Abstract
Background: The multimodality approach has significantly improved outcomes for hepatic malignancies. Microwave ablation is often used in isolation or succession, and seldom in combination with resection. Potential benefits and pitfalls from combined resection and ablation therapy in patients with complex and extensive bilobar hepatic disease have not been well defined.
Methods: A review of the University of Louisville prospective Hepato-Pancreatico-Biliary Patients database was performed with multi-focal bilobar disease that underwent microwave ablation with resection or microwave only included.
Results: One hundred and eight were treated with microwave only (MWA, n = 108) or combined resection and ablation (CRA, n = 84) and were compared with similar disease-burden patients undergoing resection only (n = 84). The groups were comparable except that the MWA group was older (p = .02) and with higher co-morbidities (diabetes, hepatitis). The resection group had larger tumours (4 vs. 3.2 and 3 cm) but the CRA group had more numerous lesions (4 vs. 3 and 2, p = .002). Short-term outcomes including morbidity (47.6% vs. 43%, p = .0715) were similar between the CRA and resection only groups. Longer operative time (164 vs. 126 min, p = .003) and need for blood transfusion (p = .001) were independent predictors of complications. Survival analyses for colorectal metastasis patients (n = 158) demonstrated better overall survival (OS) (43.9 vs. 37.6 and 30.5 months, p = .035), disease-free survival (DFS) (38 vs. 26.6 and 16.9 months, p = .028) and local recurrence-free survival (LRFS) (55.4 vs. 17 and 22.9 months, p < .001) with resection only.
Conclusion: The use of microwave ablation in addition to surgical resection did not significantly increase the morbidities or short-term outcomes. In combination with systemic and other local forms of therapy, combined resection and ablation is a safe and effective procedure.
Introduction
Hepatic resection is the mainstay of treatment for patients with primary and metastatic hepatic malignancies. Most previous surgical reports involve patients with a small number of lesions confined to either the right or left lobe of the liver. However, over the past few years, improvements in pre-operative liver function assessment and multidisciplinary treatments have extended the indications for hepatic resection to include larger tumours and multiple foci of disease. These treatments include portal vein embolisation [Citation1,Citation2], systemic or hepatic arterial chemotherapy [Citation3] and ablative therapies.
While surgical resection remains the gold standard in the management of hepatic malignancies, interstitial ablative techniques have been increasingly utilised in the treatment of unresectable hepatic malignancies. Tumour ablation is the application of chemical or thermal therapies to a tumour to achieve eradication or substantial destruction. Ablative techniques have been developed to enable local control of tumours and cytoreduction while limiting damage to surrounding healthy parenchyma in a parenchymal sparing fashion compared to resection [Citation4–6]. Microwave ablation (MWA) is now the ablative technique of choice in the treatment of both primary and metastatic unresectable hepatic lesions [Citation6]. This method utilises electromagnetic waves to induce tumoural necrosis. A recent systematic review and meta-analysis demonstrates superiority of microwave ablation for larger liver tumours but overall showed a similar efficacy of RFA (radiofrequency ablation) vs. MWA [Citation7]. The potential benefits of MWA include consistently higher intratumoural temperatures, larger ablation volumes, faster ablation times, ability to use multiple probes, improved convection profile and optimal heating of cystic masses [Citation8–10]. It is difficult to compare treatment with hepatic resection to ablative therapy because many patients undergoing ablation have poor prognostic factors including but not limited to medical co-morbidities precluding resection, multiple bilobar tumours or unresectable disease.
MWA may enhance the feasibility of surgical extirpation. With a multimodal approach including MWA, hepatic resection and chemotherapy, improved survival may be achieved in patients who were initially deemed unresectable [Citation11]. Benefits from combined resection and ablation therapy in patients with complex and extensive bilobar hepatic disease have not been well defined. Also, there is no well-defined literature for liver resection with MWA compared to liver resections only with respect to outcomes. We sought to determine if the peri-operative complication rate for combined resection and ablation therapy, liver resection only or ablation only groups were different. In addition, we sought to analyse overall survival (OS) and disease-free survival (DFS) for these cohorts.
