Abstract
Purpose: To compare local tumour control and survival rates in patients with liver metastases treated with microwave ablation (MWA), using either a low-frequency (LF) (915 MHz) or high-frequency (HF) system (2.45 GHz).
Materials and methods: The retrospective study included 221 patients (mean age: 61.7 years) with 356 malignant hepatic lesions. Ninety-four patients with 133 lesions underwent LF-MWA between September 2008 and February 2011, while 127 patients with 223 lesions were treated with HF-MWA between March 2011 and July 2013. MRI was performed after 24 h from each procedure and at 3, 6, 9, 12, 18 and 24 months post-ablation. Both groups were compared with the Fisher’s exact test. Survival rates were calculated using the Kaplan–Meier test.
Results: The mean initial ablation volume of LF-MWA was nearly half of HF-MWA (19.1 mL vs. 39.9 mL). The difference in volume between both systems was significant (p < .0001). With LF-MWA, 39/133 lesions (29.32%) progressed at follow-up while the number of lesions which progressed with HF-MWA was 10/223 (4.5%). The mean time to progression was 5.03 and 5.31 months for the lesions treated with LF-MWA and HF-MWA, respectively. The difference between both systems was significant (p = .00059). The 1-, 2- and 4-year overall survival rates for curative indication were 98.9%, 95.7% and 82.9% for LF-MWA, respectively, and were 100%, 97.6% and 92.9% for HF-MWA, respectively. The difference in survival rates was not significant (p > .05).
Conclusion: Both LF- and HF-MWA systems are effective treatment options for oligonodular liver malignant lesions, but significantly higher ablation volumes, longer time to progression and lower progression rates were observed in HF-MWA.
Introduction
For decades, surgical resection was the only available treatment option for hepatic malignancy. Newly introduced treatment modalities, with fewer complications and extended treatment spectrum, have been suggested. In recent years, several ablative techniques, such as radiofrequency ablation (RFA), laser-induced thermo-ablation (LITT), irreversible electroporation, high intensity focused ultrasound, microwave ablation (MWA) and cryotherapy, have been established [Citation1–4]. These ablative procedures are associated with a lower peri-procedural risk compared to surgical interventions and they also present an alternative treatment for patients with contraindications for surgery. In addition, some ablation techniques like microwave can be combined with the surgical approach or performed through a laparoscopic or percutaneous route.
The frequency has a strong influence on the heating rates in the tissue and microwave penetration [Citation5]. However, the present data on the exact role of ablation frequency is controversial. Only a few studies in the literature addressed the effect of frequency on the outcome following MWA. Some studies suggested that ablation with low frequencies (LF) provides larger coagulation zones than high frequencies (HF) in the liver, because a frequency of 915 MHz would provide deeper field penetration and a greater volume of microwave heat distribution [Citation6]. In other studies, ablation induced with a LF did not achieve similar large ablation zones [Citation7–9]. In an experimental study, it was shown that larger ablation areas can be achieved using a 2.45 GHz ablation device [Citation10]. A clinical study comparing both systems based on a retrospective analysis of 48 patients showed that HF-MWA achieves more predictable and faster ablation than LF-MWA [Citation11].
Owing to the controversy in the literature regarding the effect of frequency on the outcome of MWA, we performed the current study on a relatively large number of patients to comparatively evaluate the local tumour control and the survival rates in patients with liver malignancy treated with either a LF-MWA (915 MHz) or a HF-MWA (2.45 GHz) system.
Materials and methods
Patients and tumour features
The ethical committee approved the current retrospective study and informed consent was obtained from all patients before treatment. Between September 2008 and July 2013, a total of 221 patients (mean age: 61.7 ± 7.2 years, range: 26–89 years, 111 males and 110 females) with liver malignancy underwent MWA. In this study we included patients with primary and secondary liver malignant tumours, recurrent liver metastases after partial liver resection, patients with locally non-resectable tumours and patients who had general contraindications for surgery or had refused the surgical treatment option. summarises the spectrum of origin of the different malignant lesions treated in the current study.
Table 1. Distribution of primary tumours.
