Abstract
Purpose: A systematic review with conventional and network meta-analyses (NMA) was conducted to examine the outcomes of loco-regional hyperthermia (HT) with radiotherapy (RT) and/or chemotherapy (CT) in locally advanced cervix cancer, IIB–IVA (LACC).
Methods and materials: A total of 217 abstracts were screened from five databases and reported as per PRISMA guidelines. Only randomised trials with HT and RT ± CT were considered. The outcomes evaluated were complete response (CR), long-term loco-regional control (LRC), patients alive, acute and late grade III/IV toxicities.
Results: Eight articles were finally retained. Six randomised trials with HTRT (n = 215) vs. RT (n = 212) were subjected to meta-analysis. The risk difference for achieving CR and LRC was greater by 22% (p < .001) and 23% (p < .001), respectively, with HTRT compared to RT. A non-significant survival advantage of 8.4% with HTRT was noted with no differences in acute or late toxicities. The only HTCTRT vs. RT trial documented a CR of 83.3% vs. 46.7% (risk difference: 36.7%, p = .001). No other end points were reported. Bayesian NMA, incorporating 13 studies (n = 1000 patients) for CR and 12 studies for patients alive (n = 807 patients), comparing HTCTRT, HTRT, CTRT and RT alone, was conducted. The pairwise comparison of various groups showed that HTRTCT was the best option for both CR and patient survival. This was also evident on ranking treatment modalities based on the “surface under cumulative ranking” values.
Conclusions: In LACC, HTRT demonstrates a therapeutic advantage over RT without significant acute or late morbidities. On NMA, HTCTRT appears promising, but needs further confirmation through prospective randomised trials.
Introduction
Cervical cancer is the fourth commonest cancer globally [Citation1]. Of an annual incidence of 528 000 in 2012, 84.2% of cases arose in less developed regions of the world with a mortality of around 90% [Citation1,Citation2]. This can be attributed to both limited treatment facilities and to the advanced stage of disease at presentation in these countries. Radiation therapy (RT) is the mainstay of treatment for cervical cancer and several combined approaches have been implemented to improve clinical outcomes. Consequently, the use of chemotherapy (CT) concurrent with RT has led to a host of chemoradiotherapy (CTRT) schedules being evaluated in various clinical trials [Citation3]. The recent Cochrane meta-analysis of 23 CTRT trials across all stages of cervical cancer reported a 6% improvement in 5-year survival with CTRT as compared with RT. A 10% survival advantage with CTRT at 5 years in stages IB–IIA was reported. This fell to 7% in stage IIB disease and to a mere 3% in stages III–IVA [Citation3]. In terms of overall population benefit, CTRT was recently estimated to provide an absolute 5-year overall survival benefit of just 3% in cervical cancer [Citation4]. Thus, management of locally advanced cervix cancers (LACC), especially stages IIB–IVA, still poses a major therapeutic challenge.
Another option that has been explored in cervical cancers is the use of thermoradiotherapy (HTRT), that is, hyperthermia (HT) with RT. HT at 39–43 °C is a potent radio- and chemosensitiser [Citation5,Citation6]. This may be particularly relevant in LACCs, which are known to harbour a significant population of radioresistant hypoxic cells [Citation7]. In 2010, a Cochrane meta-analysis reported a possible therapeutic advantage of HTRT over RT alone in LACC [Citation8]. More recently a number of studies adding CT to HTRT have been published. Some of these clinical trials with thermochemoradiotherapy (HTCTRT) have also compared the outcomes with CTRT alone, which presently continues to be the preferred therapeutic option for LACC.
We therefore conducted a systematic review and updated the results of the Cochrane meta-analysis for HTRT vs. RT. In addition, we also looked at the outcomes of the trials reporting the use of CT in addition to HTRT or RT. With the four therapeutic options, namely, HTCTRT, HTRT, CTRT and RT alone in LACC, Bayesian network meta-analysis (NMA) was performed to synthesise the outcomes of these key approaches in LACC. Both direct and indirect evidence from various studies was combined to formulate treatment networks and perform NMA. League tables summarising the estimated odds ratios for all comparisons were generated. A surface under cumulative ranking (SUCRA) value per treatment (0–100%, where values nearer to 100% are preferred) for each outcome was estimated. These were used to amalgamate the trials and rank the outcomes of the four treatment strategies to identify the most promising approach. This could have possible implications in the future management strategies of LACCs.
Material and methods
Search strategy
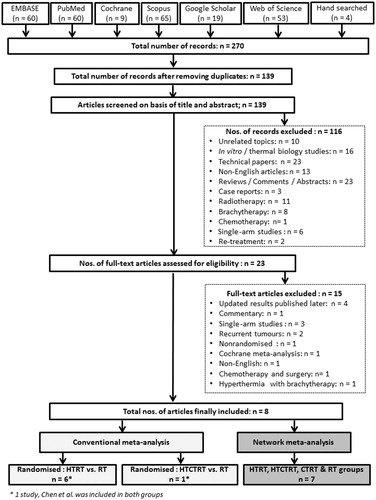
The systematic review, conventional meta-analysis and NMA were conducted in accordance with the PRISMA guidelines [Citation9] and the PRISMA extension statement for incorporating NMA [Citation10] ( and Supplementary Table 1). Six databases, namely, PubMed, EMBASE, SCOPUS, Web of Science, Google Scholar and the Cochrane library were searched. The last search was performed on 22 February 2016. The Medical Subject Headings (MeSH) terms used were “Uterine Cervical Neoplasms”, “Radiotherapy” and “Hyperthermia, Induced”. The search was not limited to any date or language. Additional papers were retrieved through hand-searching.
