Abstract
Purpose: The use of higher frequencies in percutaneous microwave ablation (MWA) may offer compelling interstitial antenna design advantages over the 915 MHz and 2.45 GHz frequencies typically employed in current systems. To evaluate the impact of higher frequencies on ablation performance, we conducted a comprehensive computational and experimental study of microwave absorption and tissue heating as a function of frequency.
Methods: We performed electromagnetic and thermal simulations of MWA in ex vivo and in vivo porcine muscle at discrete frequencies in the 1.9–26 GHz range. Ex vivo ablation experiments were performed in the 1.9–18 GHz range. We tracked the size of the ablation zone across frequency for constant input power and ablation duration. Further, we conducted simulations to investigate antenna feed line heating as a function of frequency, input power, and cable diameter.
Results: As the frequency was increased from 1.9 to 26 GHz the resulting ablation zone dimensions decreased in the longitudinal direction while remaining relatively constant in the radial direction; thus at higher frequencies the overall ablation zone was more spherical. However, cable heating at higher frequencies became more problematic for smaller diameter cables at constant input power.
Conclusion: Comparably sized ablation zones are achievable well above 1.9 GHz, despite increasingly localised power absorption. Specific absorption rate alone does not accurately predict ablation performance, particularly at higher frequencies where thermal diffusion plays an important role. Cable heating due to ohmic losses at higher frequencies may be controlled through judicious choices of input power and cable diameter.
Introduction
Microwave ablation (MWA) is being investigated for a variety of medical therapies including treatment of endometrial bleeding [Citation1], uterine myomas [Citation2], atrial fibrillation [Citation3], and cancerous tumours in the liver [Citation4–6], kidneys [Citation7], pancreas [Citation8], lungs [Citation9], and breast [Citation10]. A MWA probe typically consists of a coaxial cable feeding an interstitial antenna that radiates electromagnetic (EM) power into the tissue surrounding the antenna. Power absorption raises the local tissue temperature, leading to cell death. MWA offers a less-invasive alternative to surgical resection of tumours, allowing shortened recovery times and providing a viable treatment option for cancer patients who are not candidates for surgery.
Most studies of MWA have been conducted at 915 MHz [Citation8,Citation11–14] and 2.45 GHz [Citation2,Citation3,Citation6,Citation7,Citation9,Citation10,Citation15–20]. Similarly, most if not all commercial MWA systems to date operate at one of these two frequencies. These frequencies are logical choices because they fall within industrial, scientific, and medical (ISM) bands that are allocated for general commercial (non-telecommunications-based) use. Further rationale stems from considerations of penetration depth and the resulting specific absorption rate (SAR) pattern – both of which are relatively large at low microwave frequencies and decrease in size at higher frequencies – as well as feed cable heating, which increases with frequency.
However, there is a compelling rationale for exploring the use of frequencies above 2.45 GHz. Higher frequency MWA system designs utilise antennas with smaller dimensions, which increases flexibility in probe design and further reduces the invasiveness of the procedure. Several promising studies have been conducted at higher frequencies, including 9.2 GHz [Citation1], 10 GHz [Citation21], 14.5 GHz [Citation5,Citation22], 900 MHz–18 GHz [Citation23] and 24.125 GHz [Citation16]. A few of these studies included comparison of MWA performance at different frequencies. Luyen et al. [Citation21] demonstrated that MWA at 10 GHz yields ablation volumes in ex vivo bovine liver comparable to those of 1.9 GHz, suggesting that the development of the ablation zone is not only a function of microwave penetration and absorption depth, but also of thermal conduction. Komarov [Citation16] conducted a simulation-based study and found that a 2.45 GHz antenna produces less compact and more elliptical heating patterns than a 24.125 GHz antenna. Yoon et al. [Citation23] compared low-power ablation experiments at a number of frequencies in the 0.9–18 GHz range and found that 18 GHz operation yielded the highest tissue temperatures near the microwave applicator as well as the highest heating differentiation between cancer and fat tissue. These highlighted studies have pointed to promising performance characteristics of higher frequency MWA. The development of higher frequency MWA systems will benefit from quantitative information on how performance varies with frequency. However, none of the previous studies provides a reliable means for deriving frequency-dependent trends in MWA performance. The single-frequency studies used different antenna types, tissue environments, and input power levels, making comparisons between them difficult. Of the three multi-frequency studies, only that by Luyen et al. [Citation21] directly compares MWA performance across frequencies by using equal input power and heating time. Nonetheless, the complexity of the multi-physics problem does not permit extrapolation from only two frequency samples (1.9 and 10 GHz) to other frequencies within or above that range.
