Abstract
Purpose: Computed tomography (CT) and ultrasound-guided microwave ablations (MWA) are part of the established treatment of liver tumours. In spite of its potential advantages, magnetic resonance (MR) monitoring of MWA did not enter clinical practice because of the lack of compatible devices. The purpose of the current study was to prove the feasibility of real-time qualitative MR monitoring using a new MR-compatible MWA device.
Material and methods: We performed 27 MWA experiments with different durations (5, 10 and 15 min) on an ex vivo bovine liver model using a MR-compatible MWA device. We compared the diameters of the ablation zone as depicted on three T1-based sequences to those of the macroscopic specimen. The volume and the sphericity index of the macroscopic ablation area were calculated in order to characterise the device. Ablation pattern and artefacts on the three sequences were also taken into account.
Results: We obtained high-quality real-time images using all three sequences. The diameters as depicted on the MR sequences slightly overestimated the macroscopic ablation area but correlated significantly in all cases (p < 0.05). VIBE provided the best correlation for both short-axis diameter (r = 0.96) and long-axis diameter (r = 0.87), whereas starVIBE (r = 0.85; r = 0.72) and FLASH (r = 0.75; r = 0.84) correlated slightly less. Significantly more severe noise artefacts were observed on starVIBE compared to FLASH and VIBE sequences (p < 0.0001).
Conclusion: The current ex vivo liver model experiment suggests that real-time qualitative MR monitoring of MWA is feasible. Further research using in vivo and human models are recommended.
Introduction
In recent decades, image-guided local ablative therapies for focal malignant tumours involving parenchymatous organs have become an essential treatment option in patients with unfavourable surgical options due to difficult anatomical conditions or high perioperative risk [Citation1,Citation2]. While radiofrequency ablation (RFA) is currently the most common method, microwave ablation (MWA) is emerging as a promising alternative [Citation3–5].
In comparison to RFA, MWA is able to generate larger ablation zones in a shorter time, leading to the possibility of treating larger or more numerous tumours during a single ablation session avoiding the use of multiple simultaneous applicators. Additionally, MWA shows lower susceptibility to vascular cooling effects caused by high perfusion areas adjacent to the target tumour, which is known to be an independent predictor of incomplete tumour destruction and potential cause for tumour recrudescence [Citation6–9]. According to several comparative clinical trials, MWA already provided at least an equal local outcome, safety profile and overall survival rate compared to RFA [Citation10–13].
As ablation success highly depends on the exact conspicuity of the target tumour, MR guidance offers appealing characteristics such as excellent soft tissue resolution, sometimes even without additional use of contrast agent, and the potential of real-time thermosensitive imaging [Citation14,Citation15]. Due to the absence of ionising radiation, MR-guidance reduces life-time exposure for both patient and interventionist. Nevertheless, the challenge of MR-guided MW ablations relies on using fast sequences that are able to depict heating-related changes that can accurately predict tissue necrosis without being affected by major electric field-induced interference artefacts. Recently, a new MR-compatible MWA system combining the advantages of both techniques became commercially available [Citation16].
Therefore, the purpose of the present proof-of-principle study was to evaluate the feasibility of real-time T1-based qualitative MR imaging during MWA in an ex vivo liver model using this new MW system, first by comparing noise performance of different MR sequences, and second by calculating correlation between signal changes from MRI and ablation size at gross pathology.
Material and methods
For the present study 27 ablation experiments were performed in ex vivo bovine liver (temperature 10–15 °C) < 24 h after slaughter. A microwave generator (Medwaves, San Diego, CA, USA) and two different MR-compatible non-perfused MW applicators (14 and 16 gauge) were used at different treatment durations. During the ablation procedure three different T1-weighted MR sequences (FLASH, VIBE, starVIBE) were tested in order to prove their ability to predict the actual ablation area. After treatment the livers were cut along the ablation zone to measure the macroscopic coagulation area.
Approval from the institutional review board was not required because of the ex vivo design with bovine livers after slaughter.
