Abstract
Background: Microwave ablation (MWA) can be used to treat severe secondary hyperparathyroidism; however, its efficacy and the predictor of its efficacy are unclear. In this retrospective study we determined the predictor of efficacy of MWA and compared the efficacy of MWA and parathyroidectomy.
Materials and methods: Patients with severe secondary hyperparathyroidism who had received MWA or parathyroidectomy were enrolled in the study. Participants with MWA were divided into response and no response groups based on efficacy. Possible predictors were analysed using logistic regression to determine efficacy predictors. The participants were divided into MWA and parathyroidectomy groups, and the efficacy (including rates of achieving recommended goals for intact parathyroid hormone (iPTH), calcium, and phosphorus levels) were compared between the two groups.
Results: Thirty-one participants were enrolled for predictor analysis. Only baseline iPTH level predicted efficacy (OR 0.997, P = 0.018). The optimal threshold value of iPTH for predicting efficacy was 1493.5 pg/mL. To compare efficacy, 30 patients were enrolled in MWA (18/30) and parathyroidectomy (12/30) groups. The rates of achieving recommended goals for iPTH levels varied between 0 and 60%; a significant difference was found between the groups at 5 months (P = 0.01). However, in the parathyroidectomy group, the iPTH level and rate of iPTH <124 pg/mL (lower limit of target range) were significantly lower than in the MWA group after treatment (40–75% versus 0–16.7%).
Conclusion: Baseline iPTH level is a good predictor of MWA efficacy for severe secondary hyperparathyroidism; parathyroidectomy is more effective for severe secondary hyperparathyroidism than MWA.
Introduction
Secondary hyperparathyroidism (SHPT), one of the most common complications in patients undergoing haemodialysis, can significantly increase mortality and affect life quality [Citation1–3]. Administration of vitamin D and its analogues is the classical treatment for early SHPT [Citation4–6], whereas parathyroidectomy (PTX) is the most common therapy for severe drug-resistant SHPT; PTX significantly decreases the incidence of cardiovascular events and mortality in these patients [Citation7–11]. Thermal ablation including microwave ablation (MWA), radiofrequency ablation (RFA), and laser ablation, a minimally invasive form of treatment, have been used for several tumours including parathyroid adenoma [Citation12–14], hepatic, thyroid, and lung cancers [Citation15,Citation16].
Furthermore, MWA has been used to treat SHPT for approximately 5 years [Citation17]. However, its efficacy compared with PTX is unclear. In one study examining MWA use for SHPT [Citation17], MWA was used to treat SHPT with parathyroid nodules larger than 1.5 cm, and RFA was used for nodules smaller than 1.5 cm. In this study the authors only reported the combined result of MWA and RFA, and independent results regarding the use of MWA are not available; however, mean intact parathyroid hormone (iPTH) levels were approximately 1000 pg/mL at the 12-month follow-up after MWA or RFA (above the upper target range of approximately 600 pg/mL) [Citation18]. Therefore, the efficacy of MWA might not be very good. In another retrospective study, iPTH decreased from a pretreatment mean of 1570 pg/mL (651–6800 pg/mL) to 419 pg/mL (21–1500 pg/mL) at the 12-month follow-up [Citation19]. In that study, although the mean iPTH level was within the target range, levels varied between 21 and 1500 pg/mL, suggesting that some participants were above the upper limit of the target range.
In this retrospective study we first analysed baseline data for patients with severe SHPT undergoing MWA and determined the predictors of the efficacy of MWA for patients with severe SHPT. We then compared the efficacy and safety of MWA and PTX for treating SHPT in patients undergoing haemodialysis.
Methods
Study design
This was a retrospective cohort study of patients with severe SHPT undergoing haemodialysis who were treated with MWA or PTX between 1 September 2009 and 31 December 2014 in our haemodialysis centre.
For predictor analysis, data including age, sex, dialysis age, baseline level of iPTH, and the size and number of parathyroid gland nodules were collected. Participants with MWA were then divided into a response group and a no response group based on treatment efficacy. The efficacy criterion used to define the response group was the following: iPTH level decreased to within the target range (124–558 pg/mL) immediately after MWA and remained within that range for 6 months, with or without active vitamin D therapy. The efficacy criterion used to define the no response group was the following: iPTH level was outside the target range at a minimum of one follow-up within 6 months after MWA.
