Abstract
Purpose: Laser ablation (LA) is used as therapeutic modality for reducing the volume of large benign thyroid nodules. The aim of this retrospective study was to assess the efficacy of LA therapy in patients with benign non-functioning thyroid nodules in a 4-year follow-up and evaluate whether different compactness of nodules may influence the final shrinkage.
Patients and methods: Fifty-six euthyroid patients (42 females; mean age 54.7 ± 11.7 years) with benign cold thyroid solitary nodules or a dominant nodule within a multinodular goitre underwent LA between July 2009 and March 2012. Nodule volume, thyroid function test and ultrasound were monitored at baseline, and at 3, 6 and 12 months after the procedure, then annually.
Results: With a mean baseline volume of 15.7 ± 11.7 mL, nodule volume decreased by 55.5% (6.5 ± 5.7 mL) 4 years after LA (p < .01). Nodules had a significant decrease at 3 and 6 months, thereafter they remained stable, with an insignificant tendency to re-growth at 48 months. Thyroid functions and antibodies were unchanged throughout the follow-up. When dividing nodules into solid and spongiform, the former decreased at 6 months, remained stable up to 24 months, but showed a non-significant tendency to increase thereafter. Spongiform nodules progressively decreased up to 48 months. The difference in nodules’ reduction between solid and spongiform nodules was significant from 3 months (p = .04) and became even more significant up to 48 months (p = .001).
Conclusions: The LA technique succeeded in reducing thyroid nodules by about 50% at 4 years, but was more effective for spongiform than solid nodules.
Introduction
The management of thyroid nodules is sometimes still problematic. The decision-making depends, other than on the nodule itself, also on several other factors like patient’s age, preference, comorbidities and surgical risk factors [Citation1,Citation2]. Most thyroid nodules with benign characteristics at ultrasound and negative cytological criteria can be simply followed up [Citation1,Citation2]. However, some nodules show a progressive growth over time and become symptomatic or are associated with cosmetic concerns. Until a few years ago, since there was no medical treatment that could effectively decrease the size of the nodules, thyroidectomy or lobectomy represented the only therapeutic and definitive option in such cases [Citation3,Citation4]. However, the surgical option may be weighed by permanent hypoparathyroidism, may be not indicated for patients with high surgical risk and entails permanent hypothyroidism with variable aesthetic damage to the neck [Citation5].
Over the last 15 years, image-guided, minimally invasive techniques have been offered for the management of clinically relevant benign thyroid nodules [Citation6,Citation7]. Percutaneous ethanol injection is an effective, fast and inexpensive treatment for cystic thyroid lesions [Citation8,Citation9]. Laser ablation (LA) or radiofrequency (RF) is more commonly used, especially in Europe and the Far East, for large solid or complex thyroid nodules [Citation10–12].
In particular, LA was the first minimally invasive technique that was tested and introduced in Italy by Pacella et al. [Citation13]. Later on, several studies confirmed that LA and RF are effective, safe and cost-effective for the treatment of cold, cystic and hot thyroid nodules [Citation14–19].
The aim of the present study was to retrospectively evaluate the long-term effect of LA treatment in a 4-year follow-up, and whether pre-ablation ultrasound-detected structural differences may influence the performance of LA.
Patients and methods
We retrospectively evaluated the clinical records of patients who underwent LA from July 2009 (when the LA procedure was started) to March 2012 at “V. Fazzi” Hospital, Lecce, Italy. All patients were referred because of a palpable nodule that caused cosmetic or pressure symptoms. Patients submitted to LA needed to have: (1) two benign cytological findings; (2) normal serum thyroid-stimulating hormone (TSH), free T4 (FT4) and free T3 (FT3) concentration; (3) hypoactive appearance at 99mTc thyroid scintiscan; (4) no prior thyroid gland treatment; and (5) negative calcitonin values. Patients receiving LT4/LT3 therapies, iodine supplements and drugs interfering with thyroid function, those with a history of external radiotherapy or radioiodine exposure were excluded. As per our institution protocol, patients were monitored at 3 and 6 months, and then annually after LA. Monitoring consisted of thyroid ultrasound (US) and determination of FT4, FT3, TSH, thyroglobulin antibodies (TgAb), peroxidase antibodies (TPOAb), thyroglobulin (Tg) and calcitonin. All thyroid US images were stored. The US texture of a thyroid nodule was categorised into: (1) solid or (2) spongiform.
