising these properties, maximum thermal effect can be reached and therefore, fewer MNPs are required at the target area. Reversible hyperthermic disruption of the BRB requires a precise delivery of well-controlled thermal dose into a complex biological system that is equipped with its own thermoregulatory system. There are guidelines limiting the AC magnetic field parameters (frequency and amplitude) to prevent deleterious physiological responses [Citation35]. These responses are stimulations of peripheral and skeletal muscles, possible cardiac stimulation, arrhythmia and non-specific inductive heating of tissue. The guidelines are obtained from scientific observations and epidemiological studies. For biomedical purposes the frequency of the AC magnetic field has to be higher than 50 kHz to avoid neuromuscular electro-stimulation and lower than 10 MHz for appropriate penetration [Citation36]. Eddy current loss produced by closed currents induced by an AC magnetic flux in a conductive tissue of sufficient area can be responsible for unwanted elevation of body temperature. To avoid this undesirable discomfort, the maximum energy absorbed by the head and neck should not exceed 20 W/Kg. depicts the AC magnetic field amplitude and frequency curve that can be safely applied to the head region with a diameter of 20 cm. This curve takes into account the SAR, biological tissue conductivity values as tabulated in Barnes and Greenebaum [Citation37] for frequencies in the range of 100 kHz to 1 MHz. In this investigation, for proper penetration and efficient hyperthermic disruption of the BRB by Tf-MNPs without harmful heating of the retinal tissue (due to Eddie current losses), the AC magnetic field amplifier is set to generate an amplitude of 7.4 kA/m at 150 kHz.
Targeted drug delivery to the CNS
In order to make drug targeting a clinically viable technique, drug molecules must be propelled towards the target while being monitored by an imaging modality. This is indeed the basic idea behind the magnetic resonance navigation (MRN) project [Citation14]. In this design, MRN relies on magnetic targeting capabilities of the magnetic resonance (MR) imaging scanner to navigate non-invasive colloidal carrier systems (PLGA microparticles [Citation38], thermosensitive hydrogels [Citation39], or nanoliposomes [Citation40]) along a pre-determined suitable vascular route on their journey towards a target. The colloidal carrier system mainly consists of a limited load of MNPs and therapeutic agents. Here, the MNPs are the main key advantage of this configuration; the intrinsic magnetic properties of MNPs enable their use as contrast agents in MR imaging modalities [Citation41] thereby allowing us to track their location and assess the targeting efficacy through imaging sequences. Therefore, the MRN provides physicians with a platform that allows targeting and delivery of medications to a specific location in the human body. Once at the target, various actuation mechanisms could be envisioned to trigger the release of the therapeutic load from the colloidal system. The therapeutic molecules then diffuse through the compromised endothelium of the capillaries into the desired tissue.
Conclusion
In recent years MNPs have drawn attention for their theranostic capabilities; therefore, our findings presented in this investigation are particularly encouraging for a novel targeted drug delivery system to the eye. Evidently, we have shown effective traversing of dye molecules across the BRB, and the barrier can then recover from the thermal stress and regain full functionality. However, a more thorough investigation using drug molecules and a tumour model would allow us to examine its performance in a therapeutic setting. Today, due to the functioning barrier, drug delivery to the CNS including the eye faces a bottleneck. The present study provides preliminary evidence that hyperthermia of MNPs can open new possibilities for localised drug delivery to treat various CNS-related disorders.
Acknowledgements
The authors would like to thank Diane Vallerand and Charles Tremblay for their assistance and laboratory support during various stages of this project.
Disclosure statement
This project is supported in part by the Chaire de Recherche de l'École Polytechnique de Montréal, the Canada Foundation for Innovation (CFI), and the Natural Sciences and Engineering Research Council of Canada (NSERC) from S.M.'s Laboratory, as well as Fonds de Recherche Quebec Santé (FRQS) and the Heart and Stroke Foundation of Canada (HSFC) New Investigator Awards from H.G.'s laboratory. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Nag S, ed. The blood–brain and other neural barriers. Totowa, NJ: Humana Press, 2011.
- Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J 2010;12:348–60.
- Luo C, Deng Y-P. Retinoblastoma: Concerning its initiation and treatment. Int J Ophthalmol 2013;6:397–401.
- Rodriguez-Galindo C, Orbach DB, Van der Veen D. Retinoblastoma. Pediatr Clin North Am 2015;62:201–23.
- Zanaty M, Barros G, Chalouhi N, Starke RM, Manasseh P, Tjoumakaris SI, et al. Update on intra-arterial chemotherapy for retinoblastoma. Sci World J 2014;2014:e869604.
- Kiyatkin EA, Sharma HS. Permeability of the blood–brain barrier depends on brain temperature. Neuroscience 2009;161:926–39.
- Dokladny K, Moseley PL, Ma TY. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am J Physiol Gastrointest Liver Physiol 2006;290:G204–12.
- Kosterev VV, Kramer-Ageev EA, Mazokhin VN, van Rhoon GC, Crezee J. Development of a novel method to enhance the therapeutic effect on tumours by simultaneous action of radiation and heating. Int J Hyperthermia 2015;31:443–52.
- Goya GF, Asín L, Ibarra MR. Cell death induced by AC magnetic fields and magnetic nanoparticles: Current state and perspectives. Int J Hyperthermia 2013;29:810–18.
- Masuda H, Hirata A, Kawai H, Wake K, Watanabe S, Arima T, et al. Local exposure of the rat cortex to radiofrequency electromagnetic fields increases local cerebral blood flow along with temperature. J Appl Physiol (1985) 2011;110:142–8.
- Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL, et al. Thresholds for thermal damage to normal tissues: An update. Int J Hyperthermia 2011;27:320–43.
- Tabatabaei SN, Girouard H, Carret A-S, Martel S. Remote control of the permeability of the blood–brain barrier by magnetic heating of nanoparticles: A proof of concept for brain drug delivery. J Control Release 2015;206:49–57.
- Kong SD, Lee J, Ramachandran S, Eliceiri BP, Shubayev VI, Lal R, et al. Magnetic targeting of nanoparticles across the intact blood–brain barrier. J Control Release 2012;164:49–57.
- Bigot A, Tremblay C, Soulez G, Martel S. Magnetic resonance navigation of a bead inside a three-bifurcation PMMA phantom using an imaging gradient coil insert. IEEE Trans Robot 2014;30:719–27.
- Ohmoto Y, Fujisawa H, Ishikawa T, Koizumi H, Matsuda T, Ito H. Sequential changes in cerebral blood flow, early neuropathological consequences and blood–brain barrier disruption following radiofrequency-induced localized hyperthermia in the rat. Int J Hyperthermia 1996;12:321–34.
- Moriyama E, Salcman M, Broadwell RD. Blood–brain barrier alteration after microwave-induced hyperthermia is purely a thermal effect: I. Temperature and power measurements. Surg Neurol 1991;35:177–82.
- Tabatabaei SN, Duchemin S, Girouard H, Martel S. Towards MR-navigable nanorobotic carriers for drug delivery into the brain. In: IEEE International Conference on Robotics and Automation (ICRA). IEEE 2012, pp. 727–32.
- Friedl J, Turner E, Alexander HR. Augmentation of endothelial cell monolayer permeability by hyperthermia but not tumor necrosis factor: Evidence for disruption of vascular integrity via VE-cadherin down-regulation. Int J Oncol 2003;23:611–16.
- Song CW. Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Res 1984;44:S4721–30.
- Shivers RR, Wijsman JA. Blood–brain barrier permeability during hyperthermia. Prog Brain Res 1998;115:413–24.
- Macdonald AG. The homeoviscous theory of adaptation applied to excitable membranes: A critical evaluation. Biochim Biophys Acta 1990;1031:291–310.