To compare the efficacy of MWA and PTX, study participants meeting inclusion and exclusion criteria were divided into two groups according to the treatment they had received: MWA and PTX groups. Baseline data including relevant patient variables and serum iPTH, total calcium, and phosphorus levels were collected. These data were also collected for the following nine follow-up times: 1 week (±3 days), 1 month (±15 days), 5 months (±15 days), 9 months (±15 days), 13 months (±15 days), 17 months (±15 days), 21 months (±15 days), 25 months (±15 days), and 29 months (±15 days) after MWA or PTX. The rates of achieving the goals recommended by the Kidney Disease Improving Global Outcomes (KDIGO) guideline for iPTH, calcium and phosphorus levels [Citation18] in the MWA and PTX groups were calculated and compared. Additional complications, mainly comprising hypocalcaemia and hoarseness, were also assessed [Citation19,Citation20]. If the iPTH level was lower than 124 pg/mL at more than 80% of follow-up times, the participant was considered to have a persistently low iPTH. Participants with persistently low iPTH concentrations after treatment were asked to complete a questionnaire to determine whether they had any evidence of bone disease or pathological fracture.
The study was approved by the Institutional Review Board, Beijing Friendship Hospital, Capital Medical University, China (nos. 2016-P2-001-02 and BJFH-EC-2014-100).
Quality control
Because this study was retrospective, the greatest challenge was obtaining complete data for iPTH, calcium, and phosphorus levels of participants. Our haemodialysis centre, founded in the 1980s, is the biggest in Beijing and has been the Beijing Blood Purification Quality Control and Improvement Centre since 2014. At this centre patients are managed strictly according to international guidelines [Citation21], which in 2007 resulted in adopting the practice of checking blood biochemistry including iPTH concentrations, at 2- to 4-month intervals in every patient undergoing haemodialysis. Therefore it was possible to retrieve almost all required iPTH, calcium, and phosphorus data of the study participants from our hospital record system. A duration of follow-up of 29 months was chosen based on previous investigations: the sample size would have been too small to allow comparisons between the two groups if a longer duration of follow-up had been chosen.
Study participants
Patients with serum iPTH levels of >800 pg/mL were considered cases of severe SHPT. Patients with severe SHPT undergoing haemodialysis in our centre who had been treated with MWA between 1 September 2009 and 31 December 2014 were eligible for enrolment in the predictor analysis study. In addition, for comparing the efficacy between MWA and PTX, the following inclusion and exclusion criteria were used:
Inclusion criteria: 1) age 18–75 years, 2) underwent maintenance haemodialysis (4-h sessions three times once a week) for more than 6 months, 3) severe SHPT, defined as high serum iPTH levels (>800 pg/mL), and 4) duration of follow-up of more than 12 months.
Exclusion criteria: 1) primary or tertiary hyperparathyroidism (hyperparathyroidism after kidney transplantation);,or 2) data for iPTH, calcium, and phosphorus levels unavailable for two or more of the specified nine follow-up times after MWA or PTX.
Microwave ablation
There is no established standard for the indication of MWA for severe SHPT. In this study we adopted the indication of PTX for severe SHPT as follows: severe hyperparathyroidism that fails to respond to medical/pharmacological therapy [Citation18].
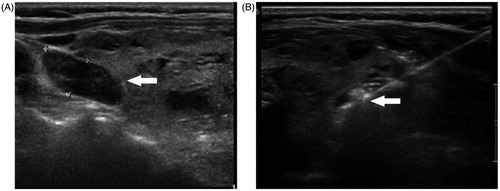
MWA was performed by a single ultrasound specialist, and the microwave therapy apparatus used was a KY2000 ablation system (Nanjing Kangyou Applied Research Institute, Nanjing, China) operating at a frequency of 2450 MHz and equipped with a 2-mm outside diameter antenna with a 5-mm tip. The procedure used was as follows (see ). First, a parathyroid ultrasound was performed to determine the blood supply of the parathyroid nodule and ablation path. Second, after sterilising the neck, local anaesthesia using 2% lidocaine hydrochloride was applied. Then 5 mL 2% lidocaine hydrochloride was diluted in 15 mL normal saline and then injected into the area around the parathyroid nodule to develop a heat insulation layer to protect the adjacent nerve and blood vessel. Third, an ablation needle was inserted into the parathyroid tissue under ultrasound guidance. Ablation was then performed, the MWA power used was between 25 and 35 W according to the target nodule size. When hypoechoic signals covered the whole nodule and no flow signal was detected ablation was terminated.
Outcome measures
Clinical data at baseline included relevant patient variables, serum iPTH, calcium, and phosphorus levels, number and size of parathyroid glands, and data concerning PTX and MWA.
The primary outcome measure was the rate of achieving recommended goals for iPTH levels at the following follow-up times: 1 week, 1 month, 5 months, 9 months, 13 months, 17 months, 21 months, 25 months, and 29 months after treatment.