A total number of 356 malignant hepatic lesions were ablated in 257 sessions. All patients were treated with percutaneous MWA systems, with internally cooled antennas, under computed tomography (CT) guidance, by two interventional radiologists with more than 5 and 12 years of experience in hepatic ablation procedures at the beginning of the study. The patients were assigned to treatment based on recommendations of an interdisciplinary tumour board. Between September 2008 and February 2011, patients were treated with the LF-MWA system (n = 94, having 133 malignant hepatic lesions) and between March 2011 and July 2013, patients were treated with the HF-MWA system (n = 127, having 223 malignant hepatic lesions).
The inclusion criteria were the general major indications for MWA: (1) primary tumour control in case of metastases; (2) a tumour with a diameter ≤5 cm; (3) a maximum of five tumours; and (4) no remaining surgical treatment options. The exclusion criteria were: (1) a tumour size >5 cm in diameter; (2) more than five tumours; (3) INR (international normalised ration) >1.5 or platelets’ count <70 000/μL; (4) significantly increased inflammatory markers; and (5) circulatory unstable patients.
MWA therapy
The LF-MWA device (Evident™, Covidien, Dublin, Ireland) used in this study generated microwaves with a frequency of 915 MHz and a maximum output power of 45 W. The available shaft lengths were 12, 17 or 22 cm, with an external diameter of 13 gauges (G) (2.4 mm) and an active applicator tip of 3.7 cm. The manufacturer’s recommendations were followed in all patients, provided that the patient could tolerate the entire duration of ablation. The mean power output range was 46.31 ± 9.48 W.
The HF-MWA system (AMICA™, Hospital Service, Aprilia, Italy) operated at a frequency of 2450 MHz and had a maximum power of 100 W. The antennas had an external diameter of 11–13 G. The active tip of the applicator was 2.0 cm wherein the distal 2.5 cm were not water-cooled. The mean power output range was 70.83 ± 24.23 W. The manufacturer’s recommendations were also followed in all patients, provided that the patient could tolerate the entire duration of ablation.
All procedures were performed under CT guidance (Somatom-Sensation 64, Siemens Healthcare, Forchheim, Germany) with the following parameters: 5 mm collimation, 30 mAs, 120 kV and 6 mm section thickness. The ablation time was recorded. A stepwise manner of antenna introduction was used to ensure an optimal area of overlapping ablation. This was performed to ensure ablation of a sufficient safety margin around the ablated tumours. The microwave antennas were introduced directly using a single puncture and without using a guiding needle. The location of the antennas was guided by CT for optimised positioning of the radiating zone of the antennas within the tumour. While positioning, the elliptic shape of the induced ablation area was taken into consideration, with the widest zone of ablation being at the level of the centre of the active antenna zone of the applicator. CT imaging of the tumour was periodically done, to check the position of the antenna and to exclude the occurrence of complications during the procedure. To prevent seeding of malignant cells during removal of the antenna and to induce local haemostasis of the electrode track, needle track ablation was routinely performed at the end of the procedure. After exclusion of complications, the patient was monitored for 8 h while the vital signs were checked continuously. If no complications occurred, patients were discharged from the hospital on the same day.
Imaging and post-interventional follow-up protocol
The follow-up studies were performed with a 1.5-T MRI system (Magnetom Avanto, Siemens, Erlangen, Germany). The assessment of the immediate treatment response was performed 24 h after ablation to demonstrate treatment success (defined as lack of nodular enhancement in the location of the ablated tumour – a circumferential enhancement around the ablation zone with oedema observed in the 24-h MRI scan was considered to be reactive), evaluate further morphologic changes and rule out delayed post-ablation complications. Complications which affected the normal course of treatment were recorded. Additional follow-up examinations were performed 3, 6, 9 and 12 months after ablation and in 6-month intervals thereafter. For three-dimensional acquisition, un-enhanced T1- and T2-weighted sequences in the transverse and coronal slice orientation were performed. In addition, 6 or 8 mm thickness and T1-weighted Gradient Echo (GE) sequences in the transverse and sagittal orientation were acquired. Then a transverse-dynamic contrast-enhanced series was acquired. Preparation of post-contrast T1-weighted sequences in the transverse slice orientation and T1-weighted GE sequences in the transverse and sagittal orientation were performed. Thus, the following sequences were performed: True FISP, HASTE, TSE, FLASH 2D, in-phase and opposed phase T1, FLASH 2D dynamic. The following parameters were assessed: treatment success, volume of ablation and local tumour response. A successful ablation was defined as a uniform hypo-intensity without enhancement in the ablation zone. The target tumour had to be completely covered by the ablation zone that included at least a 5–10 mm margin all around the expected tumour margin [Citation12]. The comparison of the volume was based on the pre-ablation imaging studies performed before the ablation procedure (before antenna insertion).