Study selection
After exclusion of duplicates from 270 abstracts, a total of 139 articles were screened based on their titles and abstracts. Topics unrelated to cervical cancers, in vitro/thermoradiobiological studies, technical papers on HT, reviews/comments, case reports, radiotherapy alone, use of brachytherapy/thermobrachytherapy, chemotherapy, single-arm studies, recurrent/retreatment and non-English articles were excluded. Two non-English papers [Citation11,Citation12] were included as English translations were made available to the authors (courtesy Dr J. van der Zee and Dr M. M. Liu). Articles that had been updated in a later publication by the same author(s) were excluded. Finally, 23 articles were shortlisted for full text review. The details of the literature search and the study selection procedures adopted are given in .
Inclusion criteria and studies included for meta-analysis
Only full text articles pertaining to randomised trials with HTRT vs. RT and HTCTRT vs. RT in newly diagnosed LACC were included for the conventional meta-analysis for these two groups [Citation11,Citation13–17]. For HTRT, all published clinical studies reporting HTRT vs. RT alone in LACC were screened. Finally, six studies were included in the conventional meta-analysis for HTRT vs. RT [Citation11,Citation13–17]. Studies that were updated by the authors in a later publication were considered. Thus, the original report by van der Zee et al. [Citation18], included in the Cochrane meta-analysis [Citation8], was replaced by updated reports from Franckena et al. [Citation13]. Harima et al. [Citation14] included in the present meta-analysis is published as a “hyperthermia classic article” and is a reproduction of the earlier article by Harima et al. [Citation19]. However, the outcome data reported in 2009 remains the same as reported in 2001. Wherever feasible, the lead authors were contacted for updates and clarifications. In addition, the toxicity profiles reported separately by Sharma et al. [Citation20] were taken into consideration during the present meta-analysis. For HTCTRT vs. RT, only one randomised study was found [Citation11]. This was a four-arm study with patients randomised to HTCTRT, HTRT, CTRT and RT groups.
For the NMA, two recently published studies were included [Citation12,Citation21] in addition to the above randomised studies with HTRTCT vs. RT and HTCTRT vs. RT. One was a randomised study with HTRT vs. CTRT [Citation21]. The other was a compilation of nine trials randomising between HTCTRT vs. CTRT reported in the meta-analysis [Citation12]. These nine trials comprised a total of 693 patients (HTCTRT vs. CTRT) and were reported in Chinese medical journals. Despite our best efforts, the full texts were not available. We therefore evaluated the end points of complete response (CR) and patient survival that were tabulated and reported in the forest plots of the meta-analysis [Citation12]. As the outcomes reported in these trials were CR and patients alive at the end of the study period, we restricted our NMA to these two key end points. The studies included in the NMA for CR and patients alive for various subgroups are detailed in Supplementary Tables 2 and 3.
Data extraction, quality assessment and critical appraisal
For conventional meta-analysis (HTRT vs. RT), the primary outcomes of interest were CR, long-term loco-regional control (LRC), patients alive at the end of their respective follow-up periods, grade III/IV acute and late toxicities. These were assessed from the six randomised trials [Citation11,Citation13–17]. For HTCTRT vs. RT, only the CR was documented by Chen et al. [Citation11]. NMA was therefore conducted from 13 studies for CR [Citation11–15,Citation21] and 12 for patients alive at the end of follow-up [Citation12–15,Citation17,Citation21].
All articles were extracted by two co-authors and critically reviewed (NRD and SG). In case of discrepancy, a consensus was reached in discussion with the third co-author (SR). Quality assessment of the trials was carried out as per Cochrane’s collaboration tool [Citation22]. The shortlisted papers were further reviewed by co-authors (NRD, SR, SG, EP and SB) to ascertain the correctness of all entries.
Statistical methods
The Comprehensive Meta-analysis Software package version 3.0 (Biostat, Englewood, NJ) was used to perform the meta-analysis [Citation23]. Effect measures were computed for all dichotomous outcomes. For efficacy, an event represented patients achieving CR, LRC and those alive at the end of their respective follow-up period. The LRC and survival estimates are given as a binary end point and not as time-to-event as the latter was not available for all studies. For toxicity, all grade III/IV acute and late toxicities were considered as an event. The odds ratio (OR), risk ratio (RR) and risk difference (RD) for each of the desired end points were computed and results given by the point estimate, the 95% confidence interval (CI), Z and p value. Heterogeneity was assessed using the I2 statistic, which represents the estimated proportion of unexplained inter-study variance prior to pooling of the studies. A random effects model was adopted for all analyses. The numbers needed to treat (NNT) were calculated for CR and LRC [Citation24]. Potential publication bias was evaluated through funnel plots and rank correlation tests with Kendall’s tau [Citation25]. A value of p < .05 was considered to be statistically significant for all tests.
