Abstract
Objectives: Breast fibroadenomata (FAD) are the most common breast lumps in women. High intensity focused ultrasound (HIFU) is a non-invasive ablative technique that can be used to treat FAD but is associated with prolonged treatment times. In the HIFU-F trial, we evaluated the change in volume over time with circumferential HIFU treatment of FAD and compared this to no treatment.
Methods: Patients ≥18 years, diagnosed with symptomatic, palpable FAD, visible on ultrasound (US) were recruited. Twenty patients were treated using US-guided HIFU under local anaesthesia. Another 20 participants underwent an US 6 months after diagnosis. Outcome measures included: reduction in treatment time compared to whole lesion ablation; feasibility to achieve a 50% reduction in volume after 6 months; decrease in volume compared to a control group and reduction in symptoms.
Results: Circumferential ablation reduced the mean treatment time by 37.5% (SD 20.1%) compared to whole lesion ablation. US demonstrated a significant mean reduction in FAD volume of 43.5% (SD 38.8%; p = 0.016, paired t-test) in the HIFU group compared to 4.6% (SD 46.0%; p = 0.530) in the control group after 6 months. This mean reduction in FAD volume between the two groups was significant in favour of the HIFU group (p = 0.002, grouped t-test). Pre-treatment pain completely resolved in 6 out of 8 patients 6 months post-treatment.
Conclusion: Circumferential HIFU ablation of FAD is feasible, with a significant reduction in pain and volume compared to control participants. It provides a simple, non-invasive, outpatient-based alternative to surgical excision for FAD.
Introduction
Breast fibroadenomata (FAD) are the most common breast lumps in women and 1 in 10 will develop a FAD during their lifetime [Citation1,Citation2]. Patients with FAD present with a palpable lump, detected during self-examination or incidentally during screening mammography or other imaging of the chest area [Citation2,Citation3]. Ultrasound (US) is the main diagnostic method used, but confirmation can only be obtained by core needle biopsy (CNB) [Citation1–3]. The management of FAD is generally limited to patient reassurance. Excision, either surgically or radiologically (e.g. by vacuum-assisted mammotomy (VAM)) is typically reserved for those women with symptomatic or rapidly growing lesions and results in scarring which may compromise cosmesis [Citation1–3]. Furthermore, surgical excision is most commonly performed under general anaesthesia with its potential complications, in addition to any possible surgical complications. VAM is licensed for diagnostic purposes (not therapeutic) but is nevertheless increasingly used as an alternative to surgery. VAM is performed under local anaesthesia, is invasive, is not suitable for all FAD and may not always be successful in removing the whole FAD due to a decrease in visibility during treatment.
High intensity focused ultrasound (HIFU) is a novel non-invasive ablative technique which has been used for the treatment of liver, kidney, prostate, brain, bone and breast tumours [Citation4–6]. During HIFU treatment, an US beam generated by a piezoelectric US transducer propagates through tissue as a high-frequency pressure wave [Citation5,Citation7]. The beam is focused onto the target tissue and the energy from the beam elevates the temperature of the focus area to 60–95 °C within seconds without causing damage to the directly adjacent tissues, leading to localised protein denaturation and coagulative necrosis [Citation5,Citation7,Citation8]. Depending on the type of application and penetration depth, US beams with a frequency in the range between 0.5 and 4.0 MHz are used [Citation4,Citation5]. HIFU is capable of providing a completely non-invasive therapy, avoiding the potential complications associated with general anaesthesia and surgery [Citation9].
A systematic review by Peek et al. [Citation10] on HIFU in the treatment of breast tumours showed that the most significant drawback with the current HIFU technique is the prolonged treatment time, which ranged from 78 to 171 min. The aim of the HIFU-F trial was to perform circumferential HIFU treatment to isolate the FAD from its blood supply, resulting in necrosis and a reduced treatment time [Citation11]. Furthermore, this is the first case–control study comparing the change in FAD volume after 6 months with and without HIFU treatment.
Materials and methods
A prospective proof-of-principle trial was set up to initially recruit and treat 20 consecutive patients with circumferential HIFU. A further 20 unselected patients were invited to have a control US scan, 6 months after their initial US scan to determine the natural change in size of their FAD when treated conservatively. Written informed consent was obtained from all patients. This study received approval from the national Research Ethics Committee (13/LO/1221).