Study design and statistical analysis
Radiologic evaluation of the pre- and post-procedural MRI was performed in consensus by two senior radiologists. The determining factor for successful or failed ablation of the tumour was the pattern of contrast enhancement. The presence of an irregular focal area of contrast enhancement was considered to be a sign of residual or recurrent disease. On the other hand, the presence of a thin symmetric rim of peripheral contrast enhancement, that is, less than 5 mm (none progressing), observed up to 6 months after ablation, was considered to be a sign of benign peri-tumoural enhancement. This was based on the parameters described in previous studies [Citation13].
Both MWA systems were compared using Fisher’s exact test. Logistic regressions for correlated data were used to assess the dependence of ablation success on all recorded tumour characteristics, numeric (including tumour size and location) and categorical (origin of the primary tumour) test whether combinations of two or more tumour traits represented significant independent predictors of successful ablation. The difference between lesions <3 cm and those measuring 3–5 cm regarding the volume change after ablation, the incidence of recurrence and the overall survival for each system was tested using the two-sample t-test. Significance was set at p = .05. Overall and progression-free survival rates were evaluated according to the end result of ablation and the nature of the primary tumour and also compared between the two MWA generators. Survival curves were evaluated by the Kaplan–Meier method. The mean survival rate for all patients started after the first MWA treatment. Statistical software (Bias for Windows, Epsilon Verlag, Darmstadt, Germany) was used. Complications were recorded and classified according to the Clavien–Dindo Classification of Surgical Complications [Citation14].
Results
MWA treatment
Overall, 126 patients with one tumour, 67 patients with two tumours, 19 patients with three tumours, 6 patients with four tumours and 3 patients with five tumours were treated. The mean number of tumours per patient was 1.65 ± 0.26 (range: 1–5). The mean maximal diameter of the metastases was 19.09 mm ±1.49 with a mean volume of 3.16 mL (SD: ±1.2 mL, range: 0.33–60.53 mL) and a mean ablation time of 18.76 min (SD: ±10.98 min, range: 1–50 min) for LF-MWA and 18.6 mm ±1.24 with a mean volume of 7.48 mL (SD: ±2.57 mL, range: 0.26–163.63 mL) and a mean ablation time of 8.28 min (SD: 3.67 min, range: 1–20 min) for HF-MWA. There were no statistically significant differences between the number and initial size of the metastases treated with the HF- or LF-MWA system (p = .481). Most of the ablated tumours were located in liver segments 7 (LF-MWA n = 47, HF-MWA n = 82) and 8 (LF-MWA n = 43, HF-MWA n = 85). Regarding the occurrence of complications according to the Clavien–Dindo Classification of Surgical Complications, Grade I complications in the form of right shoulder pain were observed in 8.7% (n = 11) of the patients treated with the HF-MWA system. None of the patients treated with the LF-MWA system experienced right shoulder pain. The pain was managed conservatively and responded well to analgesics. Other Grade I complications included small subcapsular haematoma reported in 3.2% of patients (n = 7). None of the patients included in the current study experienced Grade II, Grade III, Grade IV or Grade V complications.
Overall local tumour control
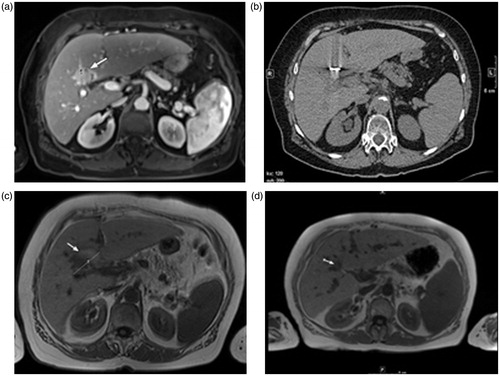
In LF-MWA (), a complete successful ablation was achieved in 120/133 (90.2%) tumours, with failure related to tumour residue in 13 (9.8%) tumours.