- Raaphorst GP, Mao J, Ng CE. Thermotolerance in human glioma cells. Int J Hyperthermia 1995;11:523–9.
- Jeliazkova-Mecheva VV, Hymer WC, Nicholas NC, Bobilya DJ. Brief heat shock affects the permeability and thermotolerance of an in vitro blood–brain barrier model of porcine brain microvascular endothelial cells. Microvasc Res 2006;71:108–14.
- Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev 2012;64:S24–36.
- Dutz S, Hergt R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int J Hyperthermia 2013;29:790–800.
- Bao Y, Wen T, Samia ACS, Khandhar A, Krishnan KM. Magnetic nanoparticles: Material engineering and emerging applications in lithography and biomedicine. J Mater Sci 2015;51:513–53.
- Brown WF. Thermal fluctuations of a single-domain particle. Phys Rev 1963;130:1677–86.
- Tabatabaei SN, Martel S. The concentration effect of magnetic iron oxide nanoparticles on temperature change for hyperthermic drug release applications via AC magnetic field. Fifth International Conference on Microtechnologies, Medicine and Biology (MMB) 2009. Available from http://wiki.polymtl.ca/nano/images/f/f8/C-2009-MRSUB-MMB-Nasr.pdf
- Recht L, Torres CO, Smith TW, Raso V, Griffin TW. Transferrin receptor in normal and neoplastic brain tissue: Implications for brain-tumor immunotherapy. J Neurosurg 1990;72:941–5.
- Pardridge WM. Drug transport across the blood–brain barrier. J Cereb Blood Flow Metab 2012;32:1959–72.
- Jiang W, Xie H, Ghoorah D, Shang Y, Shi H, Liu F, et al. Conjugation of functionalized SPIONs with transferrin for targeting and imaging brain glial tumors in rat model. PLoS One 2012;7:e37376.
- Yen LF, Wei VC, Kuo EY, Lai TW. Distinct patterns of cerebral extravasation by Evans blue and sodium fluorescein in rats. PLoS One 2013;8:e68595.
- Tabatabaei SN. Evaluation of hyperthermia using magnetic nanoparticles and alternating magnetic field. Master’s thesis, École Polytechnique de Montréal, 2010. Available from https://publications.polymtl.ca/300
- Ma M, Wu Y, Zhou J, Sun Y, Zhang Y, Gu N. Size dependence of specific power absorption of Fe3O4 particles in AC magnetic field. J Magn Magn Mater 2004;268:33–9.
- Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz). International Commission on Non-Ionizing Radiation Protection. Health Phys 1998;74:494–522.
- Prentice W. Principles of athletic training: A competency-based approach, 15th ed. New York: McGraw-Hill Education, 2013.
- Barnes FS, Greenebaum B, editors. Handbook of biological effects of electromagnetic fields, 3rd ed. Boca Raton, FL: CRC Press, 2006.
- Pouponneau P, Leroux J-C, Martel S. Magnetic nanoparticles encapsulated into biodegradable microparticles steered with an upgraded magnetic resonance imaging system for tumor chemoembolization. Biomaterials 2009;30:6327–32.
- Tabatabaei SN, Lapointe J, Martel S. Shrinkable hydrogel-based magnetic microrobots for interventions in the vascular network. Adv Robot 2011;25:1049–67.
- Taherkhani S, Mohammadi M, Daoud J, Martel S, Tabrizian M. Covalent binding of nanoliposomes to the surface of magnetotactic bacteria for the synthesis of self-propelled therapeutic agents. ACS Nano 2014;8:5049–60.
- Yallapu MM, Othman SF, Curtis ET, Gupta BK, Jaggi M, Chauhan SC. Multi-functional magnetic nanoparticles for magnetic resonance imaging and cancer therapy. Biomaterials 2011;32:1890–905.
- Nakano T, Kikugawa G, Ohara T. A molecular dynamics study on heat conduction characteristics in DPPC lipid bilayer. J Chem Phys 2010;133:154705.