In this paper we present a comprehensive study of the frequency dependence of achievable ablation zones as well as of feed line heating. We examine MWA performance at discrete frequencies between 1.9 and 26 GHz. This range was chosen to include the ISM bands at 2.45, 5.8, and 24.125 GHz. First, we used EM and thermal simulations to design a collection of floating-sleeve dipole antennas for operation in in vivo and ex vivo porcine muscle and to evaluate SAR patterns and ablation zones over frequency. Second, we fabricated a set of antennas from 1.9 to 18 GHz and performed ablation experiments in ex vivo porcine muscle. Third, we performed simulations to explore ohmic heating in coaxial feed lines embedded in in vivo tissue. Cable heating was evaluated for varied input power, operating frequency, and cable diameter.
Methods and materials
Antenna design
shows two cross sections of the coaxial floating sleeve dipole (FSD) antenna that we designed and constructed for our MWA study. The design is based on the antenna reported by Yang et al. [Citation20] for MWA at 2.45 GHz and later adapted by Luyen et al. [Citation21] for MWA at 1.9 GHz and 10 GHz. Each arm of the dipole is of length ld and the feed gap between the arms is of length lg. A floating sleeve of length lfs is used to suppress currents on the outer conductor of the cable. This reduces undesired heating along the feed line as well as decouples antenna performance from insertion depth [Citation20]. The entire structure is covered with Teflon to prevent tissue from sticking to the antenna.
Figure 1. Floating sleeve dipole (FSD) antenna used in computational and experimental studies of MWA. The length dimensions are customised for seven different frequencies between 1.9 and 26 GHz. (a) Longitudinal cross-section. (b) Transverse cross-section.
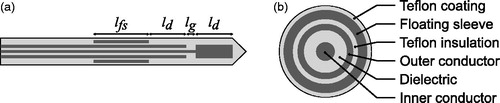
We employed CST Microwave Studio software [Citation24] to perform EM design simulations of the antennas embedded in a porcine muscle tissue model. We chose seven design frequencies between 1.9 GHz and 26 GHz, spaced by 4 GHz (or 4.1 GHz in the case of the spacing between the two lowest frequencies). The use of 1.9 GHz instead of the more logical 2 GHz as our lowest frequency was necessitated by limitations of the solid-state amplifier in our experimental MWA system. Each antenna was designed to achieve good impedance matching for power delivery efficiency and a compact specific absorption rate (SAR) pattern with negligible absorption behind the floating sleeve. These two goals were achieved by tuning ld, lg, lfs individually (shown in ) for each antenna. Antenna diameters for the investigation of MWA performance at different frequencies were chosen to be those of 50 Ω UT-085 semi-rigid coaxial cables (i.e. 2.2 mm outer diameter) from Micro-Coax (Pottsdown, PA). Hollow copper tubes with an inner diameter of 2.5 mm and an outer diameter of 3.2 mm were used for the floating sleeve. An outer covering of heat-shrink Teflon increased the outer diameter from 2.2 mm to 3.5 mm. A summary of the resulting antenna dimensions is provided in . The lengths of the dipole arms, the feed gap, and the floating sleeve all decrease with increasing frequency; this is consistent with the decrease in effective wavelength with frequency.
Table 1. Dimensions of FSD antennas.
The design process yielded FSD antennas that exhibit good impedance matching and efficient energy transfer to tissue. According to our simulation data all antennas accept at least 99.5% of the power input to the port. The 1.9 GHz antenna delivers 97.1% of input power to the tissue, and this rate decreases monotonically with frequency to 91.2% of input power delivered to the tissue by the 26 GHz antenna. The majority of the remaining power is dissipated via ohmic heating in copper, and a small amount in the Teflon insulation due to dielectric losses.