Ablation algorithm
To analyse the growth of the ablation zone, three time increments (5, 10 and 15 min) and two different applicators (14 and 16 gauge) were used. The applicator was inserted 15 cm into the liver tissue at an angle of 45°. Both output power (maximum 36 W) and microwave frequency (902–928 MHz) were automatically adjusted during the ablation process by the microwave generator according to the manufacturer’s preset protocol. To avoid interferences during imaging, each 1-min ablation cycle was followed by a 10-s break dedicated for MR imaging (1 min/10 s ablation algorithm). After each ablation procedure the total amount of energy delivered to the tissue (kJ) as displayed by the MW generator was noted.
Real-time imaging
Real-time ablation imaging was performed with a conventional high-field 3T system (Prisma-Fit, Siemens, Erlangen, Germany) using the integrated spine coil. Three different T1-based MR sequences were modified according to the 1-min/10-s ablation algorithm: (1) fast low angle shot T1-weighted gradient-echo sequence (FLASH; TR 2.47 ms, TE 0.98 ms, flip angle 8°, field of view (FOV) 250 mm, acquisition matrix 173 × 256, slice thickness 2.0 mm, acquisition time 10 s), (2) volumetric interpolated breath-hold examination T1-weighted gradient-echo sequence (VIBE; TR 4.06 ms, TE 1.35 ms, flip angle 9°, FOV 346 mm, acquisition matrix 195 × 320, slice thickness 3 mm, acquisition time 10 s) and (3) free-breathing T1-weighted gradient-echo sequence with radial data sampling (starVIBE; TR 2.47 ms, TE 1.27 ms, flip angle 9°, FOV 240 mm, acquisition matrix 224 × 224, slice thickness 3 mm, acquisition time 10 s). An additional scan with the same sequences was performed 15 min after ablation in order to evaluate the cooling changes.
Outcome and data analysis
All images were analysed on a standard PACS workstation (GE Healthcare, Barrington, IL). The maximal short (Dx), respectively long (Dy) diameter (along the needle axis) of the ablation-related signal alteration area were measured by one blinded reader on each acquisition. If the alteration margin was diffuse, the reader noted the point with the strongest contrast between altered and non-altered tissue.
After the ablation procedure, the ablated liver was cut along the needle axis and the short and long diameter were determined analogously to the MR measurements. The macroscopic evaluation was performed by a second reader, blinded to the MR measurements.
Additionally, the volume of the ablation area and its shape were calculated based on the diameters. The ablation volume was calculated using the following formula for a rotational ellipsoid:
The shape of the ablation area was assessed using the sphericity index: Dx2 /Dy2. While a perfect sphere has a sphericity index of 1.0, an ellipsoid has an index <1.0.
The effect of the microwave field on image quality was investigated by measuring image noise before and during the ablation (at every 1-min interval). The noise was measured on every MR sequence as the standard deviation (SD) of the signal intensity in the surrounding air. The noise measurement was divided in two categories: (1) overall noise (SDoverall) which was directly measured on each sequence and is composed of the inherent noise of the sequence and the noise caused by the ablation system, (2) ablation-specific noise (SDablation) which was calculated as the difference in SDoverall between the acquisitions performed before and during the ablation in order to rule out the inherent noise of the MR sequence. One-way ANOVA was performed to compare the SDoverall between the sequences. Spearman's rank correlation coefficient (r) was used to measure the correlation between time and SDablation. Spearman's test was also used to quantify how well the short-axis (Dx) and long-axis diameters (Dy) measured at the end of ablation correlated with the necrosis diameters at gross pathology. A linear regression analysis was performed to describe this relationship. A non-linear regression model (rectangular hyperbola) was used to depict the relationship between time and short-axis diameter as measured on each sequence. The coefficient of determination (r2) quantifies the variability explained by the model. Significant results were considered for p < 0.05. Excel (Microsoft) and GraphPad Prism 6 (GraphPad Software) were used for data management and statistical analysis.
Results
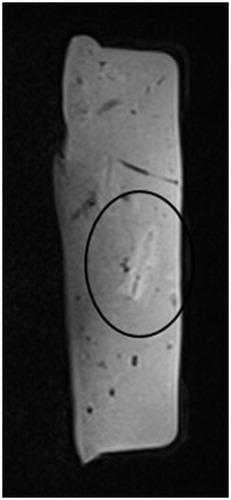
The ablation procedure was successful in all 27 experiments with clearly identifiable macroscopic ablation zones. We performed 18 ablations with a 16-gauge applicator (9 × 5 min; 9 × 10 min) and nine ablations using a 14-gauge applicator (9 × 15 min). No technical problems relating to the MWA system or the MR scanner were noted. The macroscopic characteristics of the ablation zone are shown in .