Patient selection
Patients were included if they were 18 years of age or older and had visited the one-stop Breast Clinic at Guy’s Hospital with a symptomatic FAD – either a palpable lesion or pain developing from this lesion – which was visible on US. Patients more than 25 years of age required histological confirmation of the FAD diagnosis on needle core biopsy. Patients were excluded if they had FAD of 1 cm or less, were pregnant or lactating, had received laser or radiation therapy to the ipsilateral breast, had breast implants, if epithelial atypia was seen or if there was any suspicion of phyllodes tumour. No other exclusions were applied.
Any retro-areolar FAD was treated in a lateral position. For FAD located close to the skin or pectoralis major muscle (<5 mm), local anaesthesia was injected between the FAD and the skin and/or muscle. The optimal target volume was selected in order to avoid thermal damage to skin and pectoral muscle and to cover the most central part of the FAD.
All eligible patients were identified in three ways: (1) at a multi-disciplinary meeting (MDM), where all patients who underwent CNB or fine needle aspiration cytology were discussed, (2) patients scheduled for surgical excision of a FAD and (3) patients referred to the Breast Clinic with a symptomatic breast lump requesting treatment. All patients were approached in the Breast Clinic or by telephone and received a patient information sheet (PIS) if interested in participating in the HIFU-F trial.
Primary outcome measures were the change in treatment time compared to whole lesion ablation (based on the treatment plan), feasibility of achieving a 50% reduction in volume on US after 6 months and the decrease in volume on US compared to an observation only group (control). Secondary outcomes were the complication rate and patient reported outcome measures (palpable FAD, pain symptoms before and after treatment measured with visual analogue scale (VAS) prior and after treatment).
HIFU treatment
Patients were treated using the US-guided Echopulse device (Theraclion Ltd, Malakoff, France) which is dedicated for the treatment of breast FAD and thyroid nodules. The device contained a cooling and coupling disposable unit to cool the skin and prevent burning. Breast lesions were ablated under real-time US guidance using a 7.5–12 MHz diagnostic US transducer. Therapeutic US energy was produced by a 56 mm diameter 3.0 MHz treatment transducer with a central hole measuring 11 mm for the coaxial imaging transducer. The transducer ablates an oval tissue volume of approximately 9 mm in length and 2 mm in width.
All patients were treated as a day-case and under subcutaneous local anaesthesia (1.0% lidocaine with adrenaline and 0.25–0.5% bupivacaine, ratio 1:1, mean 23.1 ml, SD 8.1 ml). In the case of FAD located close to the muscle, anaesthesia was injected deep, between the muscle and the FAD, in order to avoid pain resulting from heating of the pectoralis major muscle. Depending on the position of the FAD and the size of the breast, the patient was placed in either a supine or lateral position and an immobilisation system was used to fix the breast. After an US scan with a handheld probe to locate the FAD, the device head was positioned on top of the FAD to outline the lesion and the skin in the radial and anti-radial views (). For every radial slice, treatment pulses were visualised and the skin and FAD outlines were adjusted when required. The procedure started with a single pulse in the centre of the FAD to determine the right energy level, identified by a hyper-echoic mark visible right after or during administration of the pulse. During subsequent treatment pulses no hyper-echoic mark was required and pulses were not repeated when no mark was seen. The HIFU device calculates the energy and power level of each pulse during treatment. In the HIFU-F study, only the circumference of FADs was ablated; two circumferential rings around the FAD were treated and the centre of the FAD was deselected (). The Echopulse device treats only one central, top or bottom disc-shaped target volume (curved or horizontal) of the FAD during a treatment session. Most FAD require only one target volume as this is sufficient to cover the whole lesion. After the final pulse, the patient’s skin was observed for any treatment changes. Patients were then discharged following hospital protocol. Patients were asked to provide a pain score after the procedure for intra- and post-treatment pain on a VAS of 0–10.
Figure 1. High intensity focused ultrasound (HIFU) treatment of breast fibroadenoma using the Echopulse device (Theraclion Ltd., Malakoff, France).