Methods
This study was a retrospective review of the University of Louisville, prospectively collected hepatobiliary patient database. Institutional review board approval was obtained prior to the initiation of this study. Consecutive patients with bilobar hepatic disease who underwent MWA from May 2004 to December 2015 were identified and included in this analysis. A similar group of patients who underwent liver resections only was selected for comparison, and propensity matched for tumour burden in the combined resection and ablation groups. Tumours were regarded as resectable if the anticipated hepatic parenchymal transection plane yielded a tumour-free margin while preserving adequate hepatic remnant. MWA was used to eradicate the lesions that were not amenable to surgical resection. Patients with extrahepatic metastases were excluded. Patients with prohibitive medical co-morbidities were not resected.
All adverse events were recorded per standards and terminology set forth by the Cancer Therapy Evaluation Events, Version 3.0. Adverse events were recorded during the hospital stay and for 30 days following each treatment. All events were graded according to a 5-point grading scale. Major complications were defined as a grade 3 complication or higher. Operative mortality was defined as death within 90 days of operation.
Patients who were treated with combined MWA and hepatic resection (combined resection and ablation: CRA) were compared to patients who were treated with MWA only and resection only using chi-square, logistic regression and t-test when appropriate. Statistical analysis was performed using SPSS version 20 software (IMB, Armonk, NY). Continuous variables were compared using the Student’s t-test and categorical variables were compared using chi-square test. Three group analyses were performed with one-way ANOVA and post hoc tests were performed using the Tukey LSD (least significant difference) and Bonferroni analysis. Survival was plotted using the method of Kaplan–Meier and compared using the log-rank test. A value of p < .05 was considered a significant difference. Survival (in months) was measured from the date of initial diagnosis until death. LRFS (local recurrence-free survival – liver recurrence) was calculated up to recurrence and censured at last follow-up if no recurrence was detected. DFS was similarly calculated up to any recurrence (local or distant). Cox regression was used to determine independent predictors of outcome. Multivariate analysis was performed with the Cox proportional hazards model.
Surgical technique
Microwave ablation was performed under continuous real-time ultrasound guidance, regardless of the access used for ablation. When close to other vital structures in this study they were dissected off or packed away before ablation. The probe position was confirmed using ultrasound in two perpendicular axes to prevent erroneous placement. Once ablation was started, monitoring was performed to ensure adequate ablation and a 1 cm or greater ablation margin. The ablation device used was based on physician preference and was the Evident 915 MHz microwave ablation system (Covidien, Interventional Oncology, Boulder, CO) with a 17/22 cm antenna with a 3.7 cm active tip and 2.45 GHz Acculis V generator (Acculis Ltd/Microsulis Medical Ltd, Denmead, UK) with a 14/19 cm Accu2ipMTA applicator with radiating tip. When performed with combined resections, the resections were often done first, allowing time to inspect the hepatectomy bed after the ablations were performed. Image-guided fusion technology (cross-sectional imaging fusion with intraoperative ultrasound) was used selectively as part of a pilot project and is at this time not routinely used in our practice. Intraoperative ultrasound was performed by the operating surgeons and in this study we did not use an ultrasound contrast agent. Standard hepatectomy techniques and low CVP (central venous pressure) anaesthesia were used. Pringle manoeuvre was not used routinely for hepatectomies.
Results
Two hundred and seventy-six patients from our single institution prospective Hepato-Pancreatico-Biliary Patients database were allocated to three groups based on procedure type. The MWA group constituted 108 patients (39.4%) and underwent microwave ablation only. The CRA group and resection group included 84 (30.3%) patients each. Access in the MWA group was either laparoscopic (60, 55%) or open (46, 42%). shows the demographics of each group. A laparoscopic approach was used in patients with tumours either abutting or surrounding viscus making percutaneous ablation dangerous, or in patients with concurrent resection of primary colon cancers. The median age in the MWA group was significantly higher at 65.1 (±11.8, standard deviation) compared to 56.7 (±10.8, standard deviation) and 59.9 (±12, standard deviation) years in the CRA group and resection group (p = .002). There was not a significant difference in the patients’ sex distribution, race or BMI between the three groups. The MWA group had sicker patients overall with a significantly higher number of diabetics (22% vs. 13% vs. 7%, p = .013) and patients with a history of hepatitis (13% vs. 1.2% and 0, p = .01) compared to the other groups. However, the CRA group had a significantly higher number of patients who reported tobacco dependence (26.6% vs. 42% vs. 24%, p = .023). Other co-morbidities were similar between the three groups.
Table 1. Patient demographics.