For the NMA, outcomes for CR and “patients alive” were computed separately using a Bayesian random effects model using NetMetaXL [Citation26] and WinBUGS version 1.4.3 (MRC Biostatistics Unit, Institute of Public Health, Cambridge, UK). This allowed us to combine direct and indirect evidence from various studies [Citation27]. The direct comparisons would compare head to head within a randomised trial while the indirect comparisons would compare the results of randomised trials with common comparators [Citation28]. Models were run using 10 000 burn-in iterations followed by 10 000 sampling iterations [Citation29]. The pairwise summary paired odds ratio estimates and 95% credible interval (Cr.I) (or Bayesian confidence interval) were presented in league tables. Surface under the cumulative ranking curve (SUCRA) values were also estimated per treatment for each outcome (range 0–100%, with values nearer 100% considered preferable). Additionally, for each outcome rankograms for the various treatment options were plotted to reflect the distribution of rankings and corresponding probabilities for each intervention. Inconsistency between direct and indirect estimates was assessed when a closed loop was present by assessment of scatterplots of residuals from consistency and inconsistency models run, as well as by comparison of deviance information criteria (DIC) values between models (differences of 5 or more points were considered to indicate evidence of difference in fit) [Citation26,Citation30]. For each analysis, posterior residual deviance was compared to the total number of data points to assess adequacy of model fit.
Results
Overview of the clinical trials included in themeta-analysis
The total number of patients included in this meta-analysis (both conventional and NMA) was 1160. Of these, the conventional meta-analysis between HTRT vs. RT included 215 and 212 patients, respectively, and for HTCTRT vs. RT, there were 30 in each group. For the NMA, the numbers of patients in HTCTRT, HTRT, CTRT and RT were 323, 257, 368 and 212, respectively. In the six trials of HTRT vs. RT, 99.3% of the patients had LACC (stages IIB–IVA). All patients in the single study of HTCTRT vs. RT were also of stage IIB–IVA (). The HTRT vs. CTRT trial reported by Lutgens et al. [Citation21] included 30.1% patients below stage IIB, but all had tumours ≥4 cm. In the meta-analysis reported by Yan et al. [Citation12], all studies except one (which had patients with stages IIA–IIIB) had included patients with LACC.
Table 1. Characteristics of the studies included in the present systematic review and meta-analysis pertaining to (a) HTRT vs. RT and (b) HTCTRT vs. RT and (c) HTRT vs. CTRT.
Radiotherapy usually consisted of teletherapy (45–50 Gy) to the pelvis combined with intracavitary brachytherapy (at various dose rates). Loco-regional HT in HTRT and HTCTRT studies was usually administered after RT for around 60 min to a temperature of 40–43 °C. Single agent cisplatin was used in most of the HTCTRT and CTRT studies ().
Outcomes from trials with HTRT vs. RT (conventional meta-analysis)
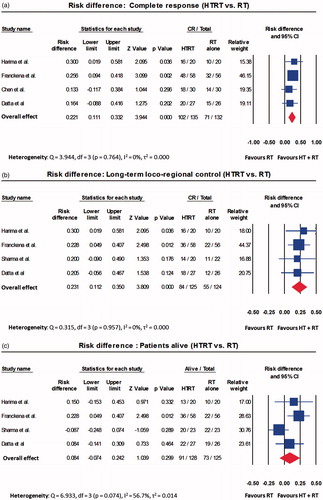
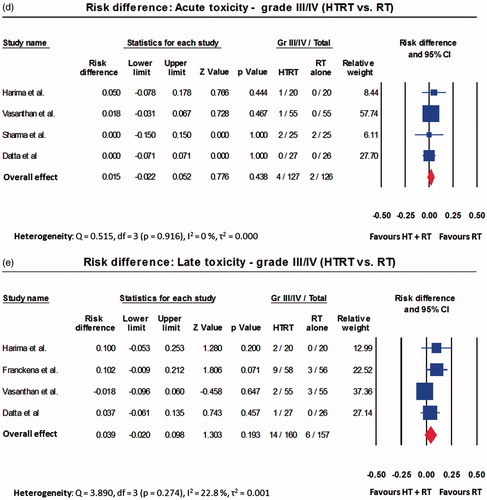
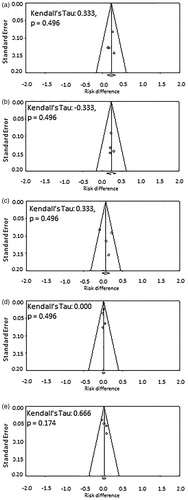
A total of 215 patients were treated with HTRT while 212 received RT alone. Those treated with HTRT had superior outcomes when compared with RT. The ORs were 2.67 (95% CI: 1.57–4.54, p < .001) for CR, 2.61 (95% CI: 1.55–4.39, p < .001) for LRC and 1.94 (95% CI: 1.10–3.40, p = .021) for patients surviving at the end of follow-up (Supplementary Figures 1(a)–(c)). The acute and late toxicities were comparable for both HTRT and RT (Supplementary Figures 1(d) and (e)). The RR also followed the same trend as OR for the above end points (Supplementary Figures 2(a)–(e)). As evident on the RD, HTRT achieved a superior CR by +22.1% (RD: 95% CI: 11.1–33.2, p < .001) and LRC by +23.1% (RD: 95% CI: 11.2–35.0, p < .001) when compared with RT alone (). A non-significant survival advantage of 8.4% was observed with HTRT () while the grade III/IV acute and late morbidities were similar in both groups (). No significant publication bias was observed (). The quality assessment of the trials is detailed in Supplementary Figure 3.
Figure 2. Forest plots depicting the risk difference for (a) complete response; (b) long-term loco-regional control; (c) number of patients alive; (d) acute toxicity; and (e) late toxicity for trials included in thermoradiotherapy (HTRT) vs. radiotherapy (RT). All computations have been performed using random effects model.
Figure 3. Funnel plots corresponding to the risk difference for (a) complete response; (b) long-term loco-regional control; (c) number of patients alive; (d) acute toxicity; and (e) late toxicity for trials included in thermoradiotherapy (HTRT) vs. radiotherapy (RT). Kendall?s τ and p values for each plot are also shown.