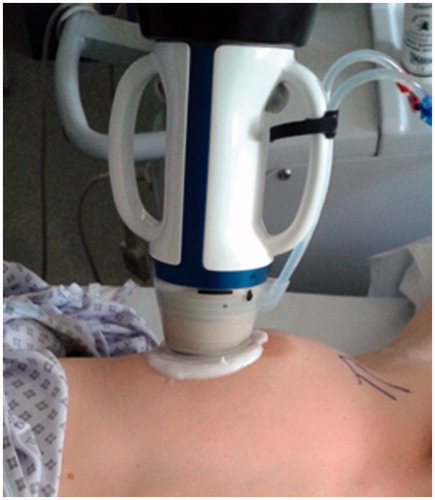
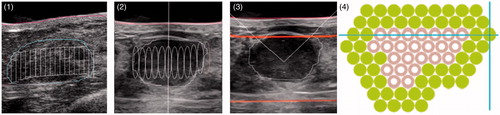
Figure 2. Treatment planning and final treatment. From left to right: (1) manual drawing of outline of fibroadenoma (FAD) (blue) and skin (red) on touchscreen unit in anti-radial position and number of treatment pulses (white) calculated by the Echopulse; (2) radial view of target volume with treatment pulses (white cylinders) calculated by the Echopulse; (3) application of treatment pulse in centre of FAD; (4) final treatment of two circumferential rings, showing completed pulses (green) and deselected pulses (grey).
Treatment time from the beginning of the first to the end of the last pulse administered was recorded, along with the number of pulses delivered. The average time to deliver a treatment pulse was calculated (including any delays between pulses due to treatment pauses) and used to estimate total treatment time for delivering the pulses required to cover the whole lesion.
Follow-up
Patients were followed up at 2 weeks, 3 and 6 months with physical examination and an US scan. The ultra-sonographers performing the US scans were not blinded but the consultant was blinded from the US results during physical examination. The decrease in FAD volume was determined using standard formulae in which V is the FAD volume and A, B and C are the longest diameters of the FAD measured on US [Citation12]:
Control group
A further 20 patients were consecutively recruited, without matching with the HIFU group, to determine the natural course in volume change of their FAD, as assessed by US, 6 months after initial presentation. Patients were recruited using the same inclusion and exclusion criteria as the HIFU treatment group. The change in volume of these patients was compared to the change in volume of patients in the HIFU treatment group.
Statistical analysis
Statistical analysis was performed using IBM SPSS statistics version 23 (SPSS Inc., Chicago, IL). A paired t-test and Wilcoxon signed rank test were used to determine the significance of the reduction in FAD volumes over time. A Kolmogorov–Smirnov test was used to determine if there were any differences in distribution between the HIFU and the control group in terms of age and initial FAD volume. A grouped t-test, Levene’s test and Mann–Whitney U-test were used to determine if there was a significant difference in volume reduction between the HIFU and control groups.
Results
Patients screened
A total of 262 patients with FAD were screened prospectively at the MDM between January 2014 and October 2014. Of these 262 patients, 122 patients (45.3%) met all inclusion criteria, 82 patients were contacted and 20 patients (7.6%) agreed to participate in the HIFU-F trial.
Patient characteristics
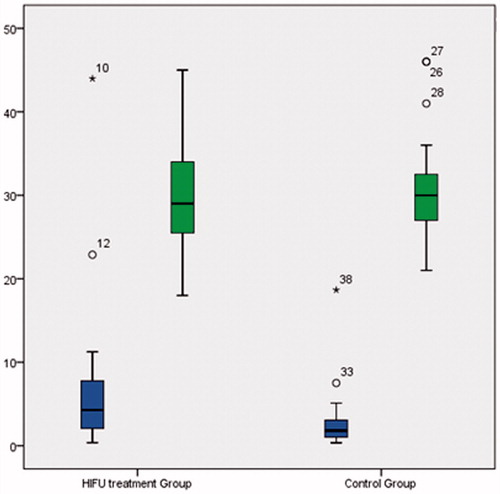
These 20 patients (HIFU group) with symptomatic palpable FAD (8 patients with pain related to FAD) successfully underwent circumferential HIFU treatment. Patients had a mean age of 30.3 years (SD 7.5 years, range 18–45 years) and mean FAD volume of 7.3 cm3 (SD 10.1 cm3, range 0.4–44.0 cm3). A further 20 patients (control group) with biopsy confirmed FAD successfully underwent a follow-up US 6 months after initial diagnosis. Patients had a mean age of 31.3 years (SD 6.5 years, range 21–46 years), not significantly different to the HIFU group (p = 0.819, Kolmogorov–Smirnov test, ). The mean FAD volume was 3.0 cm3 (SD 4.1 cm3, range 0.4–18.7 cm3), again not significantly different to the HIFU group before treatment (p = 0.082, Kolmogorov–Smirnov test, ).