The secondary outcome measure was the rate of achieving the recommended goals for serum calcium and phosphorus levels at the same follow-up times.
In accordance with the KDIGO guidelines [Citation18], the target ranges for iPTH, calcium, and phosphorus levels were 124–558 pg/mL, 2.0–2.5 mmol/L, and 0.97–1.62 mmol/L, respectively. Values within the target ranges were considered goal achievement.
Termination of follow-up
Follow-up was terminated for the following reasons: 1) death, 2) unplanned secondary PTX, MWA or other ablations for SHPT, or 3) administration of the calcimimetic agent cinacalcet as therapy for SHPT (this drug is also effective for severe SHPT [Citation22]. If the patients took this drug, the efficacies of MWA/PTX and this drug would be mixed; therefore the follow-up of patients taking cinacalcet was terminated).
Statistical analysis
Data were analysed using the Statistical Package for the Social Sciences (SPSS; IBM, Armonk, NY) version 19.0. All normally distributed continuous variables are reported as means and standard deviations. Variables with non-normal distributions are reported as medians and interquartile ranges. Categorical variables are reported with rates. Fisher’s exact test was used to compare the primary and secondary outcome measures and other categorical variables between the two groups. An independent sample t-test was used to analyse continuous variables including age, haemodialysis duration, and size of parathyroid nodules between the two groups.
Possible predictive factors were initially identified from response cases. Data on age, sex, dialysis age, size and number of parathyroid gland nodules, and baseline level of iPTH for patients in the response group were compared with data from patients in the no response group. The no response group was considered the control sample. Relationships between the response and no response groups were assessed using independent t-tests for continuous data and χ2 tests for categorical data. Factors in the two groups were further compared using binary logistic regression analysis. Odds ratios (OR), including 95% confidence intervals (95% CI), were calculated. The predictor of efficacy was analysed using receiver operating characteristic (ROC) curves to determine the threshold value for predicating efficacy. Finally, the kappa test was used to evaluate diagnostic reliability.
Results
Predictor of efficacy of MWA for severe SHPT in patients undergoing haemodialysis
Comparisons between the two groups of possible predictors
Thirty-one participants were enrolled in a predictor analysis study; 12 participants were included in the response group, and 19 were included in the no response group. Only the baseline levels of iPTH differed significantly between the two groups. There was no significant difference in age, dialysis age, size and number of parathyroid gland nodules, mean powers of MWA, and numbers of parathyroid nodules ablated between the two groups. The results are shown in .
Table 1. Comparison between the two groups regarding possible predictors.
Optimal predictive threshold of MWA efficacy for severe SHPT
Baseline levels of iPTH were analysed using logistic regression, and the results suggested that this parameter was a predictor of MWA efficacy for patients with severe SHPT (P = 0.018, OR 0.997, 95% CI 0.994–0.999), with the higher response rate having the lower level.
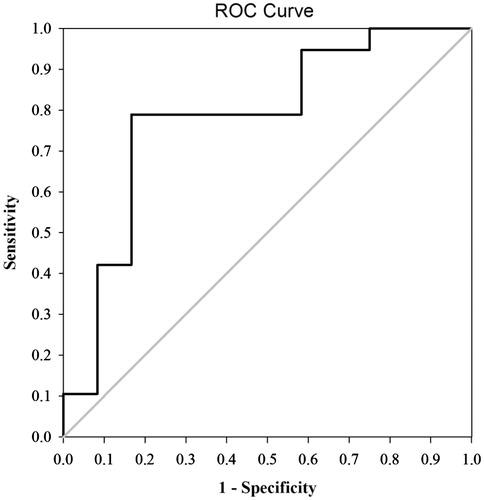
A ROC curve () was determined based on baseline iPTH levels of the 12 and 19 cases in the response and no response groups, respectively, using SPSS 19.0. The area under the ROC curve was 0.78 (), and the 95% CI was 0.60–0.96. Statistical results returned a Youden index of 0.622 (0.833 − 0.211 = 0.622), with a corresponding iPTH level of 1493.5 pg/mL, which can be considered the threshold value for predicting the efficacy of MWA for severe SHPT.
Diagnostic test for the optimal predictive threshold
The optimal threshold with an iPTH level of 1493.5 pg/mL was evaluated using the kappa test. Sensitivity, specificity, and positive and negative predictive values were 83.3%, 78.9%, 71.4%, and 88.2%, respectively. A kappa value of 0.604 represented a good degree of consistency (P = 0.001).