Figure 1. Transverse images of a 75-year-old woman with liver metastasis of colorectal cancer origin treated with low-frequency microwave ablation (LF-MWA). (a) MR image (T1 weighted) obtained 2 weeks before MWA shows a single metastasis (arrow) in segment 4 with a maximum diameter of 31.5 mm and a volume of 10.39 mL. (b) Computed tomography (CT) image obtained during MWA (30 min, 45 W). (c) MR image (T1 weighted) obtained 24 h after successful treatment shows typical coagulation (volume: 20.23 mL, max. diameter: 44.3 mm). (d) MR image obtained 48 months (volume: 5.11 mL) after MWA shows area of scarring in the ablation zone with no progress in size.
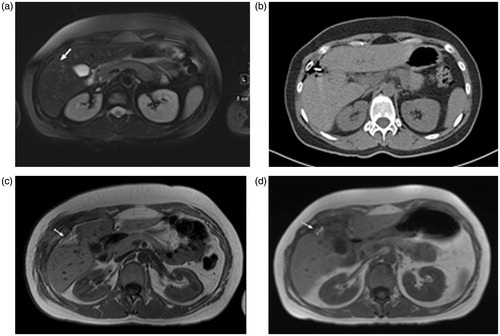
While in HF-MWA (), a complete successful ablation was achieved in 213/223 (95.5%) tumours, with failure related to tumour residue in 10 (4.5%) tumours.
Figure 2. Transverse images of a 40-year-old man with liver metastasis from colorectal cancer treated with high-frequency microwave ablation (HF-MWA). (a) MR image (T2 weighted) obtained 4 weeks prior to MWA shows metastasis (arrow) in segment 5 with a maximum diameter of 17.3 mm and a volume of 9.07 mL. (b) Computed tomography (CT) image obtained during MWA (5 min, 76 W). (c) MR image (T1 weighted) obtained 24 h after successful treatment shows typical coagulation (volume: 23.19 mL, max. diameter: 9.02 mm). (d) MR image (T1 weighted) 48 months (volume: 2.18 mL) after MWA shows area of scarring and no progression of the tumour.
The difference between both systems was statistically significant (p < .001). There was no statistically significant difference regarding the occurrence of recurrence between lesions measuring <3 cm and those measuring 3–5 cm in both the LF-MWA group (p = .16) and the HF-MWA group (p = .17).
Effect of tumour size on ablation results
The tumour size had no influence on ablation results. For LF-MWA, the maximal diameter of recurrent metastases was 11.82 mm (SD: ±5.47 mm, range: 0.68–20.35 mm) with a mean volume of 2.87 mL (SD: ±1.31 mL, range: 0.44–17.2 mL) which was lower than the mean of all ablated tumours (19.09 mm; 3.16 mL). The same observation was noted for HF-MWA and the maximal diameter of recurrent metastases was 17.93 mm (SD: ±8.35 mm, range: 4.42–30.26 mm) with a mean volume of 3.65 mL (SD: ±1.28 mL, range: 1.06–10.3 mL) which was lower than the mean of all ablated tumours (18.6 mm; 7.48 mL).
Influence of tumour location on ablation results
Most of the ablated tumours were located in liver segments 7 and 8 (40.6% for LF-MWA and 44.39% for HF-MWA). In this area HF-MWA showed a significantly higher success rate (97/99) than LF-MWA (47/54) as shown by the logistic regression analysis (p = .0097). For recurrent cases, none of the recurrent lesions was in the proximity (less than 1 cm) of a major hepatic vessel.
Influence of primary tumour origin on ablation results
For LF-MWA with a 96.93% tumour response rate, metastases from breast cancer showed the highest success rate after ablation. The success rate of hepatocellular carcinoma (HCC) lesions was 79.49% and it was 89.29% for colorectal carcinoma metastases. The differences in tumour response rate were statistically not significant (p = .328).