Outcomes with HTCTRT vs. RT (conventionalmeta-analysis)
The outcomes of HTCTRT vs. RT could only be computed from one randomised trial with 30 patients in each arm. A total of 83.3% of the patients with HTCTRT achieved a CR compared with 46.7% with RT alone (OR: 5.71, 95% CI: 1.72–18.94, p = .004; RR: 1.78, 95% CI: 1.18–2.70, p = .006; RD: 36.7%, 95% CI: 14.4–58.9, p = .001). No other parameters were reported in this study [Citation11].
Network meta-analysis for HTCTRT, HTRT, CTRT and RT groups
NMA was performed for two end points (a) CR and (b) patients alive at the end of the study period (Supplementary Tables 2 and 3). For CR, 13 studies with a total of 1000 patients resulted in six possible direct comparisons: HTCTRT vs. HTRT, HTCTRT vs. CTRT, HTCTRT vs. RT, HTRT vs. CTRT, HTRT vs. RT and CTRT vs. RT (). A total of 659/1000 patients achieved CR (). Based on the corresponding ORs, the league table and forest plots of these groups reveal a significant advantage of HTCTRT over RT (OR: 4.52, 95% Cr.I: 1.93–11.78) and over CTRT (OR: 2.91, 95% Cr.I: 1.97–4.31) for achieving CR ( and Supplementary Figure 4). HTRT also demonstrated a significantly higher probability of a CR over RT alone (OR: 2.85, 95% Cr.I: 1.63–5.08). There were no significant differences observed for the remaining three comparisons (Supplementary Figure 4). Inspection of the rankogram and SUCRA values indicate that the best possible option was HTCTRT (SUCRA = 0.952) followed by HTRT (SUCRA = 0.680), CTRT (SUCRA = 0.317) and RT (SUCRA = 0.051) (). The network inconsistency test did not indicate any potential inconsistencies (Supplementary Figure 5).
Figure 4. Network meta-analysis for complete response with trials using thermoradiotherapy (HTRT), thermochemoradiotherapy (HTCTRT), chemoradiotherapy (CTRT) and radiotherapy (RT) alone. (a) Network diagram showing the number of trials and patients included in each set of randomised groups; (b) league table with odds ratios and 95% credible interval (95% Cr.I) with pairwise comparison. Random effects model with vague priors has been used for computation of odds ratios.
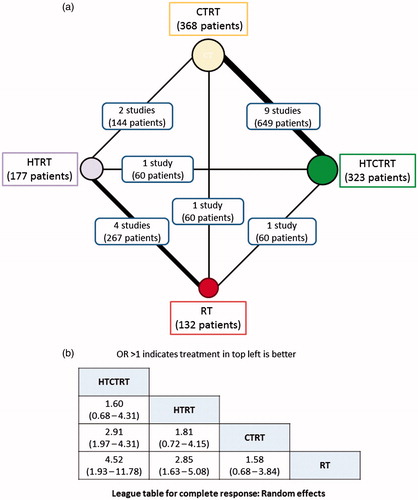
Figure 5. Rankograms for complete response computed using random effects model for subgroups of thermoradiotherapy (HTRT), thermochemoradiotherapy (HTCTRT), chemoradiotherapy (CTRT) and radiotherapy (RT) alone. The rankings have been based on the surface under the cumulative ranking (SUCRA) values with the best rank obtained by the modality with the highest SUCRA value.
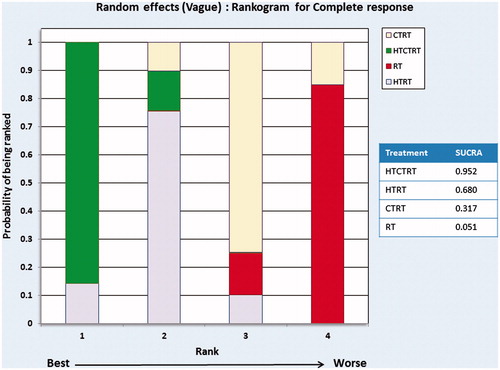
Table 2. Summary of the network meta-analysis characteristics for complete response and patients alive for various treatment arms: thermoradiotherapy (HTRT), thermochemoradiotherapy (HTCTRT), chemoradiotherapy (CTRT) and radiotherapy (RT) alone.
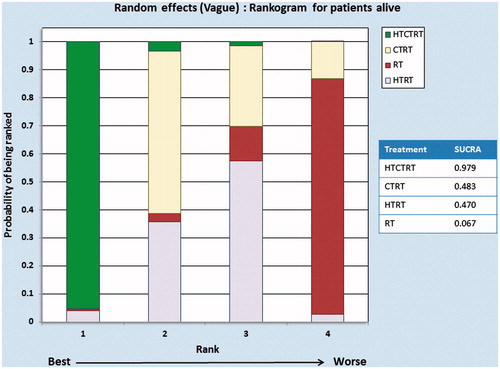
The NMA for patients remaining alive at the end of the study follow-up period was performed with data from 12 comparative studies comprising a total of 807 patients, of whom 596 patients were alive at the end of the study period: HTRT (118/170), RT (73/125), HTCTRT (202/231) and CTRT (203/281) (, ). Three direct comparisons between HTRT vs. RT (253 patients), HTCTRT vs. CTRT (470 patients) and HTRT vs. CTRT (84 patients) had data available for analysis. Three indirect comparisons between HTCTRT vs. HTRT, HTCTRT vs. RT and CTRT vs. RT were also estimated from the NMA. As evident from the league table and the forest plots, HTCTRT provides a significant advantage over CTRT (OR: 2.65, 95% Cr.I: 1.51–4.87) or RT (OR: 5.57, 95% Cr.I: 1.22–23.42) ( and Supplementary Figure 6). There were no significant differences for the remaining four comparisons between therapies. Inspection of the rankogram and SUCRA values suggests that HTCTRT (SUCRA = 0.979) may be associated with the highest patient survival. On the rankogram, HTCTRT with the best SUCRA values of 0.979 appeared to be the best option for the end point of patient survival. CTRT and HTRT were quite close to each other at 0.483 and 0.470, respectively, while RT at 0.067 was ranked as worst ().