Comparison of the efficacies of MWA versus PTX for severe SHPT in patients undergoing haemodialysis
Study participants
The participants were 30 patients with severe SHPT who had undergone haemodialysis and had been treated with MWA (18/30) or PTX (12/30). The iPTH levels in the PTX group were slightly higher than those in the MWA group at baseline; however, this difference was not statistically significant (1738.14 ± 478.52 versus 1446.34 ± 488.72 pg/mL; t = 1.17, P = 0.12). There were no significant differences in age, sex, duration of haemodialysis, or underlying renal pathology between the two groups ().
Table 2. Baseline characteristics of the study patients arranged by treatment group.
Duration of follow-up
The durations of follow-up were 23.00 (± 6.33) months and 27.00 (± 4.97) months in the MWA and PTX groups, respectively. In the MWA group, nine cases were followed up for less than 29 months; in six of these cases, this was because MWA had been administered less than 29 months (13–25 months) previously. Follow-up ended prematurely in the remaining three cases for the following reasons: underwent PTX (one case, 17 months), taking cinacalcet (two cases, 17 and 25 months, respectively). In the PTX group, the duration of follow-up of two cases was less than 29 months because PTX had been performed less than 29 months previously.
Study intervention
There was no difference in parathyroid nodule size between the two groups. There were significantly fewer parathyroid nodules per case in the MWA group than in the PTX group (t = 6.06, P < 0.001). The patient and treatment characteristics are shown in , arranged by treatment group.
Table 3. Patient and treatment characteristics arranged by treatment group.
Primary outcome measure
The rates of achieving the recommended goal for iPTH levels were low in the MWA and PTX groups, between 0% and 60% at all nine follow-ups (1 week: 33.3% versus 16.7%, P = 0.419; 1 month: 50.0% versus 16.7%, P = 0.121; 5 months: 44.4% versus 0%, P = 0.01; 9 months: 5.6% versus 16.7%, P = 0.052; 13 months: 55.6% versus 25.0%, P = 0.141; 17 months: 40.0% versus 27.3%, P = 0.68; 21 months: 33.3% versus 45.5%, P = 0.68; 25 months: 18.2% versus 60%, P = 0.08; 29 months: 22.2% versus 50.0%, P = 0.35). A significant difference in the rates of achieving this goal was found only at 5 months follow-up (8/18, 44.4% versus 0/12, 0%, P = 0.01); no significant differences were found between groups at the other eight follow-ups (all P > 0.05) ().
Figure 3. Characteristics of changes in iPTH levels arranged by treatment group. Rates of achieving the recommended goals (A) and changes in serum iPTH levels (B) arranged by treatment group. Percentage of serum iPTH levels lower than 124 pg/mL (lower limit of the target range) (C) and higher than 558 pg/mL (upper limit of the target range) (D) arranged by treatment group. *P < 0.05, compared with the PTX group. Base, baseline; w, week; m, month.
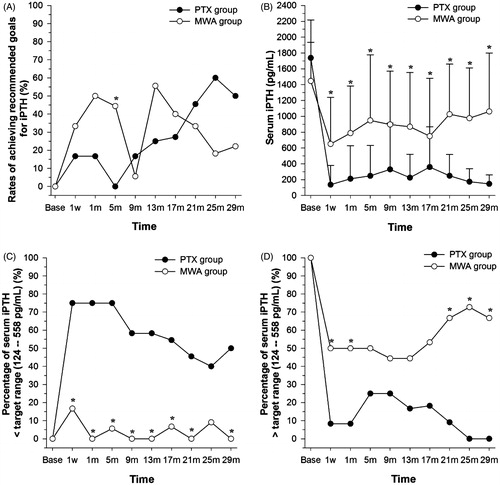
Although the rates of achieving the recommended goal were comparable between the two groups, iPTH levels were significantly lower in the PTX group than in the MWA group for all nine follow-ups (all P < 0.05; ). The mean pretreatment iPTH level in the 18 patients undergoing MWA was 1446.34 (± 488.72) pg/mL; this level decreased significantly to 650.60 (± 590.34) pg/mL at the 1-week follow-up (P = 0.03) and fluctuated between 787.83 (± 596.14) and 1061.04 (± 737.76) pg/mL for the following eight follow-ups (all P < 0.05). The mean pretreatment iPTH level in the 12 patients undergoing PTX was 1738.14 (± 478.5) pg/mL; this level decreased significantly to 137.04 (± 243.84) pg/mL at the 1-week follow-up (P < 0.001) and fluctuated between 147.60 (±111.88) and 359.55 (± 507.85) pg/mL for the following eight follow-ups (all P < 0.001).