For HF-MWA there was no significant influence on ablation results depending on the primary tumour origin. Metastases of colorectal carcinoma responded with a 93.22% success rate, 92.16% for HCC and 93.33% for breast cancer. The logistic regression analysis showed that the primary tumour origin was not a statistically significant factor correlating with the ablation result (p = .159).
Volumetric changes of the ablated area
The mean initial ablation volume was 19.09 ± 7.92 mL (range: 3.16–90.02 mL) for LF-MWA. This was approximately half the volume achieved by HF-MWA with 39.87 ± 4.17 mL (range: 2.71–103.52 mL). The difference between both systems, regarding the achieved ablation volume, was statistically significant (p < .0001). Nevertheless, the mean delivered energy for LF-MWA was significantly higher (56 652.0 ± 5129.23 joules, range: 2700–324 000 joules) compared to HF-MWA (36 028.66 ± 4285.32 joules, range: 2700–577 500 joules). However, the two ablation systems showed no statistically significant differences in volume decrease (). Furthermore, there was no statistically significant difference regarding the volume change (decrease) at follow-up between lesions measuring <3 cm and those measuring 3–5 cm in both the LF-MWA group (p = .70) and the HF-MWA group (p = .86).
Table 2. Coagulation volume in millilitres and volume decrease after ablation in percentages in parentheses.
Progression rate and mean time to progression of the ablated area
With LF-MWA, 15.79% (21/133) of the tumours progressed after 3 months, 9.02% (12 tumours) after 6 months and 4.51% (6 tumours) after 12 months. Thus, a total of 29.32% (39 tumours) progressed with a mean time to progression of 5.03 months.
With HF-MWA, 7.62% (17/223) of the tumours progressed after 3 months, 5.38% (12 tumours) after 6 months and 1.35% (3 tumours) after 12 months. Therefore, a total of 14.35% (32 tumours) progressed with a mean time to progression of 5.31 months.
The progression rate of HF-MWA was significantly lower than that of LF-MWA (p = .00059).
Survival rate
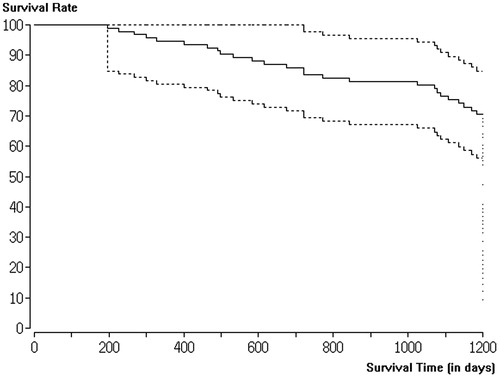
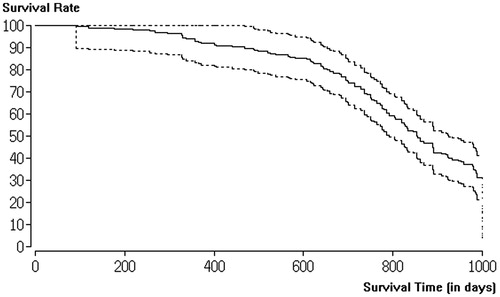
The overall survival rate of the LF-MWA group within the 1-year follow-up period was 98.93% (93/94), while at 2 years the survival rate was 95.74% (90/94) and the 4-year survival rate was 82.98% (78/94) for curative indication.
In the HF-MWA group, the overall survival rate within the 1-year follow-up period was 100%, while the 2-year survival rate was 97.63% (124/127) and the 4-year survival rate was 92.91% (118/127) for curative indication. The difference between the two groups for all indications regarding survival was not statistically significant (p > .05). In addition, there was no statistically significant difference regarding the overall survival of patients with lesions measuring <3 cm and those with lesions measuring 3–5 cm in both the LF-MWA group (p = .67) and the HF-MWA group (p = .13).
A Kaplan–Meier curve of the survival rate within the follow-up period of this study is shown in (LF-MWA) and 4 (HF-MWA). Significantly, more patients with metastases in liver segments 7 and 8 died compared to patients with metastases in other segments, regardless of the MWA system used (p < .05). Higher survival rates were observed in patients with tumour-free states after successful ablation compared to failed ablations. Neither the number of treated liver metastases per patient nor the patient sex had a significant impact on survival (p > .05).