Figure 6. Network meta-analysis for patients alive with trials using thermoradiotherapy (HTRT), thermochemoradiotherapy (HTCTRT), chemoradiotherapy (CTRT) and radiotherapy (RT) alone. (a) Network diagram showing the number of trials and patients included in each set of randomised groups; (b) league table with odds ratios and 95% credible interval (95% Cr.I) with pairwise comparison. Random effects model with vague priors has been used for computation of odds ratios.
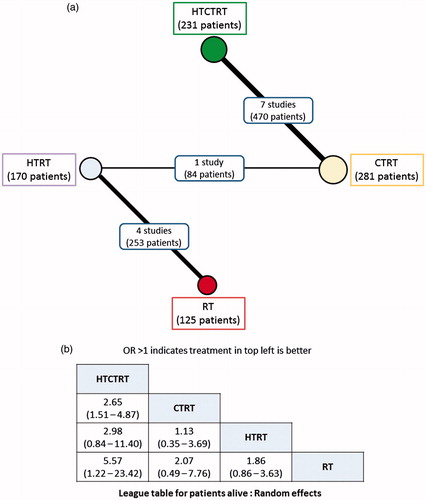
Figure 7. Rankograms for patients alive computed using random effects model for subgroups of thermoradiotherapy (HTRT), thermochemoradiotherapy (HTCTRT), chemoradiotherapy (CTRT) and radiotherapy (RT) alone. The rankings have been based on the surface under the cumulative ranking (SUCRA) values with the best rank obtained by the modality with the highest SUCRA value.
Assessing model fit and inconsistency between direct and indirect estimates for CR and patients alive using NMA
Inconsistency between direct and indirect estimates was tested by scatterplots of the residuals from consistency and inconsistency models. For patients achieving CR, the test for inconsistency was evaluated for the random effects model by comparison of the DIC values between models with a difference of 5 points considered to indicate evidence of difference in fit (as stated earlier in the statistical section). The DIC for CR for random effects (vague prior) was 141.4 while the DIC with the inconsistency model was 142.5. Since the difference between the DICs was less than 5 points, it indicates absence of any inconsistency between direct and indirect estimates of the CR from different strategies evaluated by NMA. The posterior residual deviance and number of unconstrained data points (i.e. number of study arms in the analysis) were 23.11 and 28, respectively; the similarity of these values suggests the model fit is adequate.
For the patients alive, as outcomes seen on the network diagram had no closed loops (), the inconsistency test was not indicated and or performed. However, the inconsistency plots for patients alive are depicted in Supplementary Figure 7. Values of posterior residual deviance and the number of unconstrained data points were 20.77 and 24, respectively. This similarity of values again suggests adequate model fit.
Discussion
Hyperthermia at 39–43 °C is known to be a potent hypoxic cell sensitiser and has the ability to inhibit DNA damage repair, sensitise “S” phase cells and to synergise cisplatin cytotoxicity. HT has been trialled in several randomised and single-arm studies for various sites, including cervical cancers [Citation5]. In LACC, it could plausibly act as a potential radio- and chemosensitiser and possibly enhance the efficacy of the currently accepted treatment strategies using CTRT [Citation3].
A Cochrane meta-analysis of randomised trials with HTRT vs. RT alone published in 2010 indicated that HT in addition to RT could provide clinically relevant improvements in treatment outcomes, especially in stage IIIB cervix cancer [Citation8]. As stage IIB–IVA tumours are now usually grouped under LACC, it may be appropriate to infer that HTRT could be preferred over RT in LACC without any additional acute and late toxicities.
The present systematic review was conducted to update the outcomes of the Cochrane meta-analysis [Citation8] in light of the additional published data and to explore outcomes of CT delivered concurrently with HTRT in LACC. An extensive database and hand search conducted could not identify any additional randomised trial between HTRT vs. RT other than the six trials that had been included in the earlier Cochrane meta-analysis. However, the effect measures were updated and additionally a NMA was performed to synthesise the outcomes of various randomised trials using HT and RT with or without CT.
A closer perusal of the effect measures of the HTRT vs. RT trials indicates that HTRT significantly improves the rates of CR (+22.1%) and of LRC (+23.1%) compared with RT alone. CR at the completion of treatment has been reported to be a significant predictor of long-term survival in cancer of the cervix [Citation31–33]. The 8% survival benefit in LACC without any additional acute or late treatment-related morbidity was non-significant but is between 2 and 3 times the reported survival advantage of CTRT in LACC [Citation3,Citation4]. The NNT with HTRT is 4.5 for CR and 4.3 for LRC (). An improved CR rate could be expected to translate into better survival outcomes [Citation31,Citation34,Citation35]. Thus, it is plausible that the better CR rate with HTRT would be reflected in an 8% improvement with HTRT with regard to the numbers of patients alive at the end of the respective study periods ().