The majority of the lesions in both groups were metastatic colorectal (158, 57%), followed by other metastatic disease and hepatocellular carcinoma with a few cholangiocarcinomas, and periampullary cancers (). The three groups were statistically similar with respect to diagnosis of primary lesion (p = .104). With respect to tumour burden, there were a significantly higher number of liver lesions in the CRA group (4 vs. 2 and 3 in the MWA and resection groups, respectively, p = .002). However, the size of the largest lesion was significantly higher in the resection group (4 vs. 3 and 3.2 cm in the MWA and resection groups, respectively, p = .042). The median carcinoembryonic antigen (CEA) levels for metastatic colorectal cancer between the three groups were comparable (9.9 vs. 15.6 vs. 11.7, p = .225). For patients with colorectal primaries, there was no difference in distribution of primary disease, synchronous status and node positive colon/rectal primaries (). The distribution of liver lesions differed among the three groups with left lateral segment (segments 2 and 3) lesions seen more often in the CRA group (segment 2: 25 vs. 7, 9 and segment 3: 29 vs. 9, 11, p = .001). There were also a significantly higher number of patients in the CRA group (48, 57.1% vs. 40, 36.7% and 37, 44% in the MWA group and the resection group, respectively, p = .018) who had received chemotherapy at the time of surgery (). Overall, however, the number of patients who received chemotherapy (including in an adjuvant fashion) was similar across the groups.
Table 2. Tumour characteristics/adjuvant profile among groups.
Operative parameters across the three groups are listed in . Major resections [Citation12] (defined as greater than four segments) were similar statistically between the CRA group and resection only group (34, 42% vs. 46, 55%, p = .089). As was expected, the MWA group had significantly lower median procedure time (92 vs. 147 and 162 min in the CRA and resection only groups, p = .002), less median total blood loss (125 vs. 250 and 250 ml, p = .007) and hospital stay (2 vs. 7 and 7 days, p < .001) among the three groups (). The CRA and the resection only groups were statistically similar with respect to operative parameters. Total number of trisegmentectomies/extended hepatectomies (8, 9.6% vs. 7, 8.3%, p = .090), lobectomies/hemi-hepatectomies (26, 31% vs. 39, 47%, p = .57), non-anatomical resections (18, 21.2% vs. 6, 7.1%, p = .014) and the number of major resections defined as > = 4 segments (34, 42% vs. 46, 55%, p = .089) were similar across both groups. There was one peri-operative death in the CRA group. An R1 resection (microscopically positive margin) was seen in 18 patients (12.5%), and this was only assessed for the CRA group (n = 8, 11.1%) and resection only group (n = 10, 13.8%, p = .755).
Table 3. Operative parameters and outcomes among groups.
With respect to post-operative complications, the microwave only group had a lower overall complication rate (26 vs. 57/64 complications; p < .001), total number of patients with complications (15 vs. 40/38 patients; p < .001) as well as high-grade complications (5 vs. 16/12, p = .004). High-grade complications were defined as grade 3 or higher. However, there was no difference in the complication rates, high-grade complication rates and median grade of complications between the CRA and the resection only groups ().
A total of 91 patients (36.1%) experienced 147 complications. The median complication grade was 2 and high-grade complications were seen in 36 (13%). The common complications were ileus (16, 5.7%), atrial fibrillation (8, 3%), bile leak or biloma (7, 2.5%), wound infection/drainage (9, 3.2%), pneumonia (10, 3.6%) and anastomotic leak (6, 2.2%). There were 7 (2.5%) re-admissions within 90 days and this was seen primarily in the CRA and resection only groups (4.7% vs. 3%, p = .655). Five patients underwent re-operations, 2 in the resection group and 3 in the CRA group. The causes for re-operative surgeries were biliary fistula/biloma, retained drain and anastomotic leak. None of these re-operative complications were directly attributable to microwave ablation. There were no cases of bowel injury from ablation and the anastomotic leak and fistula were related to concurrent colon resection. There was one case of secondary infection of the ablation bed 2 weeks after surgery, which resolved with antibiotics. Analysis of factors affecting overall complications and high-grade complications were analysed. shows an evaluation of the factors predictive of complications. Univariate and multivariate analysis was performed to assess the effect of co-morbidities on complication rate, high-grade complication rates and hospital stay. Among co-morbidities tobacco use was significantly associated with increased complications (p = .015) and increased high-grade complications (p = .006) on univariate analysis. Other co-morbidities including prior major abdominal surgery, type of colon/rectal resection, cardiopulmonary disease and prior chemo or other ablative therapies did not impact complications. Tumour characteristics such as size of the largest lesions or higher number of lesions were also not predictive of increased complication rates (). Patients with complications were noted to have significantly longer hospital stays (p < .001). With respect to operative parameters, on univariate analysis, total operative time (p < .001), estimated blood loss (393 vs. 249 ml, p = .004), transfusion (p < .001), extent of hepatic resection (major liver resections, p = .001) and margin status were associated with a higher rate of complications and high-grade complications. A multivariate analysis was run using the parameters on univariate analysis with a value of p ≤ 0.2. The only variables that independently predicted a higher complication rate were longer operative time (F = 5.89, p = .028, 95% CI 0.001–0.003) and the need for blood transfusion (F = 5.89, p = .001, 95% CI 0.137–0.5).