Table 1. Mean final diameters (Dx, Dy) for the three different MR sequences ± SD.
In all three sequences the MR appearance of the ablation zone consisted of an oval-shaped signal reduction area centred on the MW applicator (). The post-ablation values (Dx, Dy) for the three different MR sequences are summarised in . The correlation between the final MR diameters (Dx, Dy) and the macroscopic diameter was significant in all cases (p < 0.05). Both Dx and Dy correlated best on VIBE sequence (r = 0.96 (Dx) versus r = 0.87 (Dy)), followed by starVIBE (r = 0.85 versus r = 0.72) and FLASH (r = 0.74 versus r = 0.84). shows the linear regression model with corresponding r2.
Figure 1. Ablation zone signal changes after 0, 1, 3, 5, 7 and 10 min on each sequence with clear visibility of the MW antenna and interference-induced noise artefacts increasing with ablation time. The ablation area appears as a summation of branching signal loss lines steadily increasing perpendicular to the needle surrounded by a diffuse halo with a thickness of a few millimetres. (a) FLASH sequence: the halo was usually indistinguishable from the mostly diffuse ablation-related signal loss. This sequence was slightly more prone to needle-related artefacts and showed a high inhomogeneity of appearance between examinations. (b) VIBE sequence: in the first 5 min, a relatively sharp outer delineation of the ablation area is visible, but in the later phases it gets more diffuse, being indistinguishable from the halo. In some cases the central signal loss was almost non-apparent, the ablation area being suggested only by the extended halo. (c) starVIBE sequence: the appearance of the ablation area is similar to similar to that of VIBE, especially in the first 5 min, but the presence of the halo makes a precise delimitation of the ablation zone more difficult, especially in the later phases of ablation. Almost no needle artefacts and generally higher noise were noticeable on this sequence.
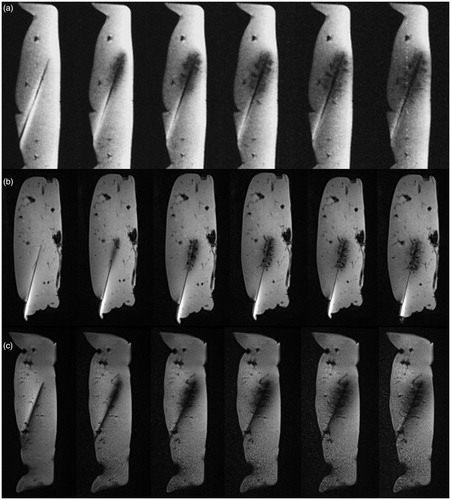
Figure 2. Correlation of Dx and Dy as measured on each MR sequence and corresponding diameters as measured on gross pathology.
Table 2. Macroscopic characteristics ± SD of the ablation zone for the different time periods and antenna sizes.
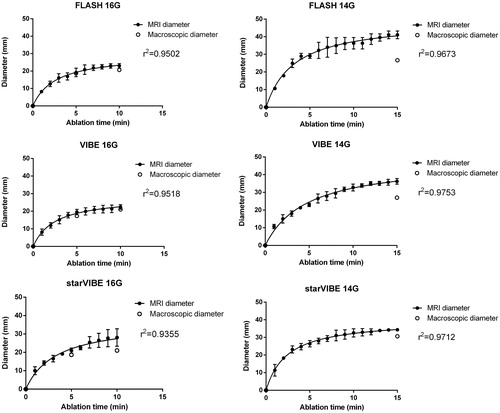
The short diameter (Dx) showed in all cases a quick increase in the first minute followed by a slower expansion in the later phase of ablation ().
Figure 3. The growth pattern of the short-axis diameters as depicted on the MR sequences with the corresponding macroscopic values as comparison. The final short diameter (Dx) in each sequence is always slightly overestimated compared to the macroscopic diameter. Only two experiments involving the 15-min FLASH (14 gauge), could be evaluated because almost no ablation-related changes could be noticed in one case.
