Abstract
Candida albicans is one of the most frequently isolated fungal pathogens causing opportunistic infections in humans. Targeted magnetic fluid hyperthermia (MFH) is a promising method in thermal therapy facilitating selective heating of pathogen cells like C. albicans. In the paper, we used meso-2,3-dimercaptosuccinic acid (DMSA)-coated magnetic nanoparticles (MNPs) and functionalised anti-C. albicans immunomagnetic nanoparticles (IMNPs) to investigate the potential of MFH in combating C. albicans cells in vitro. Using Mössbauer spectroscopy it was found that synthesised MNPs exhibited superparamagnetic phenomena. On the basis of calorimetric experiments, the maximum SAR (specific absorption rate) was found and a proper concentration of MNPs was established to control the temperature. MFH based on both DMSA-coated MNPs and functionalised anti-C. albicans IMNPs was more effective in combating C. albicans cells in vitro than thermostat hyperthermia. Especially promising results were obtained using functionalised IMNPs, which eradicated most of the pathogen colonies at the temperature of 43 °C.
Background
Candida albicans is a diploid fungus (a form of yeast) and one of the most frequently isolated fungal pathogens in humans. It lives as a harmless commensal in almost half of the human population in different body locations, but in patients with predisposing conditions, including diabetes mellitus, pregnancy or those necessitating the use of broad-spectrum antibiotics, may produce opportunistic mucosal infections such as oral, gastrointestinal and urogenital candidiasis [Citation1,Citation2]. Candida albicans biofilms readily form on the surface of implantable medical devices. Candida albicans is a highly virulent, difficult-to-treat pathogen. Currently, three groups of chemotherapeutic agents (polyenes, azoles and echinocandins) are used for the treatment of fungal infections. The polyene amphotericin B is the most active fungicidal antibiotic used only in deep-seated, life-threatening candidiasis. Due to severe side effects, the plasma concentrations of this drug should not exceed 1–2 μg/ml, and that is why it is not effective against strains for which the minimal inhibitory concentration (MIC) is above 1 μg/ml [Citation3]. The two other groups have a fungistatic effect and quite often result in strain resistance [Citation4]. The use of a combination antibiotic therapy is problematic, because of the cumulative side effects of each of the agents and lack of synergistic antifungal chemotherapeutics, allowing use of significantly reduced doses. Therefore, it is necessary to search for complementary therapies with low side effects to support treatment of fungal infections.
One of such methods can be combined antibiotic therapy with the hyperthermia procedure, especially in treatment of chronic cutaneous and mucocutaneous infections. In recent years, magnetic fluid hyperthermia (MFH) has been developed as a new and promising therapeutic technique [Citation5,Citation6]. Currently, this method is considered in cancer therapy [Citation7–9], but other applications could be developed. When magnetic nanoparticles (MNPs) coated with biocompatible molecules are exposed to an alternating magnetic field (AMF) with properly chosen frequency, a superparamagnetic heating phenomenon is observed [Citation10]. When conjugated with biomolecules such as antibodies, MNPs could form lock-and-key interactions with target molecules of the antigen on the surface of a cell [Citation11–13]. Magnetic untargeted nanoparticle hyperthermia in combating microbial cells in vitro was reported for the first time for Staphylococcus aureus [Citation14]. The use of MNP antibody targeted hyperthermia against S. aureus was the first time it was described for bacterial cutaneous infections [Citation15]. The authors developed the magnetic thermotherapy platform with MNPs coated with anti-protein A antibody via the streptavidin–biotin system which was effective at thermally inactivating S. aureus cells in vitro and in vivo. To our knowledge, currently there is no literature on the application of hyperthermia (in the temperature range of 43–45 °C) in combating fungal pathogens. Classical hyperthermia is not a promising method in combating Candida infections, because the cells of the pathogen are less sensitive to higher temperature than human cells. MNPs conjugated with anti-C. albicans antibodies can attach to and gather on the surface of the pathogen cells and, after the application of the external magnetic field, can cause heating of the immediate environment of the pathogen and induce reduction of its viability or even cell death. Such a phenomenon was previously described for bacterial cells [Citation15].
In the present study, we investigated the influence of the temperature range used in hyperthermia procedures on the viability and proliferation activity of C. albicans cells in vitro. Then, we evaluated the impact on C. albicans culture of the magnetic hyperthermia using untargeted biocompatible meso-2,3-dimercaptosuccinic acid (DMSA)-coated MNPs and targeted anti-C. albicans immunomagnetic nanoparticles (IMNPs). We tested the impact of all particular factors present in the MFH procedure on the C. albicans culture viability. This paper presents basic biological studies related to the influence of MFH on survival of fungal pathogens in vitro which is an important point for the evaluation of potential applications of this method in skin and tissue therapies.
Materials and methods
Materials
All chemicals used in the present study were of analytical grade, used without further purification and were purchased from Sigma-Aldrich, Merck or Thermo Scientific. Candida albicans strain NCPF 3153 was purchased from the National Collection of Pathogenic Fungi (Public Health England, Bristol, UK). Anti-Candida albicans type A unconjugated rabbit polyclonal antibody immunoglobulin G (IgG) was purchased from Abcam (ab53891) as a 4 mg/ml solution. Standard IgG from rabbit serum (I5006) and secondary anti-rabbit IgG (H + L)-peroxidase antibody produced in sheep (SAB3700920) were purchased from Sigma-Aldrich. The magneTherm system set for generation of AMF was purchased from nanoTherics (Stoke-on-Trent, UK) and the Pico M with OTG-MPK 5 optical sensor system used for real-time temperature monitoring from Opsens (Quebec, Canada). The scanning electron microscope (SEM) image was taken using a Quanta 3D FEG–FEI microscope. Specific absorption rate (SAR) was estimated with the use of Matlab script (Resonant Circuits Limited c/o UCL Healthcare Biomagnetics Laboratory, The Royal Institution of Great Britain, London, UK).
Synthesis of DMSA-coated MNPs andanti-C. albicans IMNPs
Synthesis of iron oxide MNPs
MNPs of iron oxide (Fe3O4) were synthesised according to the method described by Molday [Citation16] with modification by Puddu et al. [Citation17], which is based on co-precipitation of ferric and ferrous ions (with a molar ratio Fe2+/Fe3+ equal to 1:2) in a basic solution. A solution of 5.4 g of iron(III) chloride hexahydrate (Sigma-Aldrich, 236489) and 2.0 g of iron(II) chloride tetrahydrate (Sigma-Aldrich, 44939) was prepared in 25 ml of deionised water under nitrogen protection; then, the solution was slowly dropped into 250 ml of 1 M ammonium hydroxide (prepared from Sigma-Aldrich, 28%, 320145) under vigorous stirring at room temperature. When adding the solution of iron chlorides, formation of a black precipitate was observed indicating the formation of Fe3O4. The turbid black solution stayed under vigorous stirring for 10 min, after this time, the magnetite nanoparticles obtained were washed 5 times with deionised water by centrifugation at 1000 g for 5 min. Aggregated material produced during the reaction was removed by low-speed centrifugation. The mass of the nanoparticles obtained was determined by drying and weighing. Finally, MNPs were washed and resuspended in ultrapure water at a concentration of 10 mg/ml and stored in a refrigerator.