Discussion
The preferred treatment for resectable primary and secondary liver tumours has been surgical resection for decades [Citation15]. However, a considerable number of patients, with hepatic tumours, show unresectable lesions due to anatomic, technical or patient-related rationales [Citation16]. Studies showed that in only 30–35% of patients with HCC and 20–25% of patients with hepatic metastases from colorectal carcinoma, surgery can be performed [Citation17]. The number of treatment options for patients with unresectable liver tumours is increasing [Citation18], including systemic chemotherapy, ablative techniques, hepatic artery-directed therapies, external beam radiation therapy and isolated liver perfusion [Citation17,Citation19].
MWA is a comparatively new method with the ultimate goal of prolonging survival and allowing adequate tumour control [Citation20]. MWA was first used percutaneously in 1986 as an adjunct to liver biopsy [Citation21]. Since that time, MWA has been used for ablation of tumours and tissue in the treatment of many conditions including liver metastases, renal cell carcinoma, adrenal malignant carcinoma, pulmonary metastases, abdominal tumours and other unmanageable tumours with resection [Citation20,Citation22,Citation23].
In comparison to the most widely used RFA technique [Citation24], MWA has been shown to offer several advantages: larger volumes of ablation, shorter treatment time, higher temperatures and the possibility to use multiple antennas [Citation25]. The clinical intentions of ablations are curative, palliative or bridging to transplantation [Citation26]. A relevant advantage of ablation therapy is the ease of repeated intervention [Citation26], which may explain why studies showed similar 5-year survival for ablation (48%) and resection (52%) [Citation27]. Peri-interventional safety may also be improved with MWA, as previous studies reported no intra-procedural deaths and no required blood transfusions during MWA. The only reported complications were non-severe ones including local pain, skin burns and slight subscapular bleedings [Citation28,Citation29]. In addition, we used internally cooled antennas to decrease tissue charring, facilitate microwave energy deposition and avoid collateral damage [Citation22].
The size of the ablation index tumour was primarily decisive for ablation results. Also, the overall liver tumour volume is an important factor regarding success. The lowest local recurrence rates were reported for <3 cm tumours [Citation30]. Our study also showed that for tumours below this recommended volume, the tumour size had no statistically significant influence on ablation results. For both MWA systems, the mean volume of the ablation index tumour was lower for recurrent metastases compared to the volume of the successfully ablated tumours. Analysis of the 5-year survival in several prior publications showed the best results in patients with small disease [Citation30].
To preclude the remaining micro-metastases in the surrounding tissue [Citation31] in a successful ablation, the size of coagulation should be substantially larger than the target tumour, and an adequate ablation zone should be exposed with an including ablative margin of 5–10 mm around the expected tumour margin [Citation32]. To achieve this ablation margin, it is important to correctly position the antennas because the liver metastases are usually less well perfused than the surrounding liver – it is easier to ablate the tumour edge than to ablate the liver around the metastasis [Citation33]. The exact role of frequency during MWA was discussed inconsistently in prior studies. Some of them reported bigger coagulations provided by 915 MHz systems [Citation6], but other authors did not confirm this [Citation7,Citation8]. In our study, we observed significant differences in coagulation volume induced by the different high- and low-frequency systems.
The reason for this observation might be attributed to the greater power deposition in proximity to the antenna, as well as greater role of thermal conduction as suggested by Curto et al. [Citation10]. Similar to other authors, we found that LF-MWA requires a longer ablative duration to achieve the focal destruction of the ablation index tumour and create the ablative margin, which is required for recurrence prevention [Citation34]. With HF-MWA, coagulation diameters larger than 5.5 cm can be achieved in less than 8 min, which engenders shorter treatment duration and decreases total treatment time [Citation35].