A solid nodule was defined as a nodule with absent or nearly absent cystic content; those nodules with a well-defined cystic portion underwent fluid drainage before LA; a spongiform nodule was defined as a nodule with the presence of microcystic spaces intervening in more than 50% of the isoechoic partially cystic nodule.
For the purpose of this retrospective study, one endocrinologist (RN) and one radiologist (GG) with a long-standing experience in thyroid nodules, independently and in a blinded manner re-evaluated patients’ nodules into the two above-mentioned categories; discordant opinions were discussed together and a final decision was taken accordingly.
Laboratory evaluation
Serum TSH, FT3, FT4, Tg, TPOAb, TgAb and calcitonin were assessed as per the scheduled protocol (baseline, 3 and 6 months, and then annually). Serum TSH, FT3, FT4 and Tg were measured using a third-generation electrochemiluminescence immunoassay (Roche, Basel, Switzerland). Reference values were 0.27–4.2 mIU/L for TSH, 2.2–4.2 pg/mL for FT3, 0.8–1.7 ng/dL for FT4 and 0.2–70 ng/mL for Tg. TPOAb and TgAb were determined using a radioimmunoassay kit (DiaSorin, Saluggia, Italy); the reference range was 0–16 IU/mL for TPOAb and 5–100 for TgAb. Calcitonin was determined with commercially available immunoradiometric assay kits (normal values <10 ng/mL).
Thyroid sonographic evaluation was conducted at baseline and after 3 and 6 months and then annually by means of a commercially available US scanner (Esaote, Genova, Italy) equipped with a 7.5–13.0 MHz linear transducer. The nodule volume was calculated with the ellipsoid formula.
Laser ablation procedure
Light conscious sedation was obtained with intravenous midazolam (2–5 mg) in fractioned boli. After US examination of the neck and the definition of the entry point of the needles, local anaesthesia was performed with an injection of 2% xylocaine from the skin deep to the thyroid capsule. LA was carried out in a single session, inserting 21-gauge spinal needles into the target thyroid lesion under US monitoring. After the free-hand positioning of the needle tips, under US monitoring, a 300-μm-diameter plane-cut quartz optical fibre was introduced through the sheath of the needles, and the fibre tip was placed in direct contact with the tissue. Optic fibres were connected with the laser source, a continuous-wave Nd-YAG laser operating at 1064 μm with an optical beam splitting device (Elesta, Florence, Italy) and an output power of 3 W, according to a previously described technique [Citation13]. One to three needles were placed manually along the longitudinal, cranio-caudal and major nodule axis, at a distance of 10 mm each, fitting at best to the shape of the nodule. The procedure was started with a deposition energy of 1200–1800 J per fibre, in the caudal part of the nodule, 10 mm from the lower margin, the trachea and the carotid. By upward needle/fibre pull backs of 10 mm, additional energy was administered until a distance of 5–10 mm from the upper part of the nodule was reached. At US monitoring, the area under treatment was visualised as a hyperechoic zone enlarging over time due to the formation of gas microbubbles within the coagulated tissue. After treatment, the patients were given an iv injection of ketoprofen.
Statistical analysis
The Wilcoxon test was used to evaluate the absolute change in volume between two consecutive periods. An unpaired t-test was used to compare differences between solid and spongiform nodules. A value of p < .05 was considered as significant. Values are expressed as means ± SD or as a percentage. Statistical analysis was performed using SPSS (Chicago, IL).
Results
Eighty-four patients underwent LA between July 2009 and March 2012. A total of 20/84 (23.8%) were excluded from the analysis because 8 (9.5%) were lost at follow-up and 12 (14.3%) because all the six requested scheduled follow-up data were not present (12 had spongiform nodules and 8 had solid nodules); 7/84 (8.3%) patients were referred to surgery; 6 out of the 7 patients were referred to surgery because of progressive nodule enlargement, 1 patient was referred to surgery because of a tracheal perforation induced by LA; 1 patient was excluded because of pregnancy. We finally obtained 56 patients who completed the 4-year follow-up schedule: 42/56 (75%) were females; 5 (8.9%) were positive for TPOAb; 3 (5.4%) were positive for TPOAb and TgAb; and 6 (10.7%) were positive for TgAb only.
The energy delivered (joules) was 6168.1 ± 3356; 1.8 ± 0.6 fibres per patient were used with 3.3 ± 2.0 illuminations. Characteristics of nodules (volume), volume decrease and thyroid function are shown in and .
Figure 1. Nodule volume in 56 patients treated with percutaneous laser ablation for benign thyroid nodule during the 4-year follow-up.