MWA simulations
EM and thermal simulations of MWA performance were conducted using CST Microwave Studio and CST Multiphysics Studio software (Darmstadt, Germany), respectively. The simulation domain consisted of a block of porcine muscle tissue into which the antenna was inserted. In the 1.9 GHz simulations the block of tissue extended approximately 5 cm outward from the antenna in the radial direction; for all higher frequency simulations the tissue extended approximately 2 cm from the antenna. These dimensions were sufficient to avoid any boundary artefacts in both the EM and thermal simulations. Losses in copper and Teflon were taken into account. The dielectric properties of porcine muscle tissue were obtained from open-ended coaxial probe measurements (ex vivo) and incorporated into our CST software simulations using a polynomial fit to the frequency-dependent data. The mesh resolution in the EM simulations ranged from 45 mesh cells per wavelength in the tissue at 1.9 GHz down to eight cells per wavelength in the tissue at 26 GHz. The mesh was refined further within the antenna so that the coaxial conductors and floating sleeve spanned at least three mesh cells. The output of the EM simulations – namely, the spatial distribution of the volumetric rate of deposition of microwave energy in the tissue as well as the ohmic losses in the metals – was used as the input, i.e. the heat source, in the thermal solver.
We simulated MWA performance under both ex vivo and in vivo thermal conditions. We considered two different ex vivo environments: one with an ambient temperature of 20 °C to mimic those of our laboratory experiments, and one with an ambient temperature of 37 °C. These ambient temperatures also represented the initial conditions in the tissue. In both cases blood perfusion and metabolic heat generation were set to zero. We also considered two different in vivo environments in simulation: one with a blood perfusion coefficient of 2647 W/K/m3 [Citation25] and metabolic heat generation of 706 W/m3 [Citation26], and one with a blood perfusion coefficient of 71,232 W/K/m3 [Citation25] and metabolic heat generation of 10.41 W/kg [Citation27], or 10,931 W/m3. The former set of values represents typical thermal parameters for muscle tissue. The latter is representative of liver, and is included in our study to gain an understanding of the impact of higher perfusion rates on MWA performance as a function of frequency. Both in vivo environments assumed a blood temperature of 37 °C. In all simulations we assumed a thermal conductivity of 0.488 W/K/m, a density of 1050 kg/m3, and a specific heat of 3.72 kJ/K/kg [Citation25]. Thermal properties referenced porcine tissue when possible, and substituted values for human tissue otherwise. All simulations assumed 25 W of continuous power at the antenna input for a duration of 5 min.
MWA experiments
We conducted MWA experiments at five frequencies: 1.9, 6, 10, 14, and 18 GHz. The experimental set-up for the 1.9 GHz MWA experiments comprised a signal generator (HP 8350B Sweep Oscillator, now Agilent, Santa Clara, CA) connected to a high-power solid-state amplifier (DMS 7066), and reflected power was monitored using a Giga-tronics 8542C (San Ramon, CA). The same signal generator was connected to a high-power 6–18 GHz travelling wave tube (TWT) amplifier (T186-40, IFI, Ronkonkoma, NY) for the higher frequency MWA experiments. We did not conduct experiments above 18 GHz due to the limitations of the TWT amplifier’s operating range. This amplifier has a built-in reflected power monitor.
Four ablation experiments were performed at each frequency in ex vivo porcine muscle (i.e. pork loin) at room temperature (20 °C). Prior to beginning each ablation procedure we measured the input voltage standing wave ratio (VSWR) of the antenna (Agilent E8364A PNA) to verify a good impedance match at the operating frequency. During the ablation procedure 25 W of power was delivered to the antenna for a duration of 5 min. (Amplifier gain limitations precluded the delivery of power levels higher than 25 W.) Reflected power was monitored continuously to ensure that that the power returning to the amplifier remained at a safe level. The observed reflected power rose from 1 W to 2 W over the course of a typical ablation, suggesting a minimal degradation in the impedance match due to ablation-related changes in tissue dielectric properties. The VSWR measured at the conclusion of the ablation was consistent with this observation.
Cable heating simulations
We modelled a section of coaxial cable with an input port and an output port (in place of an antenna) to investigate the effects of ohmic cable heating, independent of heating due to microwave absorption associated with power radiated by the antenna. The cable was insulated with Teflon and embedded in in vivo porcine muscle tissue. Thermal properties were identical to those of the first, low-perfusion environment in which the FSD antennas were simulated (i.e. a perfusion coefficient of 2647 W/K/m3 and metabolic heat generation of 706 W/m3). We simulated cables with six different diameters (0.33 mm, 0.58 mm, 0.86 mm, 1.13 mm, 1.42 mm, 2.2 mm). Each cable was tested at 1.9, 6, 10, 14, 18, 22, and 26 GHz at input powers of 5, 10, 20, and 40 W. We performed thermal steady-state simulations and recorded the temperature at the boundary between the Teflon insulation and tissue. We recorded the temperature at the halfway point along the length of the cable, as this is where the highest temperatures were reached.