Coating of MNPs with DMSA
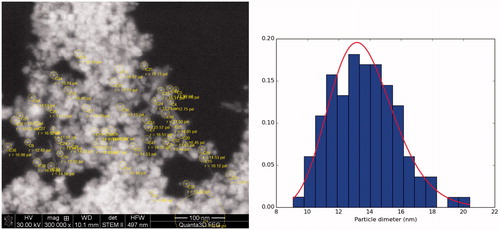
MNPs were coated with DMSA according to the process described by Fauconnier et al. [Citation18] with modification by Pisanic et al. [Citation19]. All the following steps were carried out at 25 °C, under nitrogen protection. Two starting solutions were made by adding 50 mg of DMSA (Sigma-Aldrich, D7881) to 100 ml of ultrapure deoxygenated (via nitrogen bubbling) water and separately, adding 200 ml of ultrapure deoxygenated water to 10 ml of the MNPs (10 mg/ml) suspension. Both solutions were additionally deoxygenated via nitrogen bubbling for 1 h, adjusted to pH 3 with HNO3, and mixed together under vigorous stirring and nitrogen bubbling. The mixture was left under gentle stirring for 1 h and the resulting suspension was centrifuged (900 g for 4 min), washed and resuspended in 100 ml of ultrapure water. In the next step, the pH of the suspension was adjusted to 9.5 with 1 N NaOH and left under gentle stirring for 20 min. After this time, DMSA-coated MNPs were centrifuged (900 g for 10 min), washed in ultrapure water and resuspended (10 mg/ml) in sterile phosphate buffered saline (PBS) buffer (0.1 M, pH 7.2), and stored at 4 °C under nitrogen protection. The SEM image of the synthesised DMSA-coated MNPs together with the histogram of the particle size distribution and log-normal fits can be seen in . The most likelihood method was used to estimate parameters of the log-normal distribution ().
Preparation of anti-C. albicans IMNPs
Anti-Candida albicans IMNPs were prepared by grafting anti-C. albicans type A antibody on the surface of DMSA-coated MNPs using the linker of EDC (1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride) as described in the literature [Citation12,Citation20,Citation21]. EDC is a carboxyl and amine-reactive zero-length crosslinker, which reacts with the carboxyl groups first and forms an amine-reactive O-acylisourea intermediate that quickly reacts with an amino group to form an amide bond and release an isourea by-product. In the experiment, the free carboxyl groups of DMSA chelated on the surface of MNPs were activated by the EDC solution; next, they immediately formed amino bonds with the free amino groups of the antibody. The anti-C. albicans type A unconjugated rabbit polyclonal antibody was purchased from Abcam (ab53891) as a 4 mg/ml solution. EDC in powder form was purchased from Thermo Scientific (22981). Five millilitres of DMSA-coated MNPs (1.0 mg/ml) were washed 3 times with ultrapure water using a magnet to form a sediment and resuspended in 10 ml of sterile PBS buffer (0.1 M, pH 5.6). Five millilitres of a freshly prepared EDC solution (1.0 mg/ml) in ultrapure water were added at room temperature and gently stirred for 5 min. Next, 5 mg of the antibody (as a 4 mg/ml solution) were added and gently stirred for 4 h at 4 °C. According to the literature [Citation12], approximately 96 μg of antibodies are adequate to cover the surface of 0.1 mg nanoparticles by chemical adsorption. The resulting anti-C. albicans IMNPs were washed 3 times by magnetic separation, resuspended in 2 ml of sterile PBS buffer (0.1 M, pH 7.2) and stored at 4 °C, under nitrogen protection.
Assessment of anti-C. albicans IMNP affinity forCandida cells
Affinity of anti-C. albicans IMNPs to Candida cells was checked using an indirect enzyme-linked immunosorbent assay (ELISA) method. Each well of six-well plates was coated with 1 ml of the C. albicans blastoconidium extract obtained with dithiothreitol (DTT) and incubated overnight at 4 °C. The plates were washed 3 times with 0.1 M PBS buffer pH 7.2 and dried on a filter paper. Next, the plates were blocked by adding 2 ml of PBS containing 1% bovine serum albumin (BSA) for 1 h at 37 °C. The extracts were then incubated for 1 h at 37 °C with 1.5 ml of the following reagents: anti-C. albicans IMNPs (10 μg/ml), pure anti-C. albicans antibody (2 μg/ml), irrelevant anti-rabbit IgG (2 μg/ml), heat-inactivated anti-C. albicans IMNPs (10 μg/ml) and DMSA-coated MNPs (10 μg/ml). In the next stage, the plates were washed 3 times with PBS buffer and incubated with 1.5 ml per plate of secondary anti-rabbit IgG peroxidase conjugated antibody produced in sheep (Sigma-Aldrich, SAB3700920) diluted 1:2000 in PBS containing 0.05% BSA and 0.05% Tween 20 for 1 h at 37 °C. After washing with PBS buffer, the plates were developed by addition of a peroxidase substrate – 3,3′,5,5′-tetramethylbenzidine (TMB) at a concentration of 2 × 10−4 mol/l with H2O2 at a concentration of 10 × 10−3 mol/l in phosphate-citrate buffer 0.15 M (pH 5.0). The plates were incubated with the substrate in the dark at 37 °C for 10 min. The reaction was stopped with 1 M H2SO4 and absorbance was read at 450 nm. All samples were repeated 3 times and the average results were calculated.
The interaction of AMF with MNPs
Determination of the physical parameters of MNPs
X-ray diffraction (XRD) and Mössbauer spectroscopy measurements were performed in order to determine the physical parameters of MNPs such as the crystal structure, size and internal magnetic field. The sample was dried and investigated in powder form.
The XRD patterns were obtained with a Philips X’Pert PW 3040/60 using a diffractometer with CuKα radiation at room temperature. They were fitted using the X’Pert High Score Plus programme for the Rietveld refinement method. The average particle diameter was calculated with the use of the Williamson–Hall equation:
(1)
where βtotal is the full width at half-maximum (FWHM) of the XRD peak, K is the Debye–Scherrer constant, approximately equal to 0.94 for spherical particles, λ is the X-ray wavelength, θ is the diffraction angle and η denotes the microstrain of the crystallite lattice.
The Mössbauer spectra were recorded using a constant acceleration spectrometer in the temperature range of 70–290 K. The low-temperature data were obtained with the sample mounted in a 4 K Closed Cycle Refrigerator System from Janis and SHI (Woburn, MA). A 57Co in Rh matrix source and α-Fe standard were used. The Mössbauer spectra were fitted using the least-squares procedure.
Calorimetric experiments with the use of the magneTherm system
In this experiment, we used a magneTherm system set (nanoTherics, Stoke-on-Trent, UK), which consists of a sample enclosure (with different coil configurations), a function generator and a power amplifier. The system is monitored by an output from the coil to an oscilloscope.
The heat generated in the ferrofluid exposed to AMF was quantified in terms of the unit mass of magnetic material in the solution [Citation22]:
(2)
where C is the specific heat capacity of the nanoparticle suspension (J/K ml), φ is the concentration of MNPs (mg/ml) and ΔT/Δt is the rate of change of temperature over time. In our case, the samples with DMSA-coated MNPs (φ = 5 mg/ml) were placed in 2000 μl Eppendorf tubes and then placed inside an expanded polystyrene jacket. The corrected slope method was used to ensure that appropriate regions of the data are used for calculations [Citation22]. Measurements were made in the same type of sample container for the frequency range from 100 to 1000 kHz but for the same magnetic field strength (H). Special attention was paid to temperature sensor positioning within the sample in order to find the region of highest temperature increase [Citation23]; however, since the temperature is the highest at one-third distance below the sample’s surface in our case, the thermal probe was placed centrally in order to measure the average temperature.
Numerical analysis of AMF generated in the coil
To validate the experimental results, the magnetic field strength (H [A/m]) distribution in the coil was numerically calculated and analysed using Sim4Life software (Zurich Med Tech, Zurich, Switzerland). In our case, we applied the Low Frequency Magneto-Static Algorithm, that is, the Biot–Savart law was implemented.
The knowledge of magnetic field distribution in the sample plays a crucial role during calorimetric experiments because the volumetric power dissipation from uncoated MNPs is a function of the magnetic field strength, the applied frequency (f [Hz]) and the out-of-phase component of susceptibility χ″, and can be expressed as [Citation10]
(3)
where μ0 is the permeability of free space. On the other hand, power dissipation PMNPs is related to the temperature increase rate as follows:
(4)
where ρ is the effective density and C is the specific heat capacity of the nanoparticle suspension.