Furthermore, at all nine follow-ups, the rates of iPTH levels being lower than 124 pg/mL (the lower limit of the target range) were higher in the PTX group than in the MWA group (40.0–75.0% versus 0–16.7%), and this difference was statistically significant for eight follow-ups (1 week and 1, 5, 9, 13, 17, 21, and 29 months; all P < 0.05) (). However, at all nine follow-up times the rates of iPTH levels being higher than 558 pg/mL (the upper limit of the target range) were all lower in the PTX group than in the MWA group (0–25.0% versus 44.4–72.7%), and this difference was statistically significant for five follow-up times (1 week and 1, 21, and 29 months; all P < 0.05) ().
Secondary outcome measures
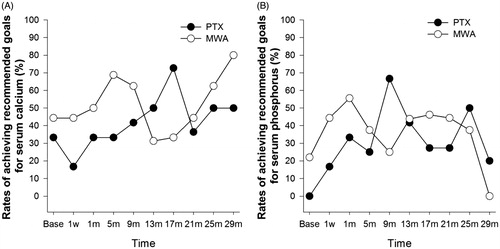
There were no significant differences in the rates of achieving the recommended goals for calcium (31.3–80.0% versus16.7–72.7%, all P > 0.05) and phosphorus (0–55.6% versus 16.7–66.7%, all P > 0.05) between the MWA and PTX groups at any follow-up time ().
Adverse events and safety
The main adverse event in both groups was hypocalcaemia. Calcium levels did not significantly differ between the MWA and PTX groups (1.77 ± 0.17 versus 1.76 ± 0.20 mmol/L; P = 0.873). Although the incidence of hypocalcaemia was lower in the MWA group than in the PTX group, this difference was not significant (8/18, 44.4% versus 9/12, 75%; P = 0.14). The time required to recover to within the normal range for serum calcium was longer in the MWA group than in the PTX group; however, this difference was not significant (5.00 ± 2.83 versus 8.56 ± 9.68 months; P = 0.32).
Hoarseness developed in one and two cases in the MWA and PTX groups, respectively. These patients recovered at 3 months (MWA group) and at 1 and 24 months (PTX group).
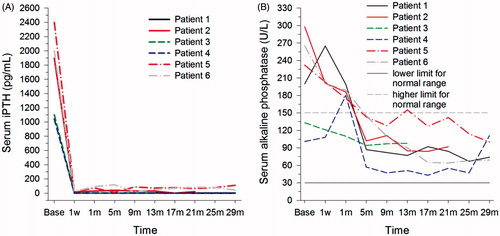
No patients in the MWA group had a persistently low iPTH level; however, six participants did have a persistently low iPTH level in the PTX group (); all of the latter six participants were still undergoing haemodialysis in our centre at the end of follow-up. Although the iPTH (1724.07 ± 537.88 versus 28.80 ± 35.29 pg/mL, P < 0.001) and alkaline phosphatase (204.83 ± 76.16 versus 112.40 ± 51.11 U/L, P < 0.001) levels decreased significantly after PTX, the latter remained within the normal range (), and no participant with persistently low iPTH concentrations reported bone pain or pathological fracture after PTX when asked to respond to a retrospective questionnaire.
Discussion
Three kinds of thermal ablation – MWA, RFA, laser ablation – have proved their efficacy in patients with primary or secondary hyperparathyroidism [Citation23,Citation24]. Each has advantages and disadvantages. Laser ablation may be ideal because of an important advantage: its ability to ablate a small, precise area using small needles, thereby minimising damage to important surrounding tissues [Citation14,Citation25]. However, unlike MWA or RFA, multiple needles are necessary for large or multiple nodules when applying laser ablation [Citation26], and the cost would be much higher. MWA can cover a larger ablative area than RFA. Therefore, MWA could be used for parathyroid nodules with diameters ≥1.5 cm, with radiofrequency being used for nodules with diameters <1.5 cm [Citation17]. Because of faster heating and higher temperatures provided by microwave energy, one advantage of MWA over RFA is that it can cover a more homogeneous, larger ablation zone that is easily predicted [Citation27].
This study suggests that baseline iPTH level is an independent predictor of the efficacy of MWA for SHPT with an optimal threshold value of 1493.5 pg/mL, a level that yields highly sensitive and specific results. Parathyroid cells will show increased endocrine function if the iPTH level is high [Citation28–30]. It is likely that some parathyroid tissue and cells remained after MWA. This is because the percutaneous ablation manoeuvre is performed under the guidance of ultrasound, not direct vision. In this study MWAs were performed by the same doctor; therefore, the remaining parathyroid tissue, if any, may be comparable after each MWA. In patients with SHPT and higher baseline levels of iPTH, parathyroid endocrine function was more active and was more likely to be unresponsive to MWA even though the remaining parathyroid tissues were comparable. The opposite appeared true for patients with SHPT and lower baseline levels of iPTH; these patients were more likely to be responsive to MWA. In addition, although MWA can be used repeatedly, we do not recommend that non-responsive patients accept it repeatedly because we consider the main reason for treatment failure to be the remaining parathyroid tissues and cells with higher active endocrine function in these patients, and it is difficult to ablate the remaining tissues under ultrasound guidance.