Results
MWA simulations: SAR as a function of frequency
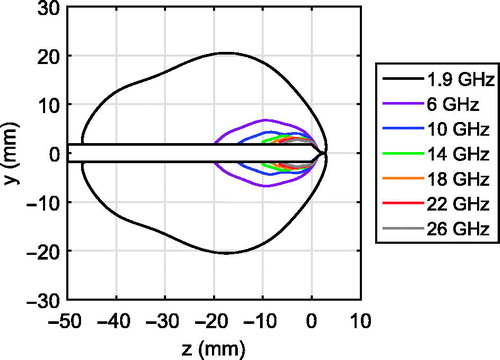
We first considered the simulated microwave power deposition patterns for the 1.9, 6, 10, 14, 18, 22, and 26 GHz FSD antennas operating in porcine muscle tissue. A comparison of normalised SAR patterns across frequency can be misleading since the peak SAR increases significantly with frequency. Instead, we plotted an alternative version of a SAR pattern that shows the volume over which 75% of the total power radiated by each antenna is absorbed. shows the contour that bounds this volume for each frequency. All of these SAR patterns are compact: none exhibit a tail extending down the feed line shaft. This confirms that the floating sleeves are effectively choking the outer-conductor currents and preventing undesired heating along the feed line. The results of indicate that the power dissipation density in the tissue increases greatly with frequency. For example, the volume over which 75% of the total power radiated by the 26-GHz antenna is absorbed is approximately 20 times smaller than the corresponding volume at 6 GHz, and is approximately 500 times smaller than the corresponding volume at 1.9 GHz.
MWA simulations: heating as a function of frequency
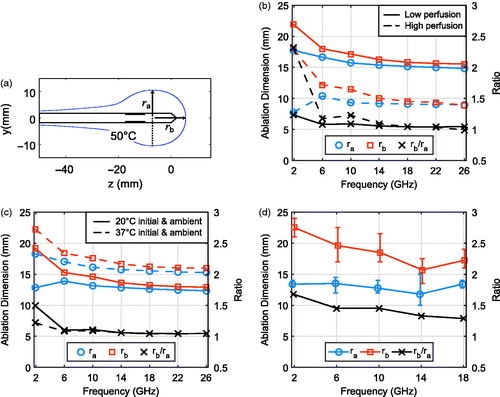
We next considered the results of in vivo thermal simulations for the 1.9, 6, 10, 14, 18, 22, and 26 GHz FSD antennas. shows the temperatures in in vivo porcine muscle tissue after 5 min of ablation at 25 W for each antenna. We observed that ablation zones of comparable lateral diameter (along the radial direction) were generated for all frequencies in the 1.9–26 GHz range. These heating patterns also reveal that the longitudinal extent of the core of the ablation zone decreased with increasing frequency. The lateral and longitudinal radii of the ablation zones are quantified in to facilitate the comparison. The ablation zone boundary is approximated here using the 50 °C temperature contour, which is a lower bound at which fatal cell destruction occurs [Citation28]. The lateral radius, ra, is defined as the distance from the centre axis of the antenna along the perpendicular to the most distant point on the 50 °C temperature contour. The longitudinal radius, rb, is defined as the distance along the antenna axis from the transverse plane where ra is measured to the point beyond the tip of the antenna where the temperature drops to 50 °C, as shown in . The quantitative data represented by the solid lines in (corresponding to porcine muscle with typical thermal parameters) supports the qualitative trends observed in . In particular, the aspect ratio of the ablation zone tends toward unity with increasing frequency. The dashed-line data in , which correspond to the temperature maps in , represent a high-perfusion environment, where the same trends manifest but with smaller overall ablation zones. The high-perfusion case also appears to restrict the maximum temperatures achieved by the 1.9 GHz FSD to much lower levels (e.g. 70 °C) compared to higher frequencies. Finally, the results of ex vivo thermal simulations for the 1.9, 6, 10, 14, 18, 22, and 26 GHz FSD antennas are shown in and . , along with the solid lines in , represent a 20 °C initial and ambient temperature, and the dashed lines in represent a 37 °C initial and ambient temperature. Ablation zones in both ex vivo environments exhibited trends over frequency comparable to those of the in vivo cases; dimensions stayed relatively stable and aspect ratios tended to unity with increasing frequency.