Candida albicans culture conditions
Candida albicans cells (strain NCPF 3153, purchased from the National Collection of Pathogenic Fungi), were stored at 4 °C on slants of Sabouraud dextrose agar. To obtain a culture in the logarithmic growth phase, the cells were passaged into liquid Yeast extract Peptone Dextrose (YPD) medium buffered to pH 7.2 with 0.165 M 3-(N-morpholino) propanesulfonic acid (MOPS) with streptomycin (Sigma-Aldrich) (0.17 mg/ml), and cultured in 2.0 ml Eppendorf round-bottom vials at 35 °C with shaking for 24 h. Before each experiment, the cells were centrifuged (1500 g, 5 min) and resuspended (1.3 × 107 cells/ml) in fresh, RPMI 1640 medium preheated to 35 °C (Sigma-Aldrich, R8755) with 2% glucose, buffered with 0.165 M MOPS to pH 7.2, without phenol red and with streptomycin (Sigma-Aldrich) (0.17 mg/ml). The cell suspension density was consistent with EN 1275:2005 [Citation24]. Next, the cell suspension was aliquoted into Eppendorf tubes and used for experiments. For the research of the influence of the alternating electromagnetic field (AMF) and the MFH the sample volume was 2000 μl, and for studying the impact of DMSA-coated MNPs and functionalised IMNPs, as a chemical factor, the sample volume was 200 μl.
Antifungal activity of anti-C. albicans polyclonal antibody
The candidacidal activity of the anti-C. albicans rabbit polyclonal antibody used for preparing IMNP was checked using exponentially growing C. albicans culture. The test was performed using two different initial cell suspensions: 2.5 × 103 cells/ml (according to the reference method for broth dilution antifungal susceptibility testing of yeast; NCCLS, document M27-A2) and 1.3 × 107 cells/ml (used in the present study for hyperthermia experiments). Briefly, 100 μl of antibody dilutions were added to 100 μl of cell suspensions in RPMI 1640 medium to obtain the final antibody concentration of 0.1, 0.5, 1.0, 2.0 and 2.5 mg/ml and incubated for 24 h at 35 °C. An irrelevant IgG from rabbit serum (Sigma-Aldrich) was used as a control. After incubation with the respective antibody dilution, the fungal cells were spread on Sabouraud dextrose agar and incubated at 35 °C. The number of colony-forming units (CFU) was recorded after 48 h of incubation.
The impact of magnetic ferrofluids on C. albicans
The impact of the ferrofluids studied in the present work (in the concentrations used in the magnetic hyperthermia experiments) on C. albicans culture was checked. The lowest concentration of nanoparticles giving the desired heating effect in the magnetic field (531.1 kHz; H = 10 kA/m) was established at 2.5 mg/ml on the basis of preliminary experiments. The 200-μl C. albicans culture samples aliquoted in Eppendorf tubes were treated with 2.5 mg/ml of DMSA-coated MNPs or with anti-C. albicans IMNPs for 1 h, diluted with fresh RPMI medium (as described above) and left at 35 °C for 24 h with shaking. Control samples were prepared in the same manner but without addition of the ferrofluids. An additional experiment was performed in which RPMI 1640 medium was supplemented with FeCl3 at a concentration ranging from 0.1 to 1.0 mM. Furthermore, experimental trials were made in which the iron chelator (bathophenanthrolinedisulfonic acid disodium salt trihydrate, Sigma, nr kat. 11890) was used in addition to studied ferrofluids and iron(III). Before performing the clonogenic assay of C. albicans culture, the nanoparticles were separated magnetically. The culture medium with yeast cells was collected, and the nanoparticles were resuspended twice in fresh medium and magnetically separated again to recover all of the cells. Whole collected broth was then centrifuged and the cell pellet was resuspended in 1.5 ml of fresh RPMI medium preheated to 35 °C. To verify if any C. albicans cells remained among the separated nanoparticles, samples of the sediment were inoculated on Petri dishes with solid medium and checked for colony-forming abilities. When any colonies grew, they were added to the corresponding results obtained for the test samples.
The impact of AMF on C. albicans
In this experiment, we used the magneTherm system set. Candida albicans culture samples in 2000 μl Eppendorf tubes were placed inside an expanded polystyrene jacket, which acted as an insulator, and exposed to an AMF (531.1 kHz; H = 10 kA/m) for 40 or 60 min. At this time, the control samples remained in an incubator under optimal conditions. Immediately after AMF exposure, all samples (treated and control) were diluted to a volume of 1.5 ml with fresh RPMI medium preheated to 35 °C, gently mixed and used for tests as described above.
Hyperthermia in a thermostat
For hyperthermia in a thermostat, the tubes were placed in a thermostat at a temperature of 43, 45 or 55 °C for 40 or 60 min. The control cultures were left for this time in the thermostat at 35 °C. After the hyperthermia experiment, all samples were diluted with RPMI 1640 medium preheated to 35 °C (Sigma, R8755) with 2% glucose, buffered with 0.165 M MOPS to pH 7.2, without phenol red and with streptomycin (Sigma) (0.17 mg/ml) to obtain 1.5 ml of the final volume. Half of the Eppendorf tubes were used immediately for tests and the other half were left in the thermostat at 35 °C with shaking for 72 h. At the indicated time points, a 20-μl sample was taken from each tube for counting cells in a haemocytometer and a 30-μl sample was placed onto a solid medium for inoculation to determine the clonogenic ability. The remaining culture volume in each tube was used for the MTT assay.
Magnetic fluid hyperthermia (MFH)
The 2000-μl C. albicans culture samples aliquoted in Eppendorf tubes, supplemented with 2.5 mg/ml of DMSA-coated MNPs or with anti-C. albicans IMNPs, were placed inside an expanded polystyrene jacket and exposed to an AMF (531.1 kHz; H = 10 kA/m) generated by the magneTherm system set. After obtaining the desired temperature (43, 45 or 55 °C), the samples were left in these conditions for 40 or 60 min. At this time, the control samples without addition of the ferrofluid remained in an incubator under optimal conditions. Immediately after the MFH treatment, the nanoparticles were separated magnetically (as described above) and then all samples (treated and control), diluted to a volume of 1.5 ml with fresh RPMI medium, were used for cell viability counting and clonogenic ability tests.
Candida albicans susceptibility testing
Determining the number of cells
To determine the cell number after the particular treatment, 20-μl samples were taken at the indicated time and the number of cells were counted under a light microscope using Bürker’s haemocytometer. Two independent counts in two haemocytometer chambers were performed for each sample repetition.
Determining the clonogenic ability
In order to determine the clonogenic capacity (CFU/ml), 30 μl of the culture suspension were taken at the indicated time points, serially diluted in a sterile YPG medium and next 30 μl of each dilution were poured onto solid Sabouraud dextrose agar. The sample volume and dilution were taken into account in the calculation of the results. Where the expected number of colonies did not exceed 10.0 CFU/ml, 30 μl of the culture suspension, without dilution, were directly applied to the plate with Sabouraud dextrose agar. After 48 h of incubation at 35 °C, the colonies were counted on the plates and expressed as CFU per 1 ml.
Viability testing in the MTT method
Cell viability (metabolic activity) was measured in the MTT test [Citation25] using the In Vitro Toxicology Assay Kit, MTT based (TOX1, Sigma). The tetrazolium dye MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was dissolved in PBS pH 7.2 buffer to obtain a concentration of 5 mg/ml. The stock solution was stored at 4 °C for up to 2 weeks. At the indicated time points, the MTT solution was added to the culture test tubes in an amount of 10% by volume of the culture medium and the incubation at 35 °C was continued for the next 4 h. After the incubation period, formazan crystals were dissolved by adding an amount of MTT Solubilisation Solution [M-8910] equal to the original culture medium volume and gentle mixing in a gyratory shaker. Next, the absorbance was spectrophotometrically measured at a wavelength of 570 nm.