The results obtained also suggest that the size and number of parathyroid nodules cannot predict efficacy. First, all parathyroid nodules detected in patients with SHPT are ablated with MWA [Citation20] if possible, as was the case in our study, unless the location is too deep or the nodules are too close to an artery. Therefore the number of parathyroid nodules cannot predict efficacy. Second, the size of the nodules has no bearing on efficacy. If nodules are detected they will be ablated where possible, unless the location is too deep or the nodules are too close to an artery. Larger parathyroid nodules may secrete more PTH per gland, but they cannot secrete more PTH per unit volume [Citation31]. Complete eradication of all cells in each patient cannot be achieved, and even if one nodule is bigger than another, the remaining parathyroid tissues after MWA may be comparable. Therefore, nodule size cannot predict the efficacy of MWA for SHPT.
In our study the results also suggested that iPTH levels decreased significantly after MWA; these levels fluctuated from 650.6 to 1061.04 pg/mL during follow-up, which is similar to the results observed in previous studies. The rates of achieving the recommended iPTH goals were comparable between the MWA and PTX groups, but both the mean iPTH level and the rate of iPTH levels of lower than 124 pg/mL were significantly lower in the PTX group than in the MWA group. The classical treatment for severe SHPT has been PTX, including total PTX, total PTX with autotransplantation, and subtotal PTX, all of which are effective for severe SHPT, improving the quality of life and decreasing the incidence of cardiovascular events and mortality [Citation8,Citation32,Citation33]. MWA, a minimally invasive treatment, has been used to treat severe SHPT and good results have been achieved [Citation20]. The mean iPTH level decreased significantly after MWA, which is consistent with previous studies [Citation17,Citation20]. The rates of achieving the recommended iPTH goals were between 0% and 60%, and there was no significant difference between the two groups. However, we considered the efficacy of PTX better than that of MWA in this study for the following reasons: Both iPTH concentrations and the rate of iPTH being lower than 124 pg/mL (the lower limit of the target range) were significantly lower in the PTX group than in the MWA group, and the rates of iPTH being higher than 558 pg/mL (the upper limit of the target range) were all lower in the PTX group than in the MWA group (0–25.0% versus 44.4–72.7%, respectively). Furthermore, the harm of iPTH levels being higher than the upper limit of the target range has been established, but the harm of iPTH levels being lower than the lower limit of the target range has not been established and, as shown in this study, six participants in the PTX group who had persistently low iPTH levels did not develop adynamic bone disease.
We consider the main reasons for the differences observed between the MWA and PTX groups to be the following: First, because PTX was performed under direct vision the parathyroid nodules were easily removed completely. In contrast, because MWA was not performed under direct vision but under ultrasound guidance some parathyroid tissue was very likely left untreated by this modality. Such residual parathyroid tissue can result in treatment failure or SHPT recurrence caused by disorders of calcium and phosphorus metabolism, and such disorders are very common in patients undergoing haemodialysis [Citation34]. Second, PTX, which has been used to treat SHPT for more than 50 years [Citation35,Citation36], is a well-established surgical procedure. However, MWA has been used to treat SHPT for only 5 years [Citation17]; thus, practitioners are still gaining the skills and experience that are necessary to improve future efficacy. Third, the efficacy of ablation was highly dependent on the performer’s technique. The efficacy will differ between interventional physicians [Citation13,Citation14]. In this study MWA was performed by a single interventional physician. It is unclear whether the finding applies to other centres.
In addition, there were significantly fewer parathyroid nodules per case in the MWA group compared to the PTX group; however, this did not affect our conclusions for the following reason: Because the MWA group averaged fewer nodules, treatment efficacy was expected be better in the MWA group than in the PTX group; however, the efficacy was comparable between the two groups, even though baseline iPTH concentrations were lower in the MWA group than in the PTX group.
Six participants in the PTX group had persistently low iPTH levels and were therefore at risk of developing adynamic bone disease; the combination of low intact parathyroid hormone and low bone alkaline phosphatase levels may be suggestive of adynamic bone disease, and the gold standard for precise diagnosis is the histomorphometric analysis of bone biopsies. Although alkaline phosphatase levels decreased significantly after PTX, they remained within normal limits in all participants with persistently low iPTH. Furthermore, none of these patients reported bone pain or pathological fracture. Therefore, these patients may not have developed adynamic bone disease, consistent with the results of previous studies [Citation34,Citation37].