Figure 3. Thermal simulation results for FSD antennas operating in (a) in vivo porcine muscle tissue, (b) ex vivo porcine muscle tissue with an initial temperature of 20 °C, and an ambient temperature of 20 °C, and (c) in vivo porcine muscle with higher perfusion and metabolic heat generation rates. The antenna is shown in white with a black outline, and the floating sleeve is highlighted in black for reference.
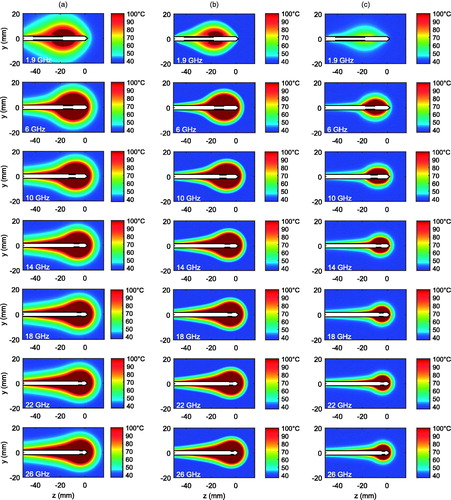
Figure 4. (a) Diagram illustrating the lateral radius, ra, and the longitudinal radius, rb, of the ablation zone, defined in simulation as the volume enclosed by the 50 °C contour. (b) Simulated in vivo ablation zone dimensions for porcine muscle with typical (solid lines) and high (dashed lines) assumed values of perfusion and metabolic heat generation, along with the ratios of their longitudinal and lateral radii, as a function of frequency. (c) Simulated ex vivo ablation zone dimensions and aspect ratios with an ambient temperature of 20 °C (solid lines) and 37 °C (dashed lines). (d) Average dimensions of ablation zones with min/max error bars, along with average aspect ratios from ex vivo ablation experiments.
MWA experiments
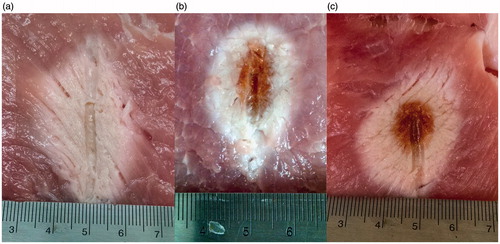
The lateral and longitudinal radii of the ablation zones created during the 20 ablation experiments were measured upon gross inspection of the bisected tissue at the completion of each ablation. Thermal coagulation alters the colour of porcine muscle tissue; we used the boundary between dark pink (unaffected tissue) and light pink/white (coagulated tissue) in measuring the dimensions of the ablation zone. shows the average, minimum, and maximum dimensions of ablation zones achieved at each frequency. The longitudinal radius of the experimental ablation zone decreases with increasing frequency; the lateral radius is fairly stable over frequency. Additionally, the average aspect ratio approaches unity with increasing frequency. All of these trends are consistent with those predicted by the simulations. The photographs of representative ablations at 1.9 GHz, 10 GHz, and 18 GHz presented in provide evidence of higher power dissipation density in higher frequency ablations. The ablation zones at 10 GHz and 18 GHz exhibit charred (dark brown) regions. There was no charred tissue in the 1.9 GHz experiments, suggesting that the temperature of the tissue closest to the 1.9 GHz antenna did not reach as high a level.