Statistical analysis
All experiments were carried out in triplicate. Results are expressed as means with standard deviation. Statistical analysis was performed using Prism version 3.0 (GraphPad Software Inc., San Diego, CA). Student’s unpaired t-test was used to compare two data sets. One-way analysis of variance (ANOVA) and Tukey’s post hoc test were used to compare multiple data sets. Statistical significance was assumed at p < 0.05.
Results
The interaction of AMF with MNPs
XRD measurements were performed to determine the crystal structure and the size of the synthesised nanoparticles. (left) shows the powder XRD pattern of the sample measured at room temperature. On the basis of the XRD reflection, which is characteristic for the inverse spinel cubic structure of magnetite (Fe3O4), we determined the lattice constant as 8.381(4) Å. Moreover, the strongly broadened Bragg peaks indicated the formation of nano-sized magnetite particles. Formula (1) was used in order to determine the average particle diameter (d) and the internal strain (η) of the investigated sample. It is evident that the grain size is equal to d = 8.9(5) nm and the crystalline strain is practically negligible (ɛ = 0.06%).
Mössbauer spectroscopy measurements were carried out and the magnetic properties of the sample were determined. The Mössbauer spectra of the MNP sample were measured at different temperatures and they are presented in (right). It can be seen that the spectra obtained at room temperature consist of four components: the first two – with low intensity related to the tetrahedral and octahedral iron position in the magnetite crystal site and the other two – dominant doublets attributed to superparamagnetic behaviour. It should be noticed that superparamagnetism is characteristic for small crystallites and is connected with single domain particles. In this case, the direction of the magnetisation vector has random orientation caused by thermal fluctuations. The average time necessary for the change of the magnetisation vector from one axis to another is called the superparamagnetic relaxation time τ and it can be expressed by the Néel–Brown formula:
(5)
where K is the magnetic anisotropy energy constant, V is the particle volume, kB is Boltzmann’s constant, T is temperature and the prefactor τ0 is typically 10−10 s.
Figure 2. Power X-ray diffraction (XRD) pattern of magnetite nanoparticles (left) and Mössbauer spectra of magnetite nanoparticles at various temperatures (right). At the room temperature (RT), the spectra consist of two doublets (middle part of the spectra) indicating superparamagnetic material, together with magnetic sextets. The lower temperatures indicate that the superparamagnetic doublet is decreasing and the magnetic sextet is dominant.
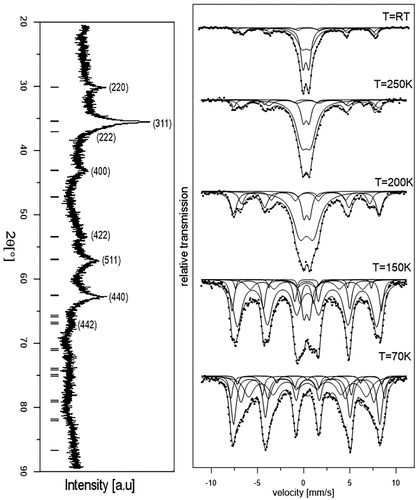
It can be seen from (right) that when the sample temperature is decreasing from room temperature to 70 K the doublet contribution in the spectrum decreases, which is typical for MNPs. When the temperature is suitably low, the fluctuations in the nanoparticles are much slower and the spectrum becomes a pure sextet pattern. The spectra obtained at 70 K and at lower temperature consist of only magnetic components, which indicate lack of magnetic fluctuations. It can be concluded that the crystalline size of our MNPs is suitably small and the relaxation time varies as a function of temperature, which indicates that our MNPs are suitable for hyperthermia application.
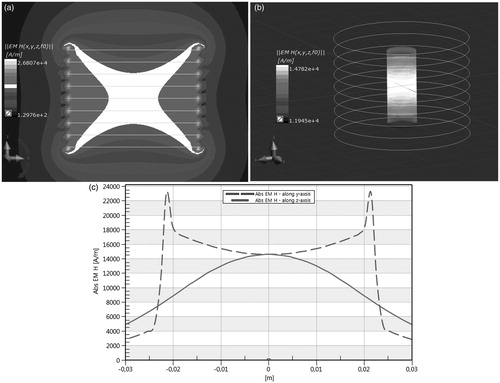
Next, we investigated experimentally the influence of AMF on the heat generation rate expressed in terms of SAR, and we found the SAR value for the DMSA-coated MNPs. The results of our experiments are shown in . shows the heating curve over time for a ferrofluid sample (concentration 5 mg/ml) subjected to AMF with amplitude H = 10 kA/m and frequency f = 531.1 kHz. shows the cross section of the magnetic field distribution in an empty nine-turn solenoid coil leading current IRMS = 65 A while the green colour indicates HRMS = 10 kA/m, which can be understood as a “working” one (see ). shows magnetic field distribution on the surface of a 2 ml cylinder (Eppendorf sample) with regard to Hmax = 14 kA/m. Moreover, the magnetic field distribution in the coil along the x axis and z axis is shown in .
Figure 3. Experimental heating curve over time for the meso-2,3-dimercaptosuccinic acid (DMSA)-coated magnetic nanoparticles (particle concentration φ = 5 mg/ml, sample volume V = 2000 μl, frequency f = 531.1 kHz, magnetic field strength Hmax = 14.78 kA/m) and specific absorption rate (SAR) evaluation using corrected slope method [Citation22].
![Figure 3. Experimental heating curve over time for the meso-2,3-dimercaptosuccinic acid (DMSA)-coated magnetic nanoparticles (particle concentration φ = 5 mg/ml, sample volume V = 2000 μl, frequency f = 531.1 kHz, magnetic field strength Hmax = 14.78 kA/m) and specific absorption rate (SAR) evaluation using corrected slope method [Citation22].](/cms/asset/b2c17e3e-4f86-4801-8b40-f3023593ba86/ihyt_a_1212277_f0003_c.jpg)
The impact of AMF on C. albicans culture
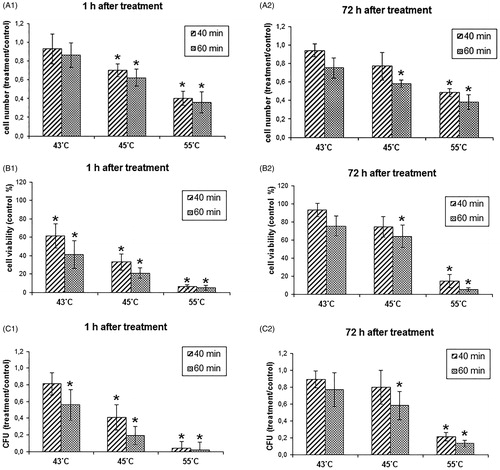
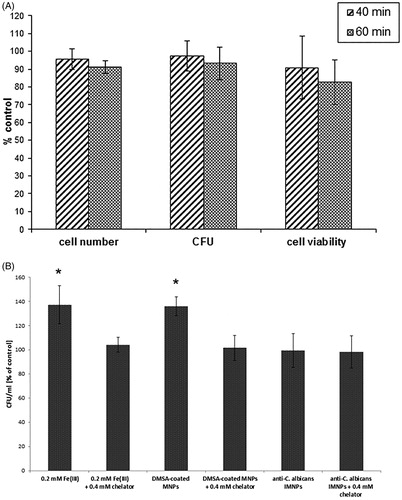
The influence of AMF was investigated by placing the samples of C. albicans culture without a magnetic ferrofluid inside a polystyrene jacket in the magneTherm system set generating the magnetic field (531.1 kHz; H = 10 kA/m) for 40 or 60 min. The parameters of AMF were established in a previous experiment as optimal for inducing the heating phenomenon by MNPs used for MFH. Under these conditions, there was no temperature rise in the samples. After 40 min of exposure to AMF, the number of cells in the suspension culture, the colony-forming ability and the metabolic activity of C. albicans cells were not significantly altered. After 60 min of exposure to the AMF, slight inhibition of the metabolic activity of cells (82.7% of the control), the ability to form colonies (93.2% of the control) and slight reduction in the number of cells (91.2% of the control) were observed (); however, these differences were not statistically significant, compared to the control samples. The viability testing of the culture was repeated after 72 h from AMF exposure, and after this time, the results obtained were at a similar level as in the control samples.