This study had some limitations. First, it was a single-centre study with a small sample, and the treatment efficacy will vary among practitioners. It is unclear whether general conclusions that are applicable to other centres can be drawn from the results. Second, this was a retrospective study. However, data were collected from our haemodialysis database and were accurate and complete. Third, three different PTX procedures were performed in the PTX group; if we had enrolled only patients who had undergone identical procedures we would have had too few patients to compare with MWA. Further large multicentre studies are needed to determine the efficacy of MWA.
In conclusion, we found in this study that baseline iPTH level was an independent predictor of MWA efficacy for severe SHPT in this population. We calculated that a threshold value of 1493.7 pg/mL yielded high sensitivity and specificity for predicting efficacy. Therefore, except for patients having lesions that are located too deep, too close to an artery or at-risk sites, who cannot be successfully treated using MWA, MWA should be applied only in selected cases with iPTH levels of less than 1493.7 pg/mL. Regarding efficacy, although there was no significant difference in the rates of achieving the recommended iPTH goals between the two groups, the iPTH level was significantly lower in the PTX group than in the MWA group. Furthermore, no patients with persistently low iPTH developed adynamic bone disease. Therefore, PTX is likely more efficacious than MWA for severe SHPT.
Disclosure statement
This work was supported by grants from the National Natural Science Foundation of China (grant no. 81300607). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Madsen CM, Jorgensen HL, Lind B, Ogarrio HW, Riis T, Schwarz P, et al. Secondary hyperparathyroidism and mortality in hip fracture patients compared to a control group from general practice. Injury 2012;43:1052–7.
- Huang C, Shapses SA, Wang X. Association of plasma parathyroid hormone with metabolic syndrome and risk for cardiovascular disease. Endocr Pract 2013;19:712–17.
- Davies EW, Matza LS, Worth G, Feeny DH, Kostelec J, Soroka S, et al. Health state utilities associated with major clinical events in the context of secondary hyperparathyroidism and chronic kidney disease requiring dialysis. Health Qual Life Outcomes 2015;13:90.
- Brancaccio D, Cozzolino M, Cannella G, Messa P, Bonomini M, Cancarini G, et al. Secondary hyperparathyroidism in chronic dialysis patients: results of the Italian FARO survey on treatment and mortality. Blood Purif 2011;32:124–32.
- Kazama JJ. Japanese society of dialysis therapy treatment guidelines for secondary hyperparathyroidism. Ther Apher Dial 2007;11:S44–S47.
- Rodriguez M, Rodriguez-Ortiz ME. Advances in pharmacotherapy for secondary hyperparathyroidism. Expert Opin Pharmacother 2015;16:1703–16.
- Ivarsson KM, Akaberi S, Isaksson E, Reihner E, Rylance R, Prutz KG, et al. The effect of parathyroidectomy on patient survival in secondary hyperparathyroidism. Nephrol Dial Transplant 2015;30:2027–33.
- Komaba H, Taniguchi M, Wada A, Iseki K, Tsubakihara Y, Fukagawa M. Parathyroidectomy and survival among Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int 2015;88:350–9.
- Tsai WC, Peng YS, Yang JY, Hsu SP, Wu HY, Pai MF, et al. Short- and long-term impact of subtotal parathyroidectomy on the achievement of bone and mineral parameters recommended by clinical practice guidelines in dialysis patients: a 12-year single-center experience. Blood Purif 2013;36:116–21.
- Lin HC, Chen CL, Lin HS, Chou KJ, Fang HC, Liu SI, et al. Parathyroidectomy improves cardiovascular outcome in nondiabetic dialysis patients with secondary hyperparathyroidism. Clin Endocrinol (Oxf) 2014;80:508–15.
- Ma TL, Hung PH, Jong IC, Hiao CY, Hsu YH, Chiang PC, et al. Parathyroidectomy is associated with reduced mortality in hemodialysis patients with secondary hyperparathyroidism. Biomed Res Int 2015;2015:639587.
- Xu SY, Wang Y, Xie Q, Wu HY. Percutaneous sonography-guided radiofrequency ablation in the management of parathyroid adenoma. Singapore Med J 2013;54:e137–40.
- Andrioli M, Riganti F, Pacella CM, Valcavi R. Long-term effectiveness of ultrasound-guided laser ablation of hyperfunctioning parathyroid adenomas: present and future perspectives. Am J Roentgenol 2012;199:1164–8.