HIFU treatment
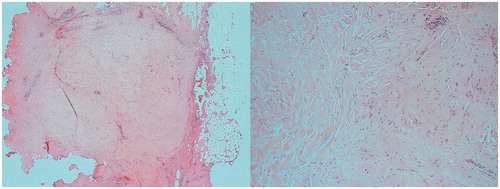
Two circumferential rings were successfully treated in 10 patients; one circumferential ring was successfully treated in 9 patients (5 of whom almost completed treatment of two rings apart from one or two pulses) due to patient movement or pain during treatment. One patient was unable to tolerate a complete circumferential ring of pulses due to pain in her arm (repetitive strain injury). Two patients underwent surgery post-HIFU due to absence of decrease in FAD size 3 and 12 months after HIFU treatment, respectively. Histology demonstrated residual FAD but with prominent areas of fibrosis (). Mean energy per HIFU treatment was 134.6 joules (SD 19.3 joules) and mean power per treatment was 33.3 watts (SD 4.8 watts).
Treatment times
The mean recorded treatment time from first sonication to last sonication was 34.6 min (SD 10.5 min). Circumferential ablation reduced the treatment time by an average of 37.5% (SD 20.1%) compared to the treatment time calculated for whole lesion ablation. Total treatment recorded time of patients being in theatre was 68.7 min (SD 16.2 min).
Pain symptoms
Eighteen of 20 patients experienced some discomfort or a burning sensation during the procedure. The mean maximum pain VAS score during treatment was 6.4 (SD 3.2). By moving to another part of the FAD, the treatment was continued with agreement of the patient in 17/18 patients. Mean maximum pain VAS score immediately after treatment was 1.6 (SD 1.9).
At 6-month follow-up, 6 out of 8 patients who experienced pre-treatment pain had complete resolution of their symptoms. Two patients developed post-treatment pain, which resolved within 3 months.
Complications
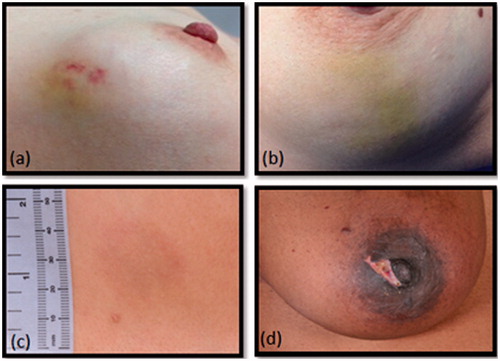
Short-term complications () at 2 weeks were: ecchymosis (n = 9), erythema (n = 6), hypo-pigmentation of the skin (n = 1), dimpling of the skin (n = 1), numbness of the skin (n = 1) and a superficial first-degree skin burn (n = 1). All short-term complications completely resolved within the first month post-treatment without the need for intervention. Hyper-pigmentation was found at 3 months in 6 patients and persisted at 6 months in 4 patients.
Volume measurements by US
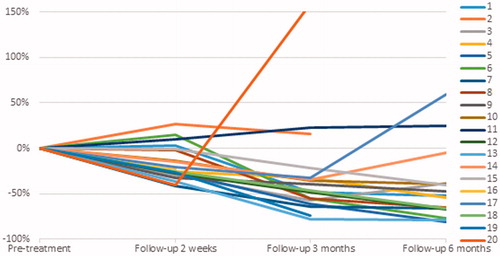
US scans at 2 weeks post-treatment showed hyper-echogenicity and oedema at the circumference of the lesion in some patients. The FAD had a mean volume of 6.1 cm3 (SD 8.4 cm3); a mean decrease in volume of 16.8% (SD 19.3%) (p = 0.021, paired t-test; Z = −2.688, p = 0.007, Wilcoxon signed rank test). At 3 months the volume was 5.0 cm3 (SD 6.5 cm3); a mean decrease in volume of 30.9% (SD 52.7%) (p = 0.022; Z = −2.535, p = 0.11). At 6 months the mean volume was 4.6 cm3 (SD 6.4 cm3), a decrease in volume of 43.5% (SD 38.8%) (p = 0.016; Z = −2.741, p = 0.006) (, ). At 6 months, 4 patients had no palpable lesion.