Figure 5. Candida albicans culture viability parameters in response to: (A) alternating magnetic field (AMF) (531.1 kHz; H = 10 kA/m); all results are not significantly different from the control (p > 0.05). (B) Number of C. albicans colony-forming units (CFU) in response to ferrofluids (meso-2,3-dimercaptosuccinic acid (DMSA)-coated magnetic nanoparticles (MNPs) and functionalised anti-C. albicans immunomagnetic nanoparticles (IMNPs)) and Fe(III) with or without addition of the iron chelator (bathophenanthrolinedisulfonic acid disodium salt trihydrate); * results significantly higher than the control (p < 0.05). Values are means of triplicate determinations ± standard deviation of the mean.
The influence of ferrofluids (DMSA-coated MNPs and anti-C. albicans IMNPs) on C. albicans culture
In the first step, the potential candidacidal activity of the polyclonal anti-C. albicans rabbit antibody was checked. The inhibitory activity of the polyclonal antibody was dependent upon the concentration ratio of the antibody to the Candida cell number. At a cell number of 2.5 × 103/ml (recommended in the reference method for antifungal activity testing, NCCLS), the MIC of the antibody was 0.5 mg/ml. In turn, at a cell number of 1.3 × 107/ml (used in the present work for testing hyperthermia effects), the antibody concentration up to 2.5 mg/ml only slightly reduced the CFU ability (approx. 85% of the control) but no clear fungicidal activity was observed (supplementary material, Figure 1).
The affinity of the polyclonal rabbit antibody and IMNPs for C. albicans cells was checked using the indirect ELISA method. The results showed high affinity of the polyclonal antibody as well as IMNPs for the tested C. albicans strain, while heat-inactivated IMNPs and DMSA-coated MNPs failed to bind with the Candida cell extract (supplementary material, Figure 2).
To determine the impact of the magnetic ferrofluids used in the present work on the clonogenicity of C. albicans cells, DMSA-coated MNPs or functionalised anti-C. albicans IMNPs (2.5 mg/ml) were added to the logarithmic growing culture and the culture was continued for 24 or 72 h at the optimal temperature (35 °C) without the magnetic field. The concentration of iron oxide nanoparticles was established on the basis of preliminary optimisation experiments, in which the concentration of the ferrofluid was fit to the selected AMF parameters to achieve an optimal ability to heat the suspension. The same concentration of the nanoparticles was used in the magnetic hyperthermia experiments. The results showed that the ferrofluid containing DMSA-coated MNPs slightly stimulated the clonogenicity of C. albicans cells (135.9% of the control) and the differences were statistically significant (). However, after supplementation with the ferrofluid containing functionalised anti-C. albicans IMNPs, no statistically significant differences, compared to the control, were noted (). To verify the hypothesis that the iron ions releasing from the DMSA-coated iron oxide nanoparticles might stimulate more intensive proliferation of C. albicans cells, the experiment was performed in which the RPMI 1640 medium was supplemented with FeCl3 at the concentrations ranging from 0.1 to 1.0 mM. Results showed that all tested concentrations of Fe(III) stimulated proliferation of yeast cells in a dose-dependent manner (data not shown). The most similar level of stimulation was obtained for the DMSA-coated MNPs (2.5 mg/ml) and the Fe(III) at the concentration of 0.2 mM (). As shown on , the use of iron chelator attenuated the effect of stimulation in all tested samples.
The impact of thermostat hyperthermia on C. albicans culture
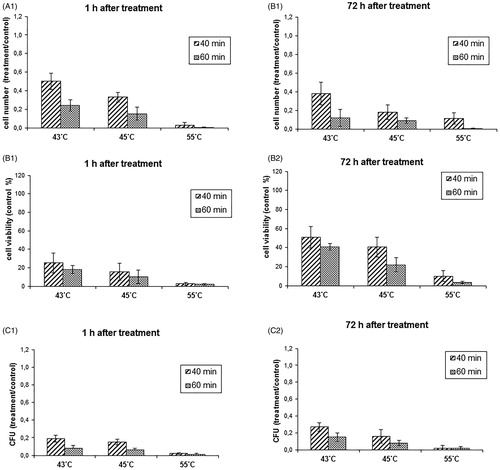
The influence of elevated temperature on C. albicans culture in the logarithmic growth phase was studied by incubating the culture in a thermostat at the temperature of 43, 45 and 55 °C for 40 or 60 min. The results obtained showed that the increased temperature inhibited the C. albicans culture at a level depending on the temperature and time of exposure (). After hyperthermia at the temperature of 43 °C, there was no significant decrease in the number of cells in the suspension culture. Treatment with the temperature of 45 °C for 60 min decreased the number of cells to approx. 60% of the control samples, whereas treatment with the temperature of 55 °C decreased the cell number to 36% of the control (). The metabolic activity measured with the MTT test was far more inhibited by the thermostat hyperthermia and decreased to approx. 20% of the control after 60 min of incubation at 45 °C and to approx. 4% after incubation at 55 °C (). The ability to form colonies decreased to approx. 19% after incubation at 45 °C and to approx. 0.02% after incubation at 55 °C (). The thermostat hyperthermia in the temperature range between 43 and 45 °C, despite transient inhibition of C. albicans cell proliferation, failed to completely eradicate the proliferative potential of the culture. It was found that cells in the culture recovered within 3 days after a single hyperthermia treatment and an increase in both metabolic activity and the colony formation ability to approx. 80% ± 10% was observed, which was not significantly lower than in the control samples (, , . The viability parameters remained at low levels, that is, below 15% of the control, only after exposure to the temperature of 55 °C.
The impact of MFH on C. albicans culture
In the next step of the work, experiments were conducted to determine the impact of the MFH on the viability parameters of C. albicans culture. DMSA-coated MNPs and functionalised anti-C. albicans IMNPs were used as ferrofluids in which paramagnetic nanoparticles interacted with AMF and caused a heating effect. The concentration of the nanoparticles, as established in preliminary experiments, was constant (2.5 mg/ml) and the initial parameters of the magnetic field (531.1 kHz; 10 kA/m) were adjusted during each experiment to obtain the desired temperature. The yeast cultures in the logarithmic growth phase were exposed to the MFH at the temperature of 43, 45 or 55 °C for 40 or 60 min. Next, nanoparticles were magnetically separated, and the remaining cell suspensions were studied to determine the number of cells, metabolic activity and ability to form colonies. The inhibitory effect against C. albicans culture obtained in the MFH procedure based on the DMSA-coated MNPs in the temperature range of 43–45 °C was stronger than in the same temperatures in the thermostat hyperthermia (). In MFH using DMSA-coated MNPs, the reduction of the cell number to the level below 40% of the control was achieved already after exposure to 43 °C for 60 min and to 45 °C for 40 and for 60 min as well. The exposure to the temperature of 55 °C for 40 and 60 min caused a more prominent decrease in the cell number to less than 0.05% of the control (). The metabolic activity measured by the MTT assay and colony-forming ability decreased to approx. 20% already after 40 min of exposure to the temperature of 43 °C and were noted at a similar level after 45 °C hyperthermia for 40 min. Exposure to 43 and 45 °C for 60 min reduced the colony-forming ability to less than 10% of the control. At the temperature of 55 °C, all parameters were below 0.05% of the control (, ). It should be emphasised that the inhibitory effect was maintained for 3 days after hyperthermia treatment, showing only a slight upward trend after 43 °C hyperthermia (, ). The inhibitory impact on the C. albicans culture (in all the studied parameters) obtained in MFH induced by interaction of DMSA-coated MNPs with magnetic field was statistically significantly stronger than the effect achieved in the thermostat hyperthermia.