Cable heating simulations
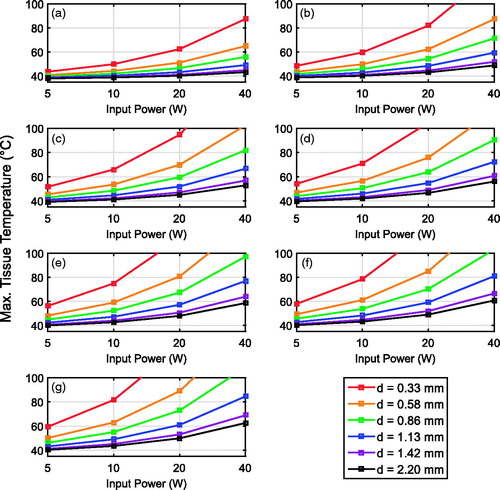
shows the dependence of peak tissue temperature at the surface of the two-port cable on operating frequency, cable diameter, and input power. Each graph in presents the results for a single frequency and has one curve for each cable diameter. For a fixed frequency, cable heating becomes more problematic for smaller diameter cables. For a fixed cable diameter, cable heating becomes more problematic at higher frequencies. Numerous previous thermal therapy studies (for example, [Citation28]) have established ∼50 °C as the temperature above which sufficient tissue coagulation for cell death can occur during short exposures (in the order of minutes). We use this lower-bound threshold for analysing the results of . For example, for the narrowest diameter tested cable (d = 0.33 mm) and highest frequency (26 GHz), even 5 W of power is likely to cause tissue damage along the feed line when no external probe cooling mechanism is used. The smallest cable diameter for which temperatures are maintained below 50 °C with 5 W of input power at 26 GHz is 0.86 mm; the 26 GHz power constraint is relaxed to 10 W for a cable of diameter 1.13 mm.
Figure 6. Simulated peak temperature in in vivo porcine muscle tissue (assuming a typical low perfusion rate associated with muscle) at the surface of an insulated coaxial cable as a function of input power, cable diameter, and operating frequency. (a) 1.9 GHz, (b) 6 GHz, (c) 10 GHz, (d) 14 GHz, (e) 18 GHz, (f) 22 GHz, and (g) 26 GHz.
Discussion
A comparison of the simulated SAR patterns with the simulated heating patterns and experimental ablation results shows that the size of the ablation zone is not determined solely by the microwave power absorption distribution. In the lateral radius of the SAR pattern shrinks from ∼23 mm at 2 GHz to ∼3 mm at 18–26 GHz. However, in , the lateral radius of the ablation zone is fairly constant across the entire frequency range. These findings are consistent with the 1.9 GHz versus 10 GHz comparison reported by Luyen et al. [Citation21] that strongly suggested a significant role of thermal conduction in creating comparably sized ablation zones at higher microwave frequencies.
As the MWA operating frequency is increased, the ratio between the lateral and longitudinal dimensions of the ablation zone tends towards unity in both simulation and experiment. This is likely explained by the outward thermal diffusion from an increasingly point-like source of deposited microwave power instead of from a large and elongated SAR distribution such as that seen at 1.9 GHz. The heat source becomes more point-like because of both decreased microwave penetration and the shorter radiators employed at higher frequencies. Thus, antennas with active lengths of the order of the intended ablation zone may lead to elliptical ablation zones while shorter antennas (with higher operating frequencies) may lead to more spherical ablation zones. These trends have been observed by other researchers. Hines-Peralta et al. [Citation4] performed MWA at 2.45 GHz in ex vivo bovine livers for a number of durations and input power levels, and reported ratios of long-axis diameter to short-axis diameter ranging from 1.1 to 3, with a typical aspect ratio of about 2.5. In contrast, the aspect ratios of ablation zones achieved in ex vivo human liver by Jones et al. [Citation5] with 14.5 GHz MWA were less than 1.2.
However, higher frequencies are also more prone to undesired shaft heating, which may lead to teardrop-shaped ablation zones. At 10 GHz and above the teardrop shape is particularly prominent in all but the high-perfusion in vivo simulations. There are three mechanisms that can cause this heating along the feed line: unchoked surface currents, heat conduction from the active part of the antenna back towards the feed line, and ohmic losses in the conductors of the feed line. The simulated SAR patterns depicted in do not show an apparent EM tailing effect at any frequency, eliminating unchoked currents as a cause of shaft heating. Our simulation-based analysis of tissue heating due to ohmic losses in isolated coaxial cables () suggests that at the power level considered in the ablation simulations and experiments of (i.e. 25 W), cable heating in the largest diameter feed line is not significant enough to raise the tissue temperature to the 100 °C level seen in . Thus, heat conduction from the antenna back towards the feed line is the likely cause for the teardrop-shaped ablation zones in simulation.
As shown in , these simulated heating tails were less evident in the experimental ablations. It is not possible in CST software to model energy transport due to steam generation and condensation, and we do not consider the reduced EM absorption of ablated tissue. Therefore, simulated temperatures near the antenna can be unrealistically high, and such high temperatures would increase the rate of heat conduction from the antenna towards the cable feed point. Steam generation and transport along the tissue grain is clearly evident in the experimental ablation results of .