Table 4. Factors affecting complications.
Follow-up
Median follow-up for this cohort was 30.1 months (range 14 days–155 months). Recurrence rate over the period of follow-up was 65% (n = 178) and was similar across the groups. Mortality rate over the follow-up period (which was different in the subgroups) was 56%. Survival analysis for the whole cohort was not done due to the mix of histologies affecting long-term outcomes differently even though the three groups were well matched.
Metastatic colorectal cancer ()
We also looked specifically at patients with colorectal metastasis (MCRC). They were well distributed across the three groups with 54, 50 and 54 patients in the MWA group, CRA group and resection only group, respectively. These three groups were well matched with respect to co-morbidities, except tobacco use, which was lower in the resection only group. In most respects they had similar characteristics as their respective larger cohorts, except that there was no difference between the three groups with respect to tumour size. The OS () was higher in the resection only group (median 43.9 months, 95% CI 29.6–58.1) compared to the MWA only group (37.6 months, 95% CI 25.1, 50) and the CRA group (30.5 months, 95% CI 23.4, 37, p = .035). Similarly, DFS was significantly higher () for the resection only group (median 38 months, 95% CI 7, 23) compared to the MWA only group (26.6 months, 95% CI 13.2, 40) and the CRA group (16.9 months, 95% CI 7.5, 23, p = .028). In addition, liver recurrence (LRFS) was also significantly higher () in the resection group (median 55.4 months, 95% CI 24.2, 66.1) compared to the MWA group (median 17.4 months, 95% CI 10.8, 22.9) and the CRA group (median 22.9 months, 95% CI 13.9, 30.8). Factors associated with a decrease in OS were a history of tobacco use (p = .05), higher number of liver lesions (p = .01) and positive margins (R1 resections, p = .05). Factors associated with worse DFS were higher number of lesions (p = .02), positive resection margins (p = .05) and higher number of positive nodes at resection of primary tumour (p = .03).
Figure 1. Kaplan–Meier survival curve: comparing overall survival between the three groups. Note: CRA: combined microwave ablation and resection; MWA: microwave ablation.
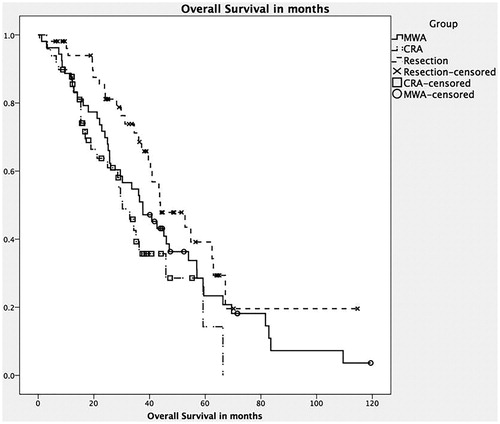
Figure 2. Kaplan–Meier survival curve: comparing disease-free survival between the three groups. Note: CRA: combined microwave ablation and resection; MWA: microwave ablation.
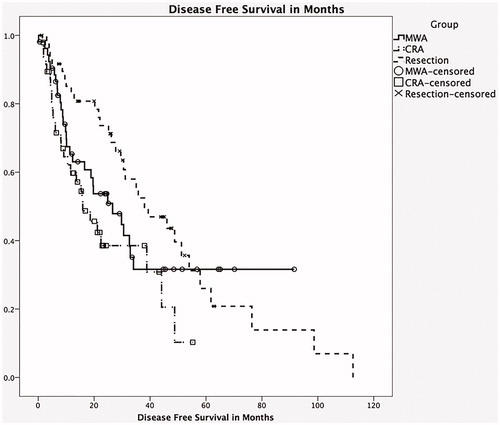
Table 5. Operative parameters and outcomes, MCRC only.