We have not included the survival figures from Vasanthan et al. [Citation16] as the published manuscript states that 17 of the total 110 patients (both groups combined) had died. Since the detailed distribution of the mortalities in the two groups was not stated, it was not possible to ascertain the exact number of patients alive in each group. Furthermore, this trial was subsequently found to have several deficiencies in terms of suboptimal radiotherapy and inadequate information regarding HT and its quality control [Citation36].
The effect measures for the end points evaluated did not show any appreciable heterogeneity, except patient survival which had heterogeneity of 56.7% in RD as indicated by I2 estimates (). A closer perusal of the forest plots for patient survival suggested that this could be attributed to a relatively higher number of patient deaths reported by Sharma et al. [Citation17] (HTRT group, 3/23 vs. RT, 1/23) (). This was verified by repeating the RD computation excluding this trial. Thereby the point estimate for RD improved from 0.08 (95% CI: −0.07 to 0.24, p: ns) to 0.17 (95% CI: 0.04–0.29, p = .009) and I2 dropped from 56.7% to 0%. The survival advantage also increased from 8% to a statistically significant 17% with no heterogeneity. Of note, 2 of the 3 patients who died in the HTRT group of this study had distant metastasis while the proportion of patients with no evidence of disease at 18 months follow-up was higher when treated with HTRT compared with RT (70% vs. 50%).
The relatively smaller number of patients in the HTRT vs. RT trials could be a reflection of the limited availability of HT facilities and also of the investigative nature of HTRT (). One of the largest series with 378 patients with stages IB2–IVA disease (89% patients in stages IIB–IVA) has been reported by Franckena et al. [Citation37]. This followed the promising results reported in the randomised Dutch Deep Hyperthermia Trial [Citation18]. A total of 77% of patients achieved CR, while 5-year local control and disease-specific survival were 53% and 47%, respectively.
A recently reported randomised study using brachytherapy vs. brachytherapy and HT following prior CTRT in patients of cervical cancer with stages II–III cervical cancer failed to demonstrate any therapeutic benefit with the addition of HT [Citation38]. The authors attributed this to (a) the relatively few stage III patients in their patient cohort and (b) heating only a small volume of cervix with interstitial HT. It is noteworthy that HT was used only during brachytherapy and not during the 5 weeks of external beam RT sessions and CT. It could be supposed that HT would be more effective in de novo bulky tumours harbouring a higher hypoxic fraction rather than in tumours that have regressed following 5–6 weeks of CTRT. This suggests that external HT should be used in LACC at the initiation of RT or CTRT to maximise the therapeutic gain of HT-induced hypoxic cell radiosensitisation.
A comparison of HTCTRT vs. RT was limited to only one study and only one end point of CR. In a multicentre, single-arm multicentre trial with HTCTRT (n = 68 patients), 89.7% of patients achieved a CR; 58.8% had a LRC while 69.1% of patients were surviving at the end of the study period (median follow-up: 81 months) [Citation39]. A total of 33% of the patients experienced grade III/IV acute toxicities in the form of leucopenia, fatigue, nausea, emesis and diarrhoea but these did not result in withholding HT treatment [Citation40]. None of the patients reported any late toxicity with HTCTRT.
Apart from these studies, the meta-analysis of nine randomised trials of HTCTRT vs. CTRT reported significantly improved 1-year (OR: 3.05, 95% CI: 1.70–6.68, p = .005) and 2-year survival (OR: 2.29, 95% CI: 1.19–4.38, p = .01) with no significant adverse effects [Citation12]. These individual trials are in non-English-language journals and could not be retrieved. In addition, a recently reported randomised study between HTRT and CTRT, which was closed prematurely after enrolling 87 of the 376 planned patients, suggested comparable outcomes with HTCTRT and CTRT in terms of event-free survival (hazard rate: 1.15, 95% CI: 0.56–2.36, p: ns) and pelvic-recurrence-free survival (hazard rate: 1.04, 95% CI: 0.48–2.44, p: ns) at 5 years [Citation21].
NMA provided us with an opportunity to synthesise the various treatment approaches using HT in LACC and simultaneously compare their outcomes. In contrast to conventional meta-analysis where only two paired interventions can be compared at a time, NMA allows both direct and indirect comparisons of the various treatment approaches. Thus, NMA was carried out to compare the outcomes of HT and RT with or without CT in LACC, using data from randomised trials. NMA is one of the methods used by health technology assessment organisations to evaluate the efficacy of various treatment options as it provides direct evidence of treatment superiority by ranking the treatment modalities using SUCRA values [Citation26,Citation28]. As evident from NMA, HTCTRT is the best option in terms of CR and also patient survival in LACC. HTRT and CTRT are closely competing strategies, with HTRT scoring well above CTRT for CR, but being similar to CTRT in terms of patient survival ( and ).
CTRT is the accepted therapeutic approach in cervical cancers but the limited survival benefits of 3–4%, especially in LACC at the cost of increased acute toxicity as reported from various randomised trials, is a matter of concern [Citation3,Citation4]. In addition, HPV positive cervix cancer has been shown to be sensitive to both HT and RT individually [Citation41–43]. As the trials included in this meta-analysis did not report outcomes by HPV tumour status, it was not possible to infer any benefit of HTRT or HTCTRT in relation to HPV positivity. Future trials with HTRT in LACC should consider HPV as a stratification factor to define treatment strategies in relation to HPV status. Thus, the hypoxic cell sensitising abilities of HT and the thermoradiobiological sensitisation of HPV positive cervical tumours should pave the way for a direct comparison of HT and RT ± CT vs. CTRT. Two clinical trials exploring HTCTRT have been registered with ClinicalTrials.gov, one as a single-arm feasibility study of HTCTRT [Citation44] and the other as a randomised phase III trial between HTCTRT vs. CTRT [Citation45]. The latter was closed due to slow accrual of patients.