Previous studies have shown that MWA is nearly always technically successful as confirmed by immediate post-ablation contrast-enhanced ultrasound (CEUS) or CT [Citation19,Citation23,Citation36,Citation37], which was also the case in our study. Tumour recurrence has been reported in various prior studies in 6–65% of the ablated tumours, mostly with colorectal cancer as the primary tumour [Citation19,Citation23,Citation28,Citation37]. This substantial discrepancy may be related to the number of treated patients – some studies only treated 6 patients with MWA [Citation36]. Also, the treatment set-up is relevant as there may be differences between percutaneously performed MWA after laparotomy and with resection. Some studies showed no recurrences at least 3 months after ablation, but the reported median time span from ablation to recurrence was 5 months. As mentioned earlier in this study, 29.32% of tumours showed a local progression with LF-MWA as opposed to 14.35% of the cases with HF-MWA. Colorectal cancer patients treated with LF-MWA developed new metastases in 6.67% of the cases, with HCC in 21.62%. HF-MWA patients with colorectal cancer developed new metastases in 10% of the cases, with HCC in 10.27%.
The results of our study indicate that the primary tumour origin seems to have no influence on the outcome. Furthermore, there is no resistance limitation of treatment dependent on histology reported in the literature. In addition, the location of the ablation index tumour is not a statistically significant factor for local tumour control. Other authors only found recurrences in liver segments 4–8 – this may be caused by the MWA treatment indications concerning non-resectability [Citation19]. We also found that most recurrences were in segments in which surgery is more complicated to perform.
This current study was limited by several factors: non-randomised non-controlled design, the two systems were set – due to technological progress – into operation at different times (LF-MWA was used earlier than HF-MWA, so there is older data) and the heterogeneity of the metastases. Beside these points, this study illustrates the potential value of MWA and includes relatively common metastatic lesions.
In conclusion, LF- and HF-MWA therapies are efficient therapeutic tools for treatment of hepatic malignant lesions. The HF-MWA induces larger ablation volumes than the LF-MWA. The progression rate for patients treated with HF-MWA is significantly lower and the time to progression is significantly longer than those treated with LF-MWA. Patients treated with HF-MWA showed better survival than those treated with LF-MWA; still the difference was not significant.
Disclosure statement
The authors of this paper declare no relationships with any companies, whose products or services may be related to the subject matter of the paper.
References
- Gillams A, Goldberg N, Ahmed M, et al. (2015). Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontieres meeting 2013. Eur Radiol 25:3438–54.
- Kim YS. (2015). Advances in MR image-guided high-intensity focused ultrasound therapy. Int J Hyperthermia 31:225–32.
- Nickfarjam A, Firoozabadi SM. (2014). Parametric study of irreversible electroporation with different needle electrodes: electrical and thermal analysis. Int J Hyperthermia 30:335–47.
- Vogl TJ, Farshid P, Naguib NN, et al. (2013). Thermal ablation therapies in patients with breast cancer liver metastases: a review. Eur Radiol 23:797–804.
- Brace CL. (2010). Microwave tissue ablation: biophysics, technology, and applications. Crit Rev Biomed Eng 38:65–78.
- Sun Y, Wang Y, Ni X, et al. (2009). Comparison of ablation zone between 915- and 2,450-MHz cooled-shaft microwave antenna: results in in vivo porcine livers. Am J Roentgenol 192:511–14.
- Hope WW, Schmelzer TM, Newcomb WL, et al. (2007). Guidelines for power and time variables for microwave ablation in a porcine liver. J Gastrointest Surg 12:463–7.
- Oshima F, Yamakado K, Nakatsuka A, et al. (2008). Simultaneous microwave ablation using multiple antennas in explanted bovine livers: relationship between ablative zone and antenna. Radiat Med 26:408–14.
- Saccomandi P, Schena E, Massaroni C, et al. (2015). Temperature monitoring during microwave ablation in ex vivo porcine livers. Eur J Surg Oncol 41:1699–705.
- Curto S, Taj-Eldin M, Fairchild D, et al. (2015). Microwave ablation at 915 MHz vs 2.45 GHz: a theoretical and experimental investigation. Med Phys 42:6152–61.
- Simo KA, Tsirline VB, Sindram D, et al. (2013). Microwave ablation using 915-MHz and 2.45-GHz systems: what are the differences? HPB (Oxford) 15:991–6.
- Wang J, Liang P, Yu J, et al. (2014). Clinical outcome of ultrasound-guided percutaneous microwave ablation on colorectal liver metastases. Oncol Lett 8:323–6.