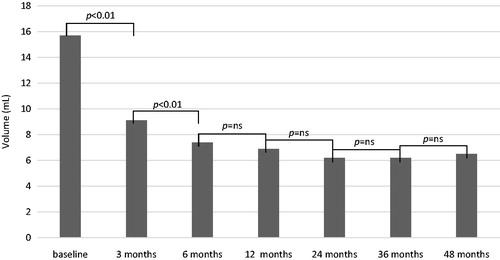
Table 1. Nodule volume and laboratory test in 56 patients treated with laser ablation for benign thyroid nodule during the 4-year follow-up.
In the whole cohort of patients, the nodules’ volume significantly decreased at 3 and 6 months, then showed to be stable from 12 months onward, with a final reduction rate of 55.5%.
Thyroid function tests and thyroid antibodies showed insignificant changes throughout the 4-year follow-up.
When dividing patients depending on the final volume decrease in respect to baseline, of the 18 patients who at 48 months had a decrease less than 50% and/or re-growth, 15 had a solid nodule and 3 a spongiform nodule (p = .02); of the 38 patients who at 48 months had a decrease more than 50% and no re-growth, 27 had a spongiform nodule and 11 a solid nodule (p = .04) ( and ). If we include in the analysis those who underwent surgery for nodule re-growth (6 patients), 1 had spongiform and 5 had solid nodules; thus, we obtain a total of 24 patients who had a decrease less than 50% and/or re-growth that in six cases led to surgery; of these 24 patients, 20 had a solid nodule and 4 had a spongiform nodule (p = .01).
Figure 2. (A) Spongiform nodule (21 mm ×17 mm ×23.5 mm, volume: 4.34 mL) before laser ablation; (B) 4 years after laser ablation (7.6 mm ×8.0 mm ×7.8 mm, volume: 0.25 mL).
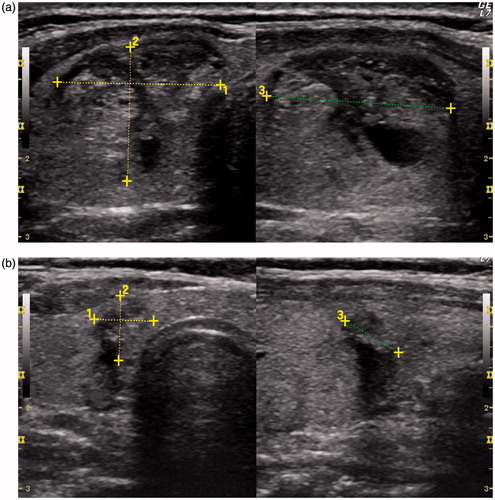
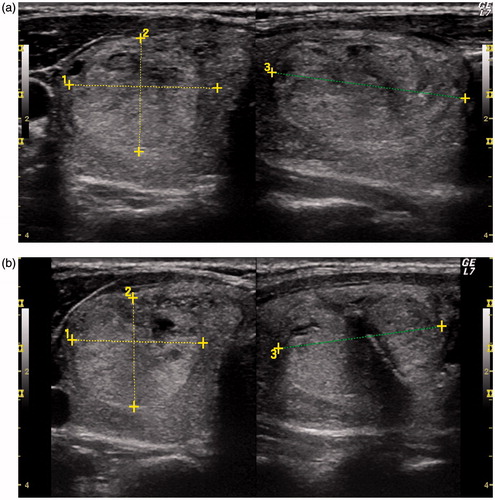
Figure 3. (A) Solid nodule (23.5 mm ×19.5 mm ×31 mm, volume: 7.4 mL) before laser ablation; (B) 4 years after laser ablation (25 mm ×19 mm ×31 mm, volume: 7.9 mL).
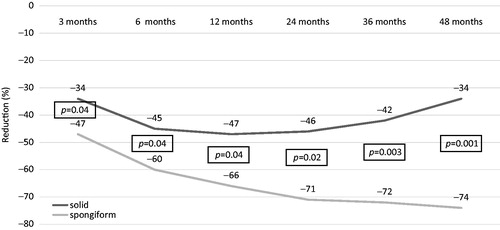
We further analysed the nodule decrease dividing patients depending on the characteristics of nodules, that is, solid vs. spongiform. With a baseline similar volume (16.8 ± 14 for spongiform and 14.3 ± 8.3 for solid), solid nodules decreased up to 6 months, remained stable up to 24 months, but tended to increase thereafter. Spongiform nodules progressively decreased up to 48 months. The difference in nodules’ reduction between solid and spongiform nodules was significant at 3 months and became more and more significant up to 48 months ().