Discussion
Over the past couple of decades, a multidisciplinary approach to primary and metastatic liver lesion has significantly changed their management [Citation13]. There has been a gradual increase in local ablative technologies, which have often been used in isolation to improve outcomes in these hepatic malignancies [Citation11].
We identified 193 patients who underwent microwave ablation only or microwave ablation with resection and compared them to 84 consecutive patients with similar tumour burden and resection technique. Most studies of combined multimodality treatment were smaller [Citation14–18] and used RFA as the primary method of ablation vs. microwave in our study [Citation19]. The use of different interstitial ablative technologies within the literature in combination with resection made comparisons difficult [Citation14,Citation20,Citation21]. In reviewing the published literature, this is one of the largest series of combined resection and interstitial ablation and the largest with respect to use of microwave ablation [Citation22]. This study also had a longer mean follow-up, leading to more robust survival and recurrence analyses.
We analysed the role of multimodality local therapy in the management of liver lesions. This study cohort had a median age of 62 years, which is comparable to prior studies of combined local therapy [Citation23]. The use of three groups with parity to contrast the operative parameters, outcomes and long-term follow-up was unique to this study. The ablation only group was noted to be significantly older and had a higher number of co-morbidities. This clearly reflects a clinical bias to perform less invasive procedures in older patients with significant medical co-morbidities and microwave ablation is a good alternative in these patients. The three groups were well matched with respect to tumour biology and origin (including primary, synchronous disease, node positive disease and CEA levels).
The CRA group had a higher number of liver lesions and the resection only group had larger lesions. This again is a reflection of using combined resection and ablation to treat extensive bilobar lesions that were unfit for either resection or ablation only. Often it is the number and distribution of liver lesions rather than the size alone, which makes them unresectable, and therefore the larger lesions are represented disproportionately in the resection only group. Left lateral segment lesions were seen more commonly in the CRA group. This is a reflection of the use of combined resection in a single or two-stage fashion in the CRA group. For more superficial lesions or lesions with easily accessed segments such as segments 2 and 3, the loss of parenchyma associated with a resection is comparatively small and a resection of these along with an ablation of a central tumour is the best parenchymal sparing approach.
Microwave ablation only is a much less invasive procedure; it was, therefore, not surprising that they had significantly lower operative times, blood loss, hospital stay, complication rates and high-grade complication rates. The resection and CRA groups, however, were similar in these respects as well as with respect to overall complication rates. Comparing only colorectal metastasis patients showed similar results. The morbidity for this study of MWA at 14.8% compares favourably with other studies reporting up to a 35% complication rate [Citation24]. Most of our microwave ablations alone were done laparoscopically under direct real-time ultrasound guidance. The overall recurrence rate at 29 months of 36% is comparable to both our study comparison cohorts as well as historic data. This once again reiterates the role of microwave ablation in local control for unresectable bilobar tumours.
There was one peri-operative death in the CRA group of a 71-year-old male with metastatic colorectal cancer who underwent a concurrent colon resection, after which he had an anastomotic leak. Longer operative time and higher blood loss, which is often a corollary for more complicated procedures, were independent predictors of complications across the groups. This study is also unique with its long follow-up in patients who underwent microwave ablation with a median follow-up of 30 months which is higher than most similar studies [Citation18]. Survival analysis was only performed on the colorectal metastasis cohort. The resection only group demonstrated an improved DFS, LRFS and OS compared to the other two groups. Firm conclusions about the true superiority of resection only cannot be made from this study given the fact that the ablation groups were older and had more co-morbidities. The median OS after microwave ablation for colorectal metastasis in this study was 37.6 months, which is comparable, if not better than most reported series with microwave ablation only [Citation18,Citation24].
The main weakness of this study is its retrospective nature. However, given the relative lacuna in the existing literature, we believe this is an important cohort of patients to look at critically. Another weakness is the long duration of the study with its attendant changes in technology and access.
Microwave ablation is a safe and efficacious alternative in patients with unresectable disease, due to tumour burden or medical co-morbidities. This study demonstrates that ablation and resection in well-selected patients can be done concurrently without any increase in complication rates or worse outcomes than resection only, therefore offering this subgroup of unresectable patients an efficacious alternative. Resectable surgical resection still offers OS, DFS and LRFS.