The outcomes of this meta-analysis should guide the future treatment strategies for LACC and encourage the resurgence of multicentre trials in LACC with addition of HT. The therapeutic efficacy of HTRT over RT has been shown quite effectively using the conventional meta-analysis for all the five study end points. Although NMA showed HTRTCT to be the preferred treatment modality over HTRT and CTRT, no randomised trial has been reported to date using these three modalities. Thus, a three-arm randomised trial comparing HTCTRT vs. HTRT vs. CTRT, with HPV status as a stratification factor, is called for. The rapid advances in treatment delivery, thermometry and treatment planning have enabled HT treatment to become a safer and more precise treatment than in the past [Citation5]. These developments, along with the encouraging results of this NMA, should encourage the possibility of integrating HT as a valuable addition to the existing modalities of RT and CT in LACC without any appreciable additive acute or late morbidity.
Conclusions
This systematic review and meta-analysis conducted in LACC to evaluate the outcomes of HT and RT with or without CT provide level I evidence of a therapeutic benefit of HTRT over RT alone. NMA further indicates that HTCTRT could be the most promising approach in LACC. This should encourage multicentre randomised clinical trials with HTRT ± CT vs. CTRT in LACC. Pre-treatment HPV status should be included as a stratification factor in all such future trial designs.
Supplemental_File.zip
Download Zip (1.1 MB)Acknowledgements
The authors gratefully acknowledge Dr B. Hutton, Ottawa Hospital Research Institute, Center for Practice Changing Research, Ottawa, Canada for his guidance and valuable inputs regarding the network meta-analysis; Dr J. van der Zee and Dr M. Franckena, Erasmus MC Cancer Institute, Rotterdam for providing updates related to their data; Dr A. Siebenhuener, University Hospital Zurich for providing access to the databases and Dr M. M. Liu, Kantonsspital Aarau for translating the Chinese articles.
Disclosure statement
The authors alone are responsible for the content and writing of the paper. There are no conflicts of interest to be declared.
References
- Ferlay J, Soerjomataram I, Ervik M, . (2013). GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon (France): International Agency for Research on Cancer; Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx [last accessed 2015 Jun 30].
- Gaffney DK, Suneja G, Ryu SY, . (2015). The cervix cancer research network: a global outreach effort on behalf of the Gynecologic Cancer InterGroup. Int J Radiat Oncol Biol Phys 92:506–08.
- Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. (2008). Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol 26:5802–12.
- Hanna TP, Shafiq J, Delaney GP, . (2015). The population benefit of radiotherapy for cervical cancer: local control and survival estimates for optimally utilized radiotherapy and chemoradiation. Radiother Oncol 114:389–94.
- Datta NR, Ordonez SG, Gaipl US, . (2015). Local hyperthermia combined with radiotherapy and-/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev 41:742–53.
- Issels RD. (2008). Hyperthermia adds to chemotherapy. Eur J Cancer 44:2546–54.
- Lim K, Chan P, Dinniwell R, . (2008). Cervical cancer regression measured using weekly magnetic resonance imaging during fractionated radiotherapy: radiobiologic modeling and correlation with tumor hypoxia. Int J Radiat Oncol Biol Phys 70:126–33.
- Lutgens L, van der Zee J, Pijls-Johannesma M, . (2010). Combined use of hyperthermia and radiation therapy for treating locally advanced cervix carcinoma. Cochrane Database Syst Rev CD006377.
- Liberati A, Altman DG, Tetzlaff J, . (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700.
- Hutton B, Salanti G, Caldwell DM, . (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162:777–84.
- Chen HW, Jei J, Wei L. (1997). A randomized trial of hyperthermoradiochemotherapy for uterine cervix. Chin J Oncol 24:249–51.
- Yan X, Liu W, Yan Z, . (2014). Efficacy and safety radio-chemotherapy combined with thermotherapy for cervical cancer: a meta-analysis. Chin J Evid-based Med 14:752–58.
- Franckena M, Stalpers LJ, Koper PC, . (2008). Long-term improvement in treatment outcome after radiotherapy and hyperthermia in locoregionally advanced cervix cancer: an update of the Dutch Deep Hyperthermia Trial. Int J Radiat Oncol Biol Phys 70:1176–82.
- Harima Y, Nagata K, Harima K, . (2009). A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma. Int J Hyperthermia 25:338–43.
- Datta NR, Bose AK, Kapoor HK. (1987). Thermoradiotherapy in the management of carcinoma cervix (stage IIIB): a controlled clinical study. Ind Med Gazette 121:68–71.
- Vasanthan A, Mitsumori M, Park JH, . (2005). Regional hyperthermia combined with radiotherapy for uterine cervical cancers: a multi-institutional prospective randomized trial of the International Atomic Energy Agency. Int J Radiat Oncol Biol Phys 61:145–53.
- Sharma S, Singhal S, Sandhu AP, . (1991). Local thermo-radiotherapy in carcinoma cervix: improved local control versus increased incidence of distant metastasis. Asia Oceania J Obstet Gynaecol 17:5–12.
- van der Zee J, González D, van Rhoon G, . (2000). Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Lancet 355:1119–25.