Table 1. Volume changes during 6-month follow-up in (a) high intensity focused ultrasound (HIFU) treatment group and (b) control group.
Control group
At the 6-month US scan the mean volume was 2.6 cm3 (SD 2.3 cm3), a non-significant change in size of 4.6% (SD 46.0%) (p = 0.530, paired t-test; Z = −0.073, p = 0.709, Wilcoxon signed rank test; ). Compared to the control group the HIFU group showed a significant change in volume over a period of 6 months (p = 0.002, grouped t-test; U = 58, p = 0.001, Mann–Whitney U-test).
Discussion
The current treatment of symptomatic or rapidly growing FAD is surgical excision, however, scarring can compromise the cosmetic outcome [Citation1–3]. VAM is licensed for diagnostic purposes (not therapeutic) but is nevertheless increasingly used as an alternative to surgery. A non-invasive alternative technique is therefore required, which allows the patient to undergo treatment without scarring, surgery and general anaesthesia and which allows for intra-operative visibility of the lesion and a low side-effect profile.
HIFU has demonstrated effectiveness in the treatment of benign breast disease using total lesion ablation [Citation13–15]. Hynynen et al. [Citation13] treated 11 FAD and found a decrease in volume of 32% (1.9–1.3 cm³) measured on T2-weighted magnetic resonance imaging after 6 months. Patients were treated with local anaesthesia and mild sedation. Slight pain was reported in 4 patients, mild pain in 2 and severe pain in 1 patient. No complications other than pain and swelling were reported. More recently, Kovatcheva et al. [Citation14] found a FAD volume decrease of 59.2 ± 18.2% (n = 42) on US after 6 months and reported complete resolution of pain in 18 patients who had pre-treatment pain. These patients were treated under conscious sedation and the mean pain score during treatment was 29.7 (SD 27.5) using a 0–100 mm VAS scale. Three superficial skin burns with blister-like aspects, and single cases of subcutaneous induration and hyper-pigmentation were reported. Cavallo Marincola et al. [Citation15] found a decrease of 50% at 3 months (n = 10). Local anaesthesia and conscious sedation were administered during treatment. The number of patients with pre-treatment pain was not reported, but none of the patients had pain at 6 months. No complications were observed at 3 months apart from swelling and hardness of the treated area.
A systematic review by Peek et al. [Citation10] showed that the most significant drawback with HIFU was the prolonged treatment time associated with it, which ranged between 78 and 171 min. The HIFU-F trial demonstrated that a high mean volume reduction of 43.5% (SD 38.8) can be achieved at 6 months with a circumferential ablation, whilst reducing mean treatment times to 34.6 min (SD 10.5). This reduction in treatment time made it possible to perform the procedure under local anaesthetic only, without the need for sedation – unlike previous studies. The treatment time for whole lesion ablation was calculated using the average time to deliver a single pulse, recorded during circumferential HIFU ablation. Although this included some variability caused by patient movement and repositioning, the calculated time might still be an under-estimation as patients with larger tumours and therefore a longer HIFU treatment time, may move more and require more time for repositioning.
It is likely that the circumferential ablation is successful through targeting the “feeding vasculature” to the FAD. This could warrant the use of Doppler US imaging to target feeding vessels in real time in future studies. In 4 patients an increase in FAD volume was seen on US post-treatment and in 1 patient the FAD did not change in size. A hypothesis about the increase in FAD size is that treatment could not be completed due to pain during treatment (n = 2), furthermore, HIFU only treats a disc and not the top and bottom of the FAD, due to distance restrictions to the skin (>5 mm) and pectoralis major (>5 mm). Two patients with increased FAD underwent surgical excision, 1 patient was happy to leave the FAD alone and the last patient was lost to follow-up.
After HIFU treatment, the FAD initially increases in size due to inflammation and oedema. This resolves within the first 2 weeks post-treatment. As seen in other trials [Citation14], the largest decrease in size is observed between the third and sixth months, followed by a slower decrease between 6 and 12 months. It is important for patients to be aware of this gradual decrease in size, as opposed to the instant removal of the lump with surgical excision, as patients might be anxious about the persistence of a lump. Patient selection is therefore a very important factor. It is important for patients to be aware of the gradual decrease in size of the FAD after treatment and accomplishing impalpability of the lump might not always be achievable.