The ablation zone was identifiable in 26 cases. One case had to be excluded (FLASH, 15 min, 14 gauge) because no ablation-related signal decrease was noticed even if the macroscopic examination suggested a successful ablation. Using ANOVA, the SDoverall for starVIBE was significantly higher (average 37.3 ± 2.3; p < 0.0001) compared to VIBE (average 14.48 ± 7.1) and FLASH (average 15.27 ± 3.58) which did not significantly differ from each other. The correlation between ablation time and SDablation was significant for FLASH (r = 0.93, p < 0.0001) and VIBE (r = 0.99, p < 0.0001). Increasing noise was visible in some starVIBE acquisitions, but overall there was no statistically significant correlation between time and SDablation for starVIBE (r = 0.2, p = 0.43). The MW applicator itself was clearly visible in all cases. The signal loss in the ablation area recovered completely 15 minutes after the procedure, and the area surrounding the applicator appeared slightly hyperintense ().
Discussion
Local ablation techniques provide a valid alternative to surgery for a large variety of tumours. While MWA of liver tumours, especially HCC, is well-established in the clinical routine, it can also be used for other organ systems such as lung or kidney [Citation17–19]. While most ablative techniques are currently performed with the help of CT or ultrasound guidance, a series of disadvantages inherent to both techniques exist. Although they are fast and relatively cheap, ultrasound guidance might be impeded by tumour inaccessibility, CT guidance is irradiating, and both techniques often provide poor tumour and ablation zone visualisation.
Because of the better soft tissue resolution and lack of radiation exposure, MR guidance might be able to overcome the disadvantages of the other techniques. While MRI has been proven to provide effective guidance for diagnostic interventions [Citation20,Citation21] and thermal ablations [Citation22], its use in association with MWA and RFA was, until recently, restricted by the lack of MR-compatible systems [Citation16,Citation23].
The purpose of the present proof-of-principle study was to show that real-time MR imaging during MWA is feasible and technically possible using a new MR-compatible MWA device (Medwaves). In order to achieve MR compatibility, some changes in the design of the MWA system were necessary. The antenna had to be designed void of magnetic material to eliminate extra artefacts that cover up the lesions, leaving only the profile of the device shaft and antenna. Additionally, the extra RF signal produced by the generator that could interfere with MRI information had to be eliminated or reduced and the cable extender had to be designed for longer reach up to 7.2 m for proper generator placement, and equipped with electromagnetic and RF interference filters to remove echo signals induced by the MR RF excitation up to 3T.
The role of MRI in guiding thermal ablation consists of targeting the tumour, monitoring the ablation and assessing the treatment response [Citation22]. As MR-guided interventions have been performed for more than 20 years, and post ablation MR follow-up is part of the clinical routine [Citation24–27], targeting and assessment of treatment response were not the subject of the current discussion. Real-time monitoring during the ablation is very important because of variation in inter- and even intra-individual ablation volumes which might need needle repositioning or longer ablation times in order to avoid incomplete ablations.
Although more thermosensitive sequences exist, T1, T2, diffusion and proton resonance frequency (PRF) are in use for thermal ablation monitoring [Citation28]. While PRF is the most common method able to provide tissue-independent quantitative temperature maps, both PRF and diffusion sequences are highly susceptible to motion which is problematic in a clinical setting. Despite the fact that they cannot show a linear relationship between temperature and signal change and are tissue dependent, T1-based sequences can instead provide satisfactory qualitative imaging with short acquisition times [Citation22,Citation28]. Real-time quantitative thermometry is feasible in clinical practice, but only on relatively static organs as shown by Chen et al. who performed proton resonance shift (PRF) MR thermometry during MWA in prostate cancer with real-time temperature mapping [Citation29]. PRF is difficult to use in a moving organ such as liver because it is based on measurements which depend on a pre-heating baseline acquisition. The acquisitions become artefacted as the position of the heated area moves away from the baseline during breathing [Citation28,Citation30]. Therefore, the results of Chen et al. are not directly transferable to our results, as more motion artefacts can be expected in a clinical setting. Terraz et al. used PRF monitoring during RF ablation of 12 liver tumours, but the lethal temperature threshold did not correlate with the size of the ablation zone on follow-up imaging [Citation15]. Because of the need for fast, motion-insensitive acquisitions and of the good experience in our centre, we have chosen T1-based sequences for ablation monitoring which are also practicable in a clinical setting [Citation24,Citation31].