Figure 7. Influence of the magnetic fluid hyperthermia (MFH) employing the meso-2,3-dimercaptosuccinic acid (DMSA)-coated magnetic nanoparticles (MNPs) on the viability parameters of the Candida albicans culture. Values are means of triplicate determinations ± standard deviation of the mean. All results are significantly different from the control (p < 0.05).
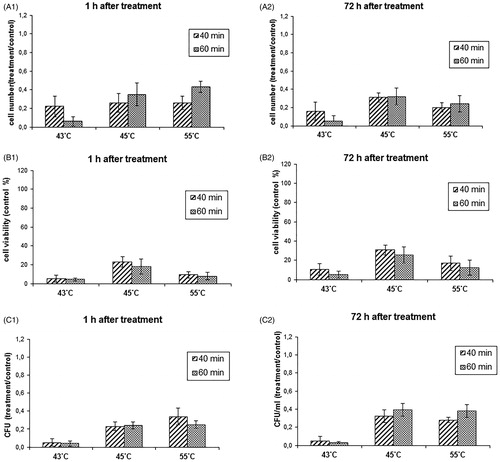
In the next stage of the work, the DMSA-coated MNPs were functionalised with anti-C. albicans polyclonal antibody, using EDC as a linker, and the anti-C. albicans IMNPs obtained, specifically binding to the cell wall of the pathogen, were used for magnetic hyperthermia. The in vitro studies revealed that functionalised anti-C. albicans IMNPs exhibited improved inhibitory activity against C. albicans at the temperature of 43 °C, compared to the DMSA-coated MNPs. After 40 min of incubation at this temperature, the number of cells in the suspension culture decreased to approx. 20 ± 10% and the metabolic activity and colony-forming ability to less than 10% (, , ). Importantly, the inhibitory effect remained at a similar level for 3 days after hyperthermia (, , ). In the case of functionalised anti-C. albicans IMNPs, hyperthermia at 45 and 55 °C gave a slightly weaker inhibitory effect than at 43 °C. It was also a little weaker than at the same temperatures obtained with the use of DMSA-coated MNPs.
Figure 8. Influence of the magnetic fluid hyperthermia (MFH) employing the functionalised anti-Candida albicans immunomagnetic nanoparticles (IMNPs) on the viability parameters of the C. albicans culture. Values are means of triplicate determinations ± standard deviation of the mean. All results are significantly different from the control (p < 0.05)
Discussion
MFH is a complex procedure involving interactions of living cells with at least two physical factors (increased temperature and AMF) and a chemical factor in the form of a ferrofluid, containing iron oxide MNPs coated with a biocompatible material (e.g. DMSA) and anti-C. albicans antibody. Before performing the experiments on the effects of MFH on the growth of C. albicans culture in vitro, it was important to investigate the influence of the particular physical and chemical factors of this procedure as a single stimulus. Therefore, we tested the impact of DMSA-coated MNPs and functionalised anti-C. albicans IMNP, AMF distribution in the samples, and hyperthermia in a thermostat on the viability parameters of C. albicans culture.
MFH efficiency is connected with the rate of heat generated by MNPs in a ferrofluid, which is caused by their magnetic properties, temperature, viscosity of the carrier liquid, magnetic field strength and the applied frequency. The phenomenon of conversion of the power losses in MNPs into heat is essential during MFH. On the basis of XRD, the size of nanoparticles was determined and the superparamagnetic properties were proved by means of Mössbauer spectroscopy. From the experimental point of view, it is very important to place the samples into a homogenous magnetic field in order to ensure the same heating in the whole volume of the sample. The AMF distribution in a sample was determined by the finite element method in order to validate its homogeneity. In the calorimetric experiments, the highest SAR value for synthesised DMSA-coated MNPs was determined (SAR =9.94 W/g, f = 531.1 kHz; H = 10 kA/m).
In the first step of the biological experiments, we tested the impact of the chemical compounds used in MFH that could affect growth of C. albicans cells. Magnetic iron oxide nanoparticles used for diagnosis or treatment should be covered with a biocompatible material to reduce their negative impact on the cells and facilitate removal thereof from the body. Among different possible substances, DMSA was chosen, because it is a non-toxic compound used in toxicology as a detoxifying agent for the treatment of heavy metal poisoning [Citation26]. The advantage of DMSA is that it is a strong chelating agent, forming stable complexes with iron oxide molecules, which are stable over a wide pH range (3–11) in buffers of ionic strength suitable for biological studies [Citation18,Citation27]. Furthermore, the SH groups of the acid remain available on the surface of coated nanoparticles and can be used to attach additional functionalising compounds, for example, an antibody [Citation11–13]. In vivo studies in mice showed that DMSA-coated nanoparticles after intravenous injection specifically accumulated in the lung tissue within 5 min to 24 h after administration [Citation28].
Our experiments revealed that the ferrofluid containing DMSA-coated MNPs as a chemical factor at a concentration suitable for MFH at optimal temperature (without the magnetic field) caused slight stimulation of C. albicans proliferation. This phenomenon is probably caused by the increased availability of iron ions released from iron oxide nanoparticles into the culture medium. This hypothesis was confirmed by the results of the experiment in which additional supplementation of the culture medium with iron(III) caused stimulation of colony-forming ability by C. albicans. The use of an iron chelator attenuated the stimulating effect of both iron supplementation and the influence of DMSA-coated MNPs. According to the literature, iron is an important limiting factor for C. albicans growth and the content of this element in the host influences the susceptibility to infections with the pathogen [Citation29,Citation30]. Candida has developed specific mechanisms to gain iron from host proteins, including haemoglobin, transferrin, lactoferrin and ferritin [Citation31,Citation32]. It is therefore not surprising that with the increased availability of iron C. albicans cells were stimulated to more intense proliferation.
In the present work, the DMSA-coated MNPs were additionally functionalised with anti-C. albicans polyclonal antibody using EDC as a linker. In the indirect ELISA method, it was established that the anti-C. albicans IMNPs obtained specifically bound to the C. albicans cell extract. Functionalised anti-C. albicans IMNPs were designed to act as smart molecules which magnetically directed into the site of infection can specifically find and attach to the pathogen cells scattered in tissues. In the case of functionalised anti-C. albicans IMNPs, no stimulation of the yeast growth was observed, and growth remained at the level of the control. This result is probably the sum of the opposing effects of the increased availability of iron and the slight fungistatic activity of the anti-C. albicans polyclonal antibody attached to the nanoparticle surface. The antibody alone, at the concentration used to prepare the nanoparticles, slightly inhibited the ability to form colonies (approx. 85% of the control). However, when bound to the nanoparticles, only part of the antibody molecules have direct access to the yeast cells. It was shown that the antibodies immobilised on nanoparticles retain approximately 50% of their binding capacity at surface saturated levels [Citation13]. The polyclonal antibody used in the present work is recommended for the diagnostics methods (e.g. immunocytochemistry or ELISA) for detection of C. albicans cells in tissue samples. The antigen binding of the antibody is uncharacterised. Antibodies are believed to play a role in the protection against Candida infections by a number of mechanisms, including the inhibition of adhesion or germ tube formation, opsonisation, neutralisation of virulence-related enzymes and direct candidacidal activity [Citation33]. In a living organism, the activity of such antibodies largely depends on recruitment of white blood cells or complement. The direct candidacidal activity of antibody in vitro is usually less effective. Summing up, the functionalised anti-C. albicans IMNPs in vitro did not show a significant impact on C. albicans growth.