- Jiang T, Chen F, Zhou X, Hu Y, Zhao Q. Percutaneous ultrasound-guided laser ablation with contrast-enhanced ultrasonography for hyperfunctioning parathyroid adenoma: a preliminary case series. Int J Endocrin 2015;2015:673604.
- Zhang NN, Lu W, Cheng XJ, Liu JY, Zhou YH, Li F. High-powered microwave ablation of larger hepatocellular carcinoma: evaluation of recurrence rate and factors related to recurrence. Clin Radiol 2015;70:1237–43.
- Hernandez JI, Cepeda MF, Valdes F, Guerrero GD. Microwave ablation: state-of-the-art review. Onco Targets Ther 2015;8:1627–32.
- Zhang JQ, Qiu M, Sheng JG, Lu F, Zhao LL, Zhang H, et al. Ultrasound-guided percutaneous thermal ablation for benign parathyroid nodules. Acad J Sec Milit Med Univ 2013;33:362–70.
- KDIGO. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2009:S1–S130.
- Yu MA, Yao L, Zhang L, Peng L, Zhuo L, Zhang Y, et al. Safety and efficiency of microwave ablation for recurrent and persistent secondary hyperparathyroidism after parathyroidectomy: a retrospective pilot study. Int J Hyperthermia 2016;32:180–6.
- Yu L, Gou CL, Li F, Feng ZY. The therapeutic effect of full ablation and partial ablation of parathyroid for secondary hyperparathyroidism: a comparison study. J Interven Radiol 2015;6:498–501.
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003;42:S1–S201.
- Bashir SO, Omer HA, Aamer MA, Somialy R, Morsy MD. Tolerance and efficacy of a low dose of the calcimimetic agent cinacalcet in controlling moderate to severe secondary hyperparathyroidism in hemodialysis patients. Saudi J Kidney Dis Transpl 2015;26:1135–41.
- Adda G, Scillitani A, Epaminonda P, Di LS, Motta F, Cecconi P, et al. Ultrasound-guided laser thermal ablation for parathyroid adenomas: analysis of three cases with a three-year follow-up. Horm Res 2006;65:231–4.
- Wang R, Jiang T, Chen Z, Chen J. Regression of calcinosis following treatment with radiofrequency thermoablation for severe secondary hyperparathyroidism in a hemodialysis patient. Intern Med 2013;52:583–7.
- Pacella CM, Mauri G, Achille G, Barbaro D, Bizzarri G, De Feo P, et al. Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab 2015;100:3903–10.
- Mauri G, Cova L, Ierace T, Baroli A, Di Mauro E, Pacella CM, et al. Treatment of etastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Inter Rad 2016;39:1023–30.
- Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol 2015;7:1054–63.
- Inic Z, Inic M, Jancic S, Paunovic I, Tatic S, Tausanovic K, et al. The relationship between proliferation activity and parathyroid hormone levels in parathyroid tumors. J Buon 2015;20:562–6.
- Duan K, Gomez Hernandez K, Mete O. Clinicopathological correlates of hyperparathyroidism. J Clin Pathol 2015;68:771–87.
- Lewin E, Olgaard K. Influence of parathyroid mass on the regulation of PTH secretion. Kidney Int Suppl 2006:102:S16–S21.
- Kakuta T, Tanaka R, Kanai G, Miyamoto Y, Inagaki M, Suzuki H, et al. Relationship between the weight of parathyroid glands and their secretion of parathyroid hormone in hemodialysis patients with secondary hyperparathyroidism. Ther Apher Dial 2008;12:385–90.
- Moldovan D, Racasan S, Kacso IM, Rusu C, Potra A, Bondor C, et al. Survival after parathyroidectomy in chronic hemodialysis patients with severe secondary hyperparathyroidism. Int Urol Nephrol 2015;47:1871–7.
- Pulgar BD, Jara CA, Gonzalez VG, Gonzalez DH. Surgical treatment of renal hyperparathyroidism. Experience in 71 patients. Rev Med Chil 2015;143:190–6.
- Stracke S, Keller F, Steinbach G, Henne-Bruns D, Wuerl P. Long-term outcome after total parathyroidectomy for the management of secondary hyperparathyroidism. Nephron Clin Pract 2009;111:c102–9.
- Stanbury SW, Lumb GA, Nicholson WF. Elective subtotal parathyroidectomy for renal hyperparathyroidism. Lancet 1960;1:793–9.
- Fe M. Total parathyroidectomy in renal hyperparathyroidism. W V Med J 1965;61:250–5.
- Jia X, Wang R, Zhang C, Cui M, Xu D. Long-term outcomes of total parathyroidectomy with or without autoimplantation for hyperparathyroidism in chronic kidney disease: a meta-analysis. Ther Apher Dial 2015;19:477–85.