The starVIBE sequence was purposefully chosen because of its ability to provide high quality free-breathing images, which is very important in a clinical setting. As opposed to usual data acquisition in parallel lines, the starVIBE sequence operates with radial sampling in the xy-plane, overlapping in the centre of the k-space and with rectilinear sampling in the z-direction with lower sensitivity to respiratory motion artefacts [Citation32,Citation33]. For this reason it is possible to perform the whole ablation process in free-breathing, leading to more patient comfort and better compliance.
In order to appreciate the ability of the aforementioned sequences to predict the extent of the ablation zone, we compared the dimensions of the MR ablation-related signal loss to the central macroscopic discoloured area, which, according to Lee et al. roughly corresponds to post-procedural coagulation necrosis [Citation34]. In all three sequences the ablation process caused an inhomogeneous central, branching signal loss area, which possibly corresponds to heated, pressured vapours fracturing the liver tissue while trying to escape, surrounded by a less pronounced, diffuse halo which might be attributable to T1 heat-induced signal loss (). In the images acquired 15 min post ablation, signal recovery was complete and the targeted area appeared slightly hyperintense, as the tissue and vapours had cooled ().
The depiction of the signal changes using starVIBE was similar in all nine examinations and a clear delimitation of the central hypointense ablation area was obvious, especially in the first 5 min of ablation. For FLASH and VIBE, a greater variability of the appearance of the ablation zone was noticed. In one case (FLASH) only a weak, diffuse signal loss was apparent despite obvious macroscopic changes.
Because of the small number of experiments we were able to calculate only an overall correlation coefficient for the different sequences but not for each time increment. The overall correlation was significant for all sequences, with VIBE showing the best results. The short diameter (Dx) as measured after 5 min corresponded well to the actual ablation diameter in all three sequences, and was overestimated in starVIBE at 10 min and in FLASH and VIBE at 15 min. Of particular interest is the case of starVIBE, which accurately predicted the ablation short diameter at 5 and 15 min, but overestimated it at 10 min. Also, high SD values were noted around the 10-min mark with this sequence. These observations might be explained by the inability of the reader to properly assess the extent of the ablation-related signal loss because of its diffuse margin. Although the same difficulties were encountered with the other sequences, they better predicted the ablation at 10 min.
The measurement of the long diameter (Dy) was less accurate, also affecting the volume and sphericity calculation. As most of the tumours treatable with local ablative techniques are characterised by a spherical appearance, the ablation zone should approximate a sphere in order to minimise destruction of the surrounding tissue. The mean overall sphericity index in the present study was 0.13 ± 0.03, indicating that the ablation zone is long and thin, similar to an ellipsoid.
Overall noise (SDoverall) was more pronounced on starVIBE sequences (average SDoverall = 37.3) than on FLASH (average SDoverall = 15.3) and VIBE (average SDoverall = 14.5). Probably because of the automatic activation of the MW generator, increasing interferences (higher noise) were noticeable in the later phases of ablation where imaging and ablation might have slightly overlapped. The correlation between time and noise increase (SDablation) was significant for VIBE and FLASH. Indeed, increasing noise was also visible in some starVIBE acquisitions, but overall there was no statistically significant correlation detectable. It is most likely that starVIBE is also susceptible to ablation specific noise that might have been overpowered by artefacts inherent to the sequence (e.g. streak artefacts), which is obvious in where generally higher noise artefacts can be seen in the later acquisitions. The noise artefacts did not have a major impact on image quality and ceased after the ablation ended.
As the number of experiments performed with each sequence was small, they can only provide a rough estimation of what could be expected from each sequence. By trying to prove the concept of MR-guided MWA before applying it on a whole-animal model or in a clinical setting, the results of the present study were limited by different factors.
The shape and size of the ablation zone might be different in an in vivo model because of the higher tissue water content and the heat-sink effect caused by blood flow [Citation35]. Another limitation was the reliance on the macroscopic appearance of the ablated zone without histological confirmation. As already mentioned, we did not perform real-time quantitative thermal imaging. For that reason, our results should be considered as a qualitative ablation assessment mainly needed in a clinical setting and not as a thermometry approach during thermal ablation [Citation15,Citation29].