Another important element that could modify the viability of fungal cells is the exposition to an AMF. Our experiments showed that AMF parameters (531.1 kHz; H = 10 kA/m), suitable for MFH, after 60 min of exposure slightly reduced the viability parameters of C. albicans culture; however, these differences were not statistically significant compared to the control. Within 3 days after AMF exposure, the metabolic activity and colony-forming ability returned to the control level. Probably, longer exposure to the studied AMF would inhibit C. albicans culture viability and proliferative activity more significantly, but prolonged exposure to AMF was not important in our study, because hyperthermia treatment lasting longer than 60 min is hardly used in practice.
The effect of increased temperature on the viability of C. albicans culture was tested using hyperthermia in a thermostat. Our research showed that increased temperature in the range of 43–45 °C, immediately after treatment, resulted in a slight decrease in the number of cells and significantly limited metabolic activity and colony formation ability (approx. 20% of the control). However, 3 days after hyperthermia treatment the cells in the culture recovered and gained the viability parameters at the control level. In our studies, the persistent inhibition of the pathogen growth was achieved at the temperature of 55 °C. Obviously, the use of such a high temperature as 55 °C in clinical practice is problematic. These results are consistent with the data available in the literature [Citation34,Citation35]. The increased temperature, depending on the range, can cause induction of thermotolerance mechanisms, allowing cells to survive under stress conditions, or cause irreversible destruction. A sudden increase in the temperature causes cell cycle arrest in the G1 phase. At that time, metabolic processes leading to thermotolerance are carried out. To achieve thermotolerance in yeast, synthesis of heat shock proteins (HSP) and accumulation of trehalose are required. These processes may occur at a temperature not exceeding 46–47 °C. According to the data presented in the literature, the sensitivity of C. albicans to increased temperature can vary depending on the strain or isolate. Observations performed on a number of C. albicans isolates revealed that at 43–45 °C growth was limited but not entirely suppressed, whereas a temperature range of 52.5–55 °C was lethal to most of the strains [Citation34–36]. However, yeast cells survived exposure to an otherwise lethal temperature of 55 °C when they had previously been exposed to 45 °C. This phenomenon was explained by induction of thermotolerance mechanisms [Citation36]. One of the most important responses of the yeast cell under thermal stress is the induction of synthesis of HSP [Citation36,Citation37]. These proteins have a variety of functions in the cell and are involved in many processes such as cell division, DNA synthesis, transcription and translation, protein folding and transport [Citation3,Citation38]. Another essential element of the protection against temperature stress in yeast is the accumulation of trehalose, a disaccharide that has a protective role for proteins and membrane lipids, stabilising them and maintaining the structural integrity of the cell [Citation34,Citation39,Citation40].
We were prompted to undertake the research on sensitivity of C. albicans cells to MFH by the results obtained for tumour cells, where the induction of cell death by MFH was due to not only elevated temperature but also to the combined effect of nanoparticles themselves administered locally to the tumour and increased temperature [Citation41–44]. For bacterial S. aureus cells, it was demonstrated that direct targeted heating of the pathogen cells is effective in their thermal inactivation [Citation15]. We assumed that the accumulation of nanoparticles in close contact with the fungal cells and the rapid temperature rise in the magnetic field could enhance the fungicidal effect, not allowing the cells to trigger thermotolerance mechanisms. The inhibitory effect obtained in MFH based on the DMSA-coated MNPs at 43–45 °C was significantly stronger than that obtained in the same temperatures in the thermostat. After 40 min of exposure to a temperature in the range of 43–45 °C, there was a decrease in the number of colonies and metabolic activity to approx. 20% of the control, and after 60 min to approx. 10% of the control. Importantly, the inhibitory effect was maintained within 3 days after the treatment, showing only a slight upward trend. The mechanisms of the enhanced inhibitory effect of MFH on C. albicans viability require further studies. One of them can be a more rapid temperature increase, not allowing induction of thermotolerance response.
In order to facilitate specific binding and direct contact of MNPs with C. albicans cells, the nanoparticles were coated with anti-C. albicans polyclonal antibodies. In this manner, functionalised nanoparticles specifically finding and binding to C. albicans cells were obtained. The resulting inhibitory effect of MFH using these functionalised nanoparticles was improved at 43 °C, compared with the DMSA-coated MNPs, which is promising for the possible applicability of this procedure in practice. In our experiments we have used the constant concentration of MNPs equal to 2.5 mg/ml to compare effectiveness of targeted and untargeted nanoparticles. Data available in the literature show that in untargeted MFH the MNPs were used at the concentration of 6.25–50 mg/ml for effective destruction of bacterial cells [Citation14], but in the antibody targeted MFH the concentration of MNPs enough to reduce viability of S. aureus cells in vitro was about 1 mg/ml [Citation15]. Targeting of the nanoparticles can significantly reduce their effective concentration, which is especially important in in vivo procedures. It should also be noted that in the case of the functionalised anti-C. albicans IMNPs hyperthermia at 45 and 55 °C gave a slightly weaker inhibitory effect than at 43 °C. It was also a little weaker than in these temperatures obtained with the use of DMSA-coated MNPs. The efficacy of the MFH employing IMNPs to a large extent depends on the binding yield and a small distance between the antibody-coated nanoparticles with the surface of pathogen cells. An antibody IgG molecule is composed of four polypeptide chains that are connected by disulphide bonds and non-covalent forces. Antibodies exhibit a strong correlation between structure and function in particular domains. In the literature it was described that increased temperature causes the unfolding of particular domains of the antibody molecule leading to denaturation which results in decreased efficiency of the antigen binding [Citation45]. Although the overall IgG denaturation usually occurs at a temperature of 60–70 °C, a strong decrease in the ellipticity, suggesting rupture of disulphide bonds due to the denaturation process, was observed at a temperature of about 55 °C [Citation46] and it was preceded by the appearance of denaturation intermediates formed due to differences in sensitivity of the disulphide bonds in the different IgG domains. In this paper it was shown that such partially unfolded intermediates undergo intensive aggregation and the rate of this process is so fast that IgG molecules become locked in aggregates before they are completely denatured. Aggregation of IMNPs was actually observed under the microscope in the samples treated with hyperthermia at higher temperatures. This phenomenon could be the reason for lower efficacy of nanoparticles coated with antibody at a higher temperature.
MFH is a promising and still developing method for local heat therapy. To the best of our knowledge, this is the first attempt at application of the MFH procedure for eradication of fungal pathogen cells. The efficacy of this procedure requires involvement of many studies from different fields of science. In contrast to tumours showing a specific location to which local hyperthermia applies, fungal infections are scattered, comprising mucous membranes or certain organs. Therefore, the present study attempted the utilisation of iron oxide nanoparticles coated with a biocompatible material (DMSA) and further functionalised with antibodies directed against C. albicans cells. The results showed that MFH had greater efficacy in eradication of yeast cells than conventional hyperthermia in a thermostat. Furthermore, the strongest reduction of cell viability and proliferative activity at 43 °C was obtained using anti-C. albicans IMNPs specifically binding to the pathogen. This is a promising result, as the temperature range of 43–45 °C is acceptable as safe in the hyperthermia treatment. In the next stage, the in vivo activity and side effects of anti-C. albicans IMNPs should be studied.
Supplemental File
Download MS Word (66.6 KB)Disclosure statement
The authors report no conflicts of interest.
References
- Liu H. (2002). Co-regulation of pathogenesis with dimorphism and phenotypic switching in Candida albicans, a commensal and a pathogen. Int J Med Microbiol 292:299–311.
- Ruhnke M. (2002). Skin and mucous membrane infections. In: Calderone RA, ed. Candida and Candidiasis. Washington (DC): ASM Press, 307–25.