- Goldberg SN, Grassi CJ, Cardella JF, et al. (2005). Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 16:765–78.
- Dindo D, Demartines N, Clavien PA. (2004). Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–13.
- Nordlinger B, Guiguet M, Vaillant J-C, et al. (1996). Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection, based on 1568 patients. Cancer 77:1254–62.
- Kitisin K, Packiam V, Steel J, et al. (2011). Presentation and outcomes of hepatocellular carcinoma patients at a western centre. HPB (Oxford) 13:712–22.
- Tsai S, Pawlik TM. (2009). Outcomes of ablation versus resection for colorectal liver metastases: are we comparing apples with oranges? Ann Surg Oncol 16:2422–8.
- Groeschl RT, Pilgrim CHC, Hanna EM, et al. (2014). Microwave ablation for hepatic malignancies: a multiinstitutional analysis. Ann Surg 259:1195–200.
- Groeschl RT, Wong RK, Quebbeman EJ, et al. (2013). Recurrence after microwave ablation of liver malignancies: a single institution experience. HPB (Oxford) 15:365–71.
- Vogl TJ, Naguib NNN, Gruber-Rouh T, et al. (2011). Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology 261:643–51.
- Tabuse Y, Tabuse K, Mori K, et al. (1986). Percutaneous microwave tissue coagulation in liver biopsy: experimental and clinical studies. Nihon Geka Hokan 55:381–92.
- Jiao D-C, Zhou Q, Han X-W, et al. (2012). Microwave ablation treatment of liver cancer with a 2,450-MHz cooled-shaft antenna: pilot study on safety and efficacy. Asian Pac J Cancer Prev 13:737–42.
- Lorentzen T, Skjoldbye B, Nolsoe C. (2011). Microwave ablation of liver metastases guided by contrast-enhanced ultrasound: experience with 125 metastases in 39 patients. Ultraschall Med 32:492–6.
- Veltri A, Gazzera C, Rotondella C, et al. (2011). Image-guided microwave ablation of hepatic tumours: preliminary experience. Radiol Med 117:378–92.
- Ong SL, Gravante G, Metcalfe MS, et al. (2009). Efficacy and safety of microwave ablation for primary and secondary liver malignancies: a systematic review. Eur J Gastroenterol Hepatol 21:599–605.
- Liang P, Yu J, Yu X-L, et al. (2012). Percutaneous cooled-tip microwave ablation under ultrasound guidance for primary liver cancer: a multicentre analysis of 1363 treatment-naive lesions in 1007 patients in China. Gut 61:1100–01.
- Otto G, Düber C, Hoppe-Lotichius M, et al. (2010). Radiofrequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Ann Surg 251:796–803.
- Liang P, Dong B, Yu X, et al. (2003). Prognostic factors for percutaneous microwave coagulation therapy of hepatic metastases. Am J Roentgenol 181:1319–25.
- Shibata T, Niinobu T, Ogata N, et al. (2000). Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer 89:276–84.
- Hammill CW, Billingsley KG, Cassera MA, et al. (2011). Outcome after laparoscopic radiofrequency ablation of technically resectable colorectal liver metastases. Ann Surg Oncol 18:1947–54.
- Gervais DA, Goldberg SN, Brown DB, et al. (2009). Society of interventional radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Interv Radiol 20:S342–7.
- Forner A, Ayuso C, Varela M, et al. (2009). Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer 115:616–23.
- Liu Z, Ahmed M, Sabir A, et al. (2007). Computer modeling of the effect of perfusion on heating patterns in radiofrequency tumor ablation. Int J Hyperthermia 23:49–58.
- Solomon SB, Sofocleous CT. (2012). The interventional radiologist role in treating liver metastases for colorectal cancer. Am Soc Clin Oncol Educ Book/ASCO Am Soc Clin Oncol 202–04.
- Hines-Peralta AU, Pirani N, Clegg P, et al. (2006). Microwave ablation: results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology 239:94–102.
- Hompes R, Fieuws S, Aerts R, et al. (2010). Results of single-probe microwave ablation of metastatic liver cancer. Eur J Surg Oncol (EJSO) 36:725–30.
- Martin RCG, Scoggins CR, McMasters KM. (2009). Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol 17:171–8.