Our analysis of cable heating highlights the trade-offs between operating frequency, input power, and cable diameter in uncooled MWA systems. In particular, the results for many combinations of small cables and high operating frequencies indicate that input power should be limited to 5 W or 10 W to prevent undesired heating of the tissue along the feed line. Such low-power operation may constrain the ablation zone to smaller volumes. Results presented by Jones et al. [Citation5] from ex vivo ablations with 10 W of input power for 3 min using a 14.5 GHz antenna suggest that even at these low power levels significant volumes of tissue can be ablated. They achieved a mean ablation diameter of 17.5 mm.
These results present a compelling case for the use of higher frequency MWA. The chief clinical advantage of higher frequency MWA devices is that they may comprise smaller radiators that still efficiently couple power to surrounding tissue. A smaller antenna allows more flexible routing near organ boundaries or around major blood vessels. Further, smaller radiators permit the treatment of smaller tumours with less ablative heating of adjacent healthy tissue than would occur with lower frequency devices that have less compact SAR patterns. Higher frequency MWA devices also provide greater heating differentiation between cancerous and fatty tissues [Citation23]. Yoon et al. further suggested that high frequency MWA (specifically 18 GHz) is well-suited to treating small cancers without damage to adjacent fatty tissues. Our study supports this use by also demonstrating experimentally that ablation zones with clinically relevant dimensions can be achieved with 18 GHz MWA.
Conclusion
This paper presents numerical and experimental results that elucidate the qualitative and quantitative performance characteristics of MWA at frequencies beyond 2.45 GHz. Although microwave power deposition occurred in substantially smaller volumes as frequency increased from 1.9 to 26 GHz, the lateral dimensions of the resulting ablation zones were comparable in size over frequency for a constant input power and ablation duration. Increasing the frequency of operation led to ablation zones that were more compact in the longitudinal direction. This study underscores the limitations of using SAR distributions as a stand-alone metric for predicting ablation performance; at higher frequencies, thermal effects of heat conduction and steam transport play an important role in determining the characteristics of the ablation zone.
Higher frequency MWA carries with it a caveat of potential heating along the feed line as a result of cable losses that are higher than those typically encountered in lower frequency MWA. These cable heating effects were quantified through simulations of two-port coaxial cables embedded in in vivo porcine muscle tissue. Examination of the peak tissue temperature achieved as a function of operating frequency, input power, and cable diameter revealed that all three factors within the ranges studied significantly impact the heating of feed cables and therefore trade-offs need to be made among those variables to control antenna shaft temperatures.
Disclosure statement
This study was supported by the US National Science Foundation under grant ECCS-1406090 and the Wisconsin Alumni Research Foundation (WARF) Accelerator Program. The authors alone are responsible for the content and writing of this paper.
References
- Hodgson DA, Feldberg IB, Sharp N, Cronin N, Evans M, Hirschowitz L. Microwave endometrial ablation: development, clinical trials and outcomes at three years. Br J Obstet Gynaecol 1999;106:684–94.
- Kanaoka Y, Yoshida C, Fukuda T, Kajitani K, Ishiko O. Transcervical microwave myolysis for uterine myomas assisted by transvaginal ultrasonic guidance. J Obstet Gynaecol Res 2009;35:145–51.
- Qian P, Barry MA, Nguyen T, Ross D, Kovoor P, Mcewan A, et al. A novel microwave catheter can perform noncontact circumferential endocardial ablation in a model of pulmonary vein isolation: noncontact circumferential microwave catheter ablation. J Cardiovasc Electrophysiol 2015;26:799–804.
- Hines-Peralta AU, Pirani N, Clegg P, Cronin N, Ryan TP, Liu Z, et al. Microwave ablation: results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology 2006;239:94–102.
- Jones RP, Kitteringham NR, Terlizzo M, Hancock C, Dunne D, Fenwick SW, et al. Microwave ablation of ex vivo human liver and colorectal liver metastases with a novel 14.5 GHz generator. Int J Hyperthermia 2012;28:43–54.
- Kuang M, Lu MD, Xie XY, Xu HX, Mo LQ, Liu GJ, et al. Liver cancer: increased microwave delivery to ablation zone with cooled-shaft antenna – experimental and clinical studies. Radiology 2007;242:914–24.
- Liang P, Wang Y, Zhang D, Yu X, Gao Y, Ni X. Ultrasound guided percutaneous microwave ablation for small renal cancer: initial experience. J Urol 2008;180:844–8.