Our preliminary experience suggests that some improvements might be necessary for an efficient clinical workflow. It would be preferable to have an intercalated synchronisation system between the MR scanner and the MWA generator to avoid interferences at the end of long ablation periods. Also, it is unclear whether the size of the ablation zone is influenced by the length of the imaging break, and what might be the optimal imaging/ablation ratio. Furthermore, the initial experience in a clinical setting is likely to demonstrate the limitations of using the relatively small gantry of a 3T scanner compared to an open MR device.
In conclusion, our experiments using ex vivo bovine liver suggest that it is possible to perform MW ablations under real-time qualitative MR imaging using a MR-compatible MWA antenna and a determined scanning algorithm. Although there was not a perfect superposition between the macroscopic and MR changes, the T1 sequences we used provided a satisfactory, relatively artefact-free prediction of the ablation area. Nevertheless, further research with in vivo and human models is necessary to investigate potential technical barriers with this new device that might not be obvious on an ex vivo liver model. It can be assumed that MWA under MR guidance with the possibility of real-time imaging will have an essential impact on the emerging field of local ablation techniques.
Acknowledgements
We thank the company Medwaves for providing the MW antenna for the present experimental single-centre ex vivo study.
Disclosure statement
The authors and involved institutions have no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Nishikawa H, Inuzuka T, Takeda H, Nakajima J, Matsuda F, Sakamoto A, et al. Comparison of percutaneous radiofrequency thermal ablation and surgical resection for small hepatocellular carcinoma. BMC Gastroenterol 2011;11:143.
- Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008;47:82–9.
- McCarley JR, Soulen MC. Percutaneous ablation of hepatic tumors. Semin Intervent Radiol 2010;27:255–60.
- Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology 2007;72:S124–31.
- Lubner MG, Brace CL, Hinshaw JL, Lee FT. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 2010;21:192–203.
- Brace CL, Laeseke PF, Sampson LA, Frey TM, van der Weide, Daniel W, Lee FT. Microwave ablation with a single small-gauge triaxial antenna: in vivo porcine liver model. Radiology 2007;242:435–40.
- Bhardwaj N, Dormer J, Ahmad F, Strickland AD, Gravante G, West K, et al. Microwave ablation of the liver: a description of lesion evolution over time and an investigation of the heat sink effect. Pathology 2011;43:725–31.
- Kang TW, Lim HK, Lee MW, Kim Y, Choi D, Rhim H. Perivascular versus nonperivascular small HCC treated with percutaneous RF ablation: retrospective comparison of long-term therapeutic outcomes. Radiology 2014;270:888–99.
- Lu DSK, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol 2003;14:1267–74.
- Dong B, Liang P, Yu X, Su L, Yu D, Cheng Z, et al. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: results in 234 patients. Am J Roentgenol 2003;180:1547–55.
- Lu M, Xu H, Xie X, Yin X, Chen J, Kuang M, et al. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a retrospective comparative study. J Gastroenterol 2005;40:1054–60.
- Ding J, Jing X, Liu J, Wang Y, Wang F, Wang Y, et al. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol 2013;82:1379–84.
- Iannitti DA, Martin RCG, Simon CJ, Hope WW, Newcomb WL, McMasters KM, et al. Hepatic tumor ablation with clustered microwave antennae: the US phase II trial. HPB (Oxford) 2007;9:120–4.
- Rempp H, Loh H, Hoffmann R, Rothgang E, Pan L, Claussen CD, et al. Liver lesion conspicuity during real-time MR-guided radiofrequency applicator placement using spoiled gradient echo and balanced steady-state free precession imaging. J Magn Reson Imaging 2014;40:432–9.
- Terraz S, Cernicanu A, Lepetit-Coiffé M, Viallon M, Salomir R, Mentha G, et al. Radiofrequency ablation of small liver malignancies under magnetic resonance guidance: progress in targeting and preliminary observations with temperature monitoring. Eur Radiol 2010;20:886–97.
- Hoffmann R, Rempp H, Eibofner F, Kessler D, Blumenstock G, Weiss J, et al. In vitro artefact assessment of a new MR-compatible microwave antenna and a standard MR-compatible radiofrequency ablation electrode for tumour ablation. Eur Radiol 2016;26:771–9.
- Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology 2012;262:43–58.
- Smith SL, Jennings PE. Lung radiofrequency and microwave ablation: a review of indications, techniques and post-procedural imaging appearances. Br J Radiol 2015;88(1046):20140598.
- Moreland AJ, Ziemlewicz TJ, Best SL, Hinshaw JL, Lubner MG, Alexander ML, et al. High-powered microwave ablation of t1a renal cell carcinoma: safety and initial clinical evaluation. J Endourol 2014;28:1046–52.
- Zangos S, Müller C, Mayer F, Naguib NN, Nour-Eldin NA, Hansmann M, et al. Retrospective 5-year analysis of MR-guided biopsies in a low-field MR system. Rofo 2009;181:658–63.
- Hoffmann R, Thomas C, Rempp H, Schmidt D, Pereira PL, Claussen CD, et al. Performing MR-guided biopsies in clinical routine: factors that influence accuracy and procedure time. Eur Radiol 2012;22:663–71.
- McDannold NJ, Jolesz FA. Magnetic resonance image-guided thermal ablations. Top Magn Reson Imaging 2000;11:191–202.
- Dong J, Zhang L, Li W, Mao S, Wang Y, Wang D, et al. 1.0 T open-configuration magnetic resonance-guided microwave ablation of pig livers in real time. Sci Rep 2015;5:13551.
- Vogl TJ, Müller PK, Hammerstingl R, Weinhold N, Mack MG, Philipp C, et al. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: technique and prospective results. Radiology 1995;196:257–65.
- Schima W, Ba-Ssalamah A, Kurtaran A, Schindl M, Gruenberger T. Post-treatment imaging of liver tumours. Cancer Imaging 2007;7:28–36.
- Vilgrain V. Advancement in HCC imaging: diagnosis, staging and treatment efficacy assessments: hepatocellular carcinoma: imaging in assessing treatment efficacy. J Hepatobiliary Pancreat Sci 2010;17:374–9.
- Clasen S, Boss A, Schmidt D, Fritz J, Schraml C, Claussen CD, et al. Magnetic resonance imaging for hepatic radiofrequency ablation. Eur J Radiol 2006;59:140–8.
- Rieke V, Butts Pauly K. MR thermometry. J Magn Reson Imaging 2008;27:376–90.
- Chen JC, Moriarty JA, Derbyshire JA, Peters RD, Trachtenberg J, Bell SD, et al. Prostate cancer: MR imaging and thermometry during microwave thermal ablation-initial experience. Radiology 2000;214:290–7.
- Winter L, Oberacker E, Paul K, Ji Y, Oezerdem C, Ghadjar P, et al. Magnetic resonance thermometry: methodology, pitfalls and practical solutions. Int J Hyperthermia 2016;32:63–75.
- Wichmann JL, Beeres M, Borchard BM, Naguib NNN, Bodelle B, Lee C, et al. Evaluation of MRI T1-based treatment monitoring during laser-induced thermotherapy of liver metastases for necrotic size prediction. Int J Hyperthermia 2014;30:19–26.
- Azevedo RM, de Campos, Rafael O P, Ramalho M, Herédia V, Dale BM, Semelka RC. Free-breathing 3D T1-weighted gradient-echo sequence with radial data sampling in abdominal MRI: preliminary observations. Am J Roentgenol 2011;197:650–7.
- Chandarana H, Block TK, Rosenkrantz AB, Lim RP, Kim D, Mossa DJ, et al. Free-breathing radial 3D fat-suppressed T1-weighted gradient echo sequence: a viable alternative for contrast-enhanced liver imaging in patients unable to suspend respiration. Invest Radiol 2011;46:648–53.
- Lee JD, Lee JM, Kim SW, Kim CS, Mun WS. MR imaging-histopathologic correlation of radiofrequency thermal ablation lesion in a rabbit liver model: observation during acute and chronic stages. Korean J Radiol 2001;2:151–8.
- Hines-Peralta AU, Pirani N, Clegg P, Cronin N, Ryan TP, Liu Z, et al. Microwave ablation: results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology 2006;239:94–102.