Figure 4. The volume reduction of solid (n = 26) and spongiform (n = 30) nodules over 4-year follow-up.
Adverse events: 4/56 patients (7.1%) experienced periprocedural pain, 3/56 patients transient vocal cord paresis (5.4%). One patient, 50 days after the procedure, started to have symptoms related to a tracheal stenosis (dyspnoea and stridor); after tracheoscopy, a tracheal perforation was diagnosed and the patient underwent a total thyroidectomy plus tracheal suture.
Discussion
US-guided LA has been used for at least a decade in the treatment of symptomatic or steadily growing thyroid nodules that are benign at cytological assessment. Several randomised controlled trials have been published; in all but one, LA treatment was compared with no treatment; the time to follow-up ranged from 6 to 96 months; results showed that nodule volume decreased by 44–55%, and that local symptoms and cosmetic complaints had significant improvements [Citation20–23]. Only one randomised controlled study compared LA vs. levothyroxine suppressive therapy and vs. no intervention, demonstrating the overwhelming superiority of LA (42.7% decrease) compared to levothyroxine (non-significant shrinkage), and no intervention (non-significant increase) [Citation3]. The present retrospective 4-year follow-up study confirmed that a significant shrinkage in nodules’ volume can be obtained, as compared to baseline (−41% at 3 months), that the volume was significantly reduced up to 6 months (−53%) and then remained substantially stable up to 48 months (−55%).
In the 3-year multicentre prospective randomised trial by Papini et al. [Citation21], no clinical or US baseline feature appeared as a predictive factor of a thyroid nodule volume reduction >50%, although the probability of a nodule volume reduction >50% seemed to be greater, even if not significantly, in nodules with a minimal fluid component than in solid nodules. In the 3-year follow-up retrospective study by Valcavi et al. [Citation24], 11/122 patients (9%) experienced a significant nodule re-growth: of these 11 nodules, 8 were solid, whereas 3 were spongiform (p = ns). Gambelunghe et al. [Citation25] demonstrated that LA obtained different effects in a single nodule and in a nodular conglomerate, as the latter showed a smaller reduction at 6 months and a significant tendency to re-growth at 12 months. Compared to the above-mentioned studies evaluating the US characteristics of thyroid nodules, the novelty presented in the present study derived from differentiating solid from spongiform nodules. The performance of LA in these two structurally different nodules was significantly different. While solid nodules tended to re-growth from 2 years onward, spongiform ones continued to shrink. Then, the mean of values we obtained mixing together these two populations of nodules, demonstrated a positive long-term effect, yet mainly due to the progressively reduced volume of the spongiform nodules. This finding was enforced by the evidence that those patients who underwent surgery for nodule’s enlargement more frequently carried a solid nodule. The clue that LA performed better in spongiform than solid nodules certainly needs more confirmation, but it may be logically explained by the different densities of the nodules treated with elevated temperature [Citation26]. It may be speculated that solid nodules, due to their compactness, attenuate the heat diffusion more than spongiform ones; indeed, the thermal energy required to induce cell death varies in different tissues. The exact temperature at which the tissue is not viable any more is multifactorial and tissue-specific; indeed, the critical temperature at the edge of the coagulative zone showed relevant variation of the thermal dose required to induce cell death in normal neoplastic tissues [Citation27].
Severe adverse events due to LA procedures as subcapsular haematoma, light-headedness, skin burn, cervical swelling and cystic transformation have been previously reported, but are very rare [Citation24,Citation28,Citation29]. In our series of patients, one of the first treated patients had a tracheal perforation (1 × 2 cm on the right posterior-lateral part of the second and third tracheal ring); this risk, as the risk of laryngeal nerve damage appears higher for the period of operator training, and during this stage, thyroid nodules should be approached very carefully [Citation30]. We also observed few cases of transient vocal cord paresis; therefore, our experience was not different from the data of 1531 laser-treated patients from eight Italian thyroid referral centres that showed a 0.9% rate of complications, with half of these represented by transient voice changes and half by minor events [Citation31].
Laser ablation is relatively inexpensive. The treatment is performed by a team of two physicians and one nurse in an outpatient setting. The time requested for the complete procedure is 30–40 min, and the rough cost of optical fibres and disposables is about €600 [US$806] per patient. There is no cost-effective analysis that compares LA vs. surgery, however, one study comparing RF (which is more expensive than LA) vs. haemithyroidectomy found RF cheaper by far [Citation32].
In a randomised trial, whether or not patients with thyroid autoimmunity