- Carrafiello G, Ierardi AM, Piacentino F, Lucchina N, Dionigi G, Cuffari S, et al. Microwave ablation with percutaneous approach for the treatment of pancreatic adenocarcinoma. Cardiovasc Intervent Radiol 2012;35:439–42.
- Sun YH, Song PY, Guo Y, Sheng LJ. Computed tomography-guided percutaneous microwave ablation therapy for lung cancer. Genet Mol Res 2015;14:4858–64.
- Zhou W, Zha X, Liu X, Ding Q, Chen L, Ni Y, et al. US-guided percutaneous microwave coagulation of small breast cancers: a clinical study. Radiology 2012;263:364–73.
- Hurter W, Reinbold F, Lorenz WJ. A dipole antenna for interstitial microwave hyperthermia. IEEE Trans Microw Theory Tech 1991;39:1048–54.
- Liang P-C, Lai H-S, Shih TT-F, Wu C-H, Huang K-W. Initial institutional experience of uncooled single-antenna microwave ablation for large hepatocellular carcinoma. Clin Radiol 2015;70:e35–40.
- Pisa S, Cavagnaro M, Bernardi P, Lin JC. A 915-MHz antenna for microwave thermal ablation treatment: physical design, computer modeling and experimental measurement. IEEE Trans Biomed Eng 2001;48:599–601.
- Ringe KI, Lutat C, Rieder C, Schenk A, Wacker F, Raatschen H-J. Experimental evaluation of the heat sink effect in hepatic microwave ablation. PLoS ONE 2015;10:e0134301.
- Cavagnaro M, Pinto R, Lopresto V. Numerical models to evaluate the temperature increase induced by ex vivo microwave thermal ablation. Phys Med Biol 2015;60:3287–311.
- Komarov VV. Numerical study and optimization of interstitial antennas for microwave ablation therapy. Eur Phys J Appl Phys 2014;68:10901.
- McWilliams BT, Schnell EE, Curto S, Fahrbach TM, Prakash P. A directional interstitial antenna for microwave tissue ablation: theoretical and experimental investigation. IEEE Trans Biomed Eng 2015;62:2144–50.
- Niemeyer DJ, Simo KA, McMillan MT, Seshadri RM, Hanna EM, Swet JH, et al. Optimal ablation volumes are achieved at submaximal power settings in a 2.45-GHz Microwave Ablation System. Surg Innov 2015;22:41–5.
- Saito K, Hosaka S, Okabe S, Yoshimura H, Ito K. A proposition on improvement of a heating pattern of an antenna for microwave coagulation therapy: introduction of a coaxial-dipole antenna. Electron Commun Jpn Part Commun 2003;86:16–23.
- Yang D, Bertram JM, Converse MC, O’Rourke AP, Webster JG, Hagness SC, et al. A floating sleeve antenna yields localized hepatic microwave ablation. IEEE Trans Biomed Eng 2006;53:533–7.
- Luyen H, Gao F, Hagness SC, Behdad N. Microwave ablation at 10.0 GHz achieves comparable ablation zones to 1.9 GHz in ex vivo bovine liver. IEEE Trans Biomed Eng 2014;61:1702–10.
- Hancock CP, Dharmasiri N, White M, Goodman AM. The design and development of an integrated multi-functional microwave antenna structure for biological applications. IEEE Trans Microw Theory Tech 2013;61:2230–41.
- Yoon J, Cho J, Kim N, Kim D-D, Lee E, Cheon C, et al. High-frequency microwave ablation method for enhanced cancer treatment with minimized collateral damage. Int J Cancer 2011;129:1970–8.
- CST AG. CST Studio Suite 2013. Framingham, MA: CST.
- Duck FA. Physical properties of tissue: a comprehensive reference book. London: Academic Press, 1990.
- Gordon RG, Roemer RB, Horvath SM. A mathematical model of the human temperature regulatory system – transient cold exposure response. IEEE Trans Biomed Eng 1976;23:434–44.
- IT’IS Foundation. Tissue Properties Database V1.0. 2011. Available from http://dx.doi.org/10.13099/ViP-Database-V1.0 (accessed 1 Jun 2016).
- Heisterkamp J, van Hillegersberg R, IJzermans JNM. Critical temperature and heating time for coagulation damage: implications for interstitial laser coagulation (ILC) of tumors. Lasers Surg Med 1999;25:257–62.