Six out of 8 patients showed a resolution of pain symptoms post-treatment, this is most likely due to the treatment damaging the local sensory pain receptors thereby blocking the pain feedback pathway. Clearly FADs can cause pain and HIFU can be used if the pain is located in the FAD. Kovatcheva et al. [Citation14] also reported a resolution in FAD pain after HIFU treatment. For the control group the only measured outcome was change in volume and we did not assess pain scores. Volume was evaluated using an US to determine the FAD size after 6 months. Patients of both the control and the HIFU groups were recruited using the same inclusion and exclusion criteria without selection. However, since this study was not randomised, the control group might have been less symptomatic compared to the HIFU group, since they did not request treatment.
After 6 months the FAD became impalpable in 4 patients and in the other 16 patients, the lump was still palpable but was more diffuse and therefore harder to feel. During follow-up, however, patients were not concerned about the residual lump. On US the FAD was seen to be fragmented in some cases and more integrated with the surrounding tissue. Patients were very conscious of their treated FAD due to their participation in the trial, sometimes resulting in an over-estimation of the size of the lesion, compared to the size measured on US.
All complications recorded at 2 weeks completely disappeared within a month post-treatment without the need of any additional treatment. One patient developed a superficial first-degree skin burn during HIFU treatment; caused by either the micro-foam used for immobilisation of the breast or air between the probe and the skin. The patient required no treatment and the burn completely resolved within 1 month post-treatment. This patient was not concerned with this complication and attended the HIFU clinic 1 month later for further HIFU treatment of a contralateral FAD. When placing the treatment probe on the skin, care is needed to make sure the probe is not placed on the site of local anaesthesia injection. Small air bubbles might be left at this site and when located in the US beam this might cause cavitation and in the worst case cause a blister or skin burn. Altered skin pigmentation was seen in 4 patients at 3 months and in 4 patients at 6 months. Skin pigmentation was more common in patients with a darker pigment colour, however, skin pigmentation also occurred in a patient with pale skin.
Altered skin pigmentation can be caused by a high treatment power or a shorter distance between the FAD and the skin resulting in overheating of the skin. However, the mean treatment power and distance between skin and FAD within these 6 patients was less than the average for the complete HIFU group (33.0 W/site vs. 33.3 W/site and 3.7 mm vs. 4.7 mm for the patients altered with skin pigmentation and the HIFU-F trial group, respectively). Another possible explanation is that these patients did not feel much discomfort during treatment and therefore treatment could continue without interruptions, resulting in overheating of the skin. This theory could be correct for the first 3 patients but the latter 3 patients did feel more pain, resulting in more treatment breaks. Although none of the 6 patients were concerned with hyperpigmentation, further investigation is required to determine the cause of the altered pigmentation. It is important to inform future patients of this potential complication as was done in our study.
Compared to the control group, in the HIFU group there was a significant reduction in volume of the FAD over a period of 6 months. The significant difference in volume demonstrated that HIFU as a non-invasive technique can be used for the treatment of FAD.
However, there are a few drawbacks of the technique. Even with assistance of an immobilisation system, it was difficult to position and immobilise patients with smaller breasts. A more advanced immobilisation system is required to be able to perform more accurate treatment and faster positioning of patients. Furthermore, even with local anaesthesia, patients were found to have discomfort during treatment. This might be a result of pulses being administered on local sensory pain receptors. This would also explain why, when further pulses were applied at a painful location, the area was not be as uncomfortable as before. Treatment pulses given at the border of the FAD and the surrounding tissue could be more painful as well. More anaesthesia in the form of pre-treatment oral painkillers, topical cream or pectoral blocks should be evaluated in future studies.
The ideal patient for HIFU treatment would therefore be one who has a symptomatic palpable FAD located at least 5 mm from both the skin and the pectoralis major and with a size of about 10–30 mm. These inclusion criteria are similar to those used for VAM. Furthermore, patients should accept an approximated 50% decrease in volume over a period of 6 months as an alternative to surgical scarring.
Conclusion
Circumferential HIFU ablation of FAD is feasible with a significant reduction in treatment time (mean 37.5%, SD 20.1) and a significant reduction in volume of the lesion at 6 months (mean 43.5%, SD 38.8%). Furthermore, a resolution of pain symptoms (6/8 patients) and minor short-term complications were found at 6 months follow-up. HIFU has a role in the treatment of FAD, which requires further economic and clinical evaluation.