Conclusion
The use of microwave ablation in addition to surgical resection did not significantly increase the morbidities or short-term outcomes. Surgical resection is the treatment of choice and MWA is a useful adjunct for local control in patients who are otherwise unresectable either alone or in combination with resection. In patients with extensive bilobar disease, performing a concurrent ablation with planned resection has the advantages of fewer procedures, greater visualisation and easier access in case of ablation-related complications.
Combined resection and interstitial ablation is safe and effective in providing good local control in otherwise unresectable liver disease. This study has demonstrated the safety of this procedure when compared to resection only, thus, making a strong case for concurrent ablation and resection vs. subsequent ablation.
Disclosure statement
The authors report no conflicts of interest.
References
- Azoulay D, Castaing D, Krissat J, et al. (2000). Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg 232:665–72.
- Jaeck D, Oussoultzoglou E, Rosso E, et al. (2004). A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg 240:1037–49; discussion 1049–51.
- Bismuth H, Adam R, Lévi F, et al. (1996). Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 224:509–20; discussion 520–22.
- Pearson AS, Izzo F, Fleming RY, et al. (1999). Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg 178:592–99.
- Wood TF, Rose DM, Chung M, et al. (2000). Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol 7:593–600.
- Boutros C, Somasundar P, Garrean S, et al. (2010). Microwave coagulation therapy for hepatic tumors: review of the literature and critical analysis. Surg Oncol 19:e22–e32.
- Facciorusso A, Di Maso M, Muscatiello N. (2016). Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia 32:339–44.
- Simon CJ, Dupuy DE, Mayo-Smith WW. (2005). Microwave ablation: principles and applications. Radiographics 25(Suppl 1): S69–S83.
- Martin RC, Scoggins CR, McMasters KM. (2007). Microwave hepatic ablation: initial experience of safety and efficacy. J Surg Oncol 96:481–86.
- Wright AS, Lee FT Jr, Mahvi DM. (2003). Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann Surg Oncol 10:275–83.
- Sindram D, Lau KN, Martinie JB, et al.(2010). Hepatic tumor ablation. Surg Clin North Am 90:863–76.
- Reddy SK, Barbas AS, Turley RS, et al. (2011). A standard definition of major hepatectomy: resection of four or more liver segments. HPB (Oxford) 13:494–502.
- Nesbitt C, Glendinning RJ, Byrne C, et al. (2007). Factors that influence treatment strategies in advanced colorectal cancer. Eur J Surg Oncol 33(Suppl 2):S88–S94.
- Kornprat P, Jarnagin WR, DeMatteo RP, et al. (2007). Role of intraoperative thermoablation combined with resection in the treatment of hepatic metastasis from colorectal cancer. Arch Surg 142:1087–92.
- Fioole B, Jansen MC, van Duijnhoven FH, et al. (2006). Combining partial liver resection and local ablation of liver tumours: a preliminary Dutch experience. World J Surg Oncol 4:46.
- Bilchik AJ, Wood TF, Allegra D, et al. (2000). Cryosurgical ablation and radiofrequency ablation for unresectable hepatic malignant neoplasms: a proposed algorithm. Arch Surg 135:657–62; discussion 662–64.
- Elias D, Goharin A, El Otmany A, et al. (2000). Usefulness of intraoperative radiofrequency thermoablation of liver tumours associated or not with hepatectomy. Eur J Surg Oncol 26:763–69.
- Leung U, Kuk D, D’Angelica MI, et al. (2015). Long-term outcomes following microwave ablation for liver malignancies. Br J Surg 102:85–91.
- de Jong MC, van Vledder MG, Ribero D, et al. (2011). Therapeutic efficacy of combined intraoperative ablation and resection for colorectal liver metastases: an international, multi-institutional analysis. J Gastrointest Surg 15:336–44.
- Pawlik TM, Izzo F, Cohen DS, et al. (2003). Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol 10:1059–69.
- de Jong KP, Wertenbroek MW. (2011). Liver resection combined with local ablation: where are the limits? Dig Surg 28:127–33.
- Lloyd DM, Lau KN, Welsh F, et al. (2011). International multicentre prospective study on microwave ablation of liver tumours: preliminary results. HPB (Oxford) 13:579–85.
- Gleisner AL, Choti MA, Assumpcao L, et al. (2008). Colorectal liver metastases: recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection-radiofrequency ablation. Arch Surg 143:1204–12.
- Stattner S, Jones RP, Yip VS, et al. (2013). Microwave ablation with or without resection for colorectal liver metastases. Eur J Surg Oncol 39:844–49.