- Burnie JP, Carter TL, Hodgetts SJ, et al. (2006). Fungal heat-shock proteins in human disease. Fems Microbiol Rev 30:53–88.
- Kontoyiannis DP, Lewis RE. (2002). Antifungal drug resistance of pathogenic fungi. Lancet 359:1135–44.
- Hilger I. (2013). In vivo applications of magnetic nanoparticle hyperthermia. Int J Hyperthermia 29:828–34.
- Kozissnik B, Bohorquez AC, Dobson J, et al. (2013). Magnetic fluid hyperthermia: advances, challenges, and opportunity. Int J Hyperthermia 29:706–14.
- Dudeck O, Bogusiewicz K, Pinkernelle J, et al. (2006). Local arterial infusion of superparamagnetic iron oxide particles in hepatocellular carcinoma – a feasibility and 3.0 T MRI study. Invest Radiol 41:527–35.
- Jordan A, Scholz R, Maier-Hauff K, et al. (2006). The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J Neurooncol 78:7–14.
- Maier-Hauff K, Rothe R, Scholz R, et al. (2007). Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: results of a feasibility study on patients with glioblastoma multiforme. J Neuro-Oncol 81:53–60.
- Carrey J, Mehdaoui B, Respaud M. (2011). Simple models for dynamic hysteresis loop calculations of magnetic single-domain nanoparticles: application to magnetic hyperthermia optimisation. J Appl Phys 109:083921.
- Weissleder R, Kelly K, Sun EY, et al. (2005). Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol 23:1418–23.
- Zhang S, Bian Z, Gu C, et al. (2007). Preparation of anti-human cardiac troponin I immunomagnetic nanoparticles and biological activity assays. Colloid Surface B 55:143–8.
- Koh I, Wang X, Varughese B, et al. (2006). Magnetic iron oxide nanoparticles for biorecognition: evaluation of surface coverage and activity. J Phys Chem B 110:1553–8.
- Thomas LA, Dekker L, Kallumadil M, et al. (2009). Carboxylic acid-stabilised iron oxide nanoparticles for use in magnetic hyperthermia. J Mater Chem 19:6529–35.
- Kim MH, Yamayoshi I, Mathew S, et al. (2013). Magnetic nanoparticle targeted hyperthermia of cutaneous Staphylococcus aureus infection. Ann Biomed Eng 41:598–609.
- Molday RS. (1984). US Patent 4,452,773.
- Puddu M, Paunescu D, Stark WJ, et al. (2014). Magnetically recoverable, thermostable, hydrophobic DNA/silica encapsulates and their application as invisible oil tags. ACS Nano 8:2677–85.
- Fauconnier N, Pons JN, Roger J, et al. (1997). Thiolation of maghemite nanoparticles by dimercaptosuccinic acid. J Colloid Interface Sci 194:427–33.
- Pisanic TR II, Blackwell JD, Shubayev VI, et al. (2007). Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials 28:2572–81.
- Staros JV, Wright RW, Swingle DM. (1986). Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal Biochem 156:220–2.
- Grabarek Z, Gergely J. (1990). Zero-length crosslinking procedure with the use of active esters. Anal Biochem 185:131–5.
- Wildeboer RR, Southern P, Pankhurst QA. (2014). On the reliable measurement of specific absorption rates and intrinsic loss parameters in magnetic hyperthermia materials. J Phys D Appl Phys 47:495003.
- Wang SY, Huang SJ, Borca-Tasciuc DA. (2013). Potential sources of errors in measuring and evaluating the specific loss power of magnetic nanoparticles in an alternating magnetic field. IEEE Trans Magn 49:255–62.
- Standarisation EC. EN 1275: chemical disinfectants and antiseptics. (2005). Quantitative suspension test for the evaluation of basic fungicidal or basic yeasticidal activity of chemical disinfectants and antiseptics. Test method and requirements (phase 1).
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63.
- Graziano JH. (1986). Role of 2,3-dimercaptosuccinic acid in the treatment of heavy metal poisoning. Med Toxicol Adv Drug 1:155–62.
- Rooney JP. (2007). The role of thiols, dithiols, nutritional factors and interacting ligands in the toxicology of mercury. Toxicology 234:145–56.
- Chaves SB, Lacava LM, Lacava ZGM, et al. (2002). Light microscopy and magnetic resonance characterisation of a DMSA-coated magnetic fluid in mice. IEEE Trans Magn 38:3231–3.
- Baillie GS, Douglas LJ. (1998). Iron-limited biofilms of Candida albicans and their susceptibility to amphotericin B. Antimicrob Agents Chemother 42:2146–9.
- Fratti RA, Belanger PH, Ghannoum MA, et al. (1998). Endothelial cell injury caused by Candida albicans is dependent on iron. Infect Immun 66:191–6.
- Knight SA, Vilaire G, Lesuisse E, et al. (2005). Iron acquisition from transferrin by Candida albicans depends on the reductive pathway. Infect Immun 73:5482–92.
- Almeida RS, Wilson D, Hube B. (2009). Candida albicans iron acquisition within the host. Fems Yeast Res 9:1000–12.
- Moragues MD, Omaetxebarria MJ, Elguezabal N, et al. (2003). A monoclonal antibody directed against a Candida albicans cell wall mannoprotein exerts three anti-C. albicans activities. Infect Immun 71:5273–9.
- Arguelles JC. (1997). Thermotolerance and trehalose accumulation induced by heat shock in yeast cells of Candida albicans. Fems Microbiol Lett 146:65–71.
- Pinjon E, Sullivan D, Salkin I, et al. (1998). Simple, inexpensive, reliable method for differentiation of Candida dubliniensis from Candida albicans. J Clin Microbiol 36:2093–5.
- Zeuthen ML, Howard DH. (1989). Thermotolerance and the heat-shock response in Candida albicans. J Gen Microbiol 135:2509–18.
- Sandini S, Melchionna R, Bromuro C, et al. (2002). Gene expression of 70 kDa heat shock protein of Candida albicans: transcriptional activation and response to heat shock. Med Mycol 40:471–8.
- Piecuch A, Oblak E. (2013). (Mechanisms of yeast resistance to environmental stress). Postepy Hig Med Dosw (Online) 67:238–54.
- Jain NK, Roy I. (2009). Effect of trehalose on protein structure. Protein Sci 18:24–36.
- Mahmud SA, Hirasawa T, Furusawa C, et al. (2012). Understanding the mechanism of heat stress tolerance caused by high trehalose accumulation in Saccharomyces cerevisiae using DNA microarray. J Biosci Bioeng 113:526–8.
- Prasad NK, Rathinasamy K, Panda D, et al. (2007). Mechanism of cell death induced by magnetic hyperthermia with nanoparticles of gamma-MnxFe2−xO3 synthesised by a single step process. J Mater Chem 17:5042–51.
- Marcos-Campos I, Asin L, Torres TE, et al. (2011). Cell death induced by the application of alternating magnetic fields to nanoparticle-loaded dendritic cells. Nanotechnology 22:205101.
- Goya GF, Asin L, Ibarra MR. (2013). Cell death induced by AC magnetic fields and magnetic nanoparticles: current state and perspectives. Int J Hyperthermia 29:810–18.
- Petryk AA, Giustini AJ, Gottesman RE, et al. (2013). Magnetic nanoparticle hyperthermia enhancement of cisplatin chemotherapy cancer treatment. Int J Hyperthermia 29:845–51.
- Vermeer AWP, Bremer MGEG, Norde W. (1998). Structural changes of IgG induced by heat treatment and by adsorption onto a hydrophobic Teflon surface studied by circular dichroism spectroscopy. Bba-Gen Subjects 1425:1–12.
- Vermeer AWP, Norde W. (2000). The thermal stability of immunoglobulin: unfolding and aggregation of a multi-domain protein. Biophys J 78:394–404.