HIFU-F Trialists’ Group
HIFU-F Trialists’ Group: Prof. Michael Douek (King’s College London, Guy’s and St Thomas’ NHS Foundation Trust), [email protected]; Miss Mirjam Peek (King’s College London, Guy’s and St Thomas’ NHS Foundation Trust), [email protected]; Mr Muneer Ahmed (King’s College London), [email protected]; Miss Julie Scudder (Guy’s and St Thomas’ NHS Foundation Trust), [email protected]; Prof. Rose Baker (University of Salford), [email protected]; Prof. Sarah Pinder (King’s College London, Guy’s and St Thomas’ NHS Foundation Trust), [email protected]; Mr Ashutosh Kothari (Guy’s and St Thomas’ NHS Foundation Trust), [email protected]; Mr Hisham Hamed (Guy’s and St Thomas’ NHS Foundation Trust), [email protected]; Mr Tibor Kovacs (Guy’s and St Thomas’ NHS Foundation Trust), [email protected]; Mrs Sarah MacWilliams (St Bartholomew’s Hospital); Mr Bauke Anninga (King’s College London, Guy’s and St Thomas’ NHS Foundation Trust), [email protected]; Mr Petros Charalampoudis (Guy’s and St Thomas’ NHS Foundation Trust), [email protected], Lorna Cook (King’s College London, Guy’s and St. Thomas’ NHS Foundation Trust), [email protected], Ali Zada (University of Twente), [email protected].
Disclosure statement
The authors report no conflicts of interest.
Funding
This work was supported by an unrestricted educational grant from Theraclion Ltd (Malakoff, France).
References
- Cerrato F, Labow BI. (2013). Diagnosis and management of fibroadenomas in the adolescent breast. Semin Plast Surg 27:23–5.
- Sperber F, Blank A, Metser U. et al. (2003). Diagnosis and treatment of breast fibroadenomas by ultrasound-guided vacuum-assisted biopsy. Arch Surg 138:796–800.
- Greenberg R, Skornick Y, Kaplan O. (1998). Management of breast fibroadenomas. J Gen Intern Med 13:640–45.
- Wu F, Wang ZB, Cao YD. et al. (2003). A randomised clinical trial of high-intensity focused ultrasound ablation for the treatment of patients with localised breast cancer. Br J Cancer 89:2227–33.
- Schmitz AC, Gianfelice D, Daniel BL, et al. (2008). Image-guided focused ultrasound ablation of breast cancer: current status, challenges, and future directions. Eur Radiol 18:1431–41.
- Maloney E, Hwang JH. (2015). Emerging HIFU applications in cancer therapy. Int J Hyperthermia 31:302–09.
- Haar GT, Coussios C. (2007). High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia 23:89–104.
- Kim SH, Jung SE, Kim HL, et al. (2010). The potential role of dynamic MRI in assessing the effectiveness of high-intensity focused ultrasound ablation of breast cancer. Int J Hyperthermia 26:594–603.
- Payne A, Todd N, Minalga E. et al. (2013). In vivo evaluation of a breast-specific magnetic resonance guided focused ultrasound system in a goat udder model. Med Phys 40:073302.
- Peek MC, Ahmed M, Napoli A. et al. (2015). Systematic review of high-intensity focused ultrasound ablation in the treatment of breast cancer. Br J Surg 102:873–82; discussion 882.
- Peek MC, Ahmed M, Douek M. (2015). High-intensity focused ultrasound for the treatment of fibroadenomata (HIFU-F) study. J Ther Ultrasound 3:6.
- Krekel NM, Zonderhuis BM, Stockmann HB. et al. (2011). A comparison of three methods for nonpalpable breast cancer excision. Eur J Surg Oncol 37:109–15.
- Hynynen K, Pomeroy O, Smith DN. et al. (2001). MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology 219:176–85.
- Kovatcheva R, Guglielmina JN, Abehsera M. et al. (2015). Ultrasound-guided high-intensity focused ultrasound treatment of breast fibroadenoma – a multicenter experience. J Ther Ultrasound 3:1.
- Cavallo Marincola B, Pediconi F, Anzidei M. et al. (2015). High-intensity focused ultrasound in breast pathology: non-invasive treatment of benign and malignant lesions. Expert Rev Med Devices 12:191–9.