- Harima Y, Nagata K, Harima K, . (2001). A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma. Int J Hyperthermia 17:97–105.
- Sharma S, Sandhu AP, Patel FD, . (1990). Side-effects of local hyperthermia: results of a prospectively randomized clinical study. Int J Hyperthermia 6:279–85.
- Lutgens LC, Koper PC, Jobsen JJ, . (2016). Radiation therapy combined with hyperthermia versus cisplatin for locally advanced cervical cancer: results of the randomized RADCHOC trial. Radiother Oncol DOI:10.1016/j.radonc.2016.02.010.
- Higgins JP, Altman DG, Gotzsche PC, . (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928.
- Comprehensive Meta-analysis Software, version 3.0. Available from: http://www.meta-analysis.com/index.php [last accessed 25 May 2015].
- Schünemann HJ, Oxman AD, Vist GE, . (2008). Interpreting results and drawing conclusions. In: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley, 359–89.
- Begg CB, Mazumdar M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–101.
- Brown S, Hutton B, Clifford T, . (2014). A Microsoft-Excel-based tool for running and critically appraising network meta-analyses – an overview and application of NetMetaXL. Syst Rev 3:110.
- NetMetaXL. Available from: http://www.netmetaxl.com/.
- Xiong T, Turner RM, Wei Y, . (2014). Comparative efficacy and safety of treatments for localised prostate cancer: an application of network meta-analysis. BMJ Open 4:e004285.
- Caldwell DM, Ades AE, Higgins JP. (2005). Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 331:897–900.
- Hutton B, Salanti G, Chaimani A, . (2014). The quality of reporting methods and results in network meta-analyses: an overview of reviews and suggestions for improvement. PLoS One 9:e92508.
- Datta NR, Pasricha R, Singh U, . (2004). Predictors of survival end points in patients with cancer of the cervix on long-term follow-up: inferences and implications from an audit of patients treated with a specific radiotherapy protocol. Clin Oncol (R Coll Radiol) 16:536–42.
- Chen SW, Liang JA, Yang SN, . (2003). The adverse effect of treatment prolongation in cervical cancer by high-dose-rate intracavitary brachytherapy. Radiother Oncol 67:69–76.
- Hong JH, Chen MS, Lin FJ, . (1992). Prognostic assessment of tumor regression after external irradiation for cervical cancer. Int J Radiat Oncol Biol Phys 22:913–17.
- Saibishkumar EP, Patel FD, Sharma SC, . (2006). Prognostic value of response to external radiation in stage IIIB cancer cervix in predicting clinical outcomes: a retrospective analysis of 556 patients from India. Radiother Oncol 79:142–46.
- Jacobs AJ, Faris C, Perez CA, . (1986). Short-term persistence of carcinoma of the uterine cervix after radiation. An indicator of long-term prognosis. Cancer 57:944–50.
- Jones EL, Prosnitz LR, Dewhirst MW, . (2005). In regard to Vasanathan et al. (Int J Radiat Oncol Biol Phys (2005). 61:145–153). Int J Radiat Oncol Biol Phys 63:644.
- Franckena M, Lutgens LC, Koper PC, . (2009). Radiotherapy and hyperthermia for treatment of primary locally advanced cervix cancer: results in 378 patients. Int J Radiat Oncol Biol Phys 73:242–50.
- Zolciak-Siwinska A, Piotrkowicz N, Jonska-Gmyrek J, . (2013). HDR brachytherapy combined with interstitial hyperthermia in locally advanced cervical cancer patients initially treated with concomitant radiochemotherapy – a phase III study. Radiother Oncol 109:194–99.
- Westermann A, Mella O, Van Der Zee J, . (2012). Long-term survival data of triple modality treatment of stage IIB–III–IVA cervical cancer with the combination of radiotherapy, chemotherapy and hyperthermia – an update. Int J Hyperthermia 28:549–53.
- Westermann AM, Jones EL, Schem BC, . (2005). First results of triple-modality treatment combining radiotherapy, chemotherapy, and hyperthermia for the treatment of patients with stage IIB, III, and IVA cervical carcinoma. Cancer 104:763–70.
- Datta NR, Kumar P, Singh S, . (2006). Does pretreatment human papillomavirus (HPV) titers predict radiation response and survival outcomes in cancer cervix? – a pilot study. Gynecol Oncol 103:100–5.
- Datta NR, Singh S, Kumar P, . (2015). Human papillomavirus confers radiosensitivity in cancer cervix: a hypothesis toward a possible restoration of apoptotic pathways based on clinical outcomes. Future Oncol 11:1363–71.
- Oei AL, van Leeuwen CM, Ten Cate R, . (2015). Hyperthermia selectively targets human papillomavirus in cervical tumors via p53-dependent apoptosis. Cancer Res 75:5120–29.
- Westermann AM. (2013). Cisplatin combined with radiation therapy and hyperthermia in treating patients with stage II, stage III, or stage IV cervical cancer: ClinicalTrials.gov; ClinicalTrials.gov identifier: NCT00008112. Available from: https://clinicaltrials.gov/ct2/show/NCT00008112?term=NCT00008112&rank = 1 [last accessed 30 Jun 2015].
- Dewhirst MW. (2013). Cisplatin and radiation therapy with or without hyperthermia therapy in treating patients with cervical cancer: ClinicalTrials.gov; ClinicalTrials.gov identifier: NCT00085631. Available from: https://clinicaltrials.gov/ct2/show/NCT00085631?term=NCT00085631&rank =1 [last accessed 30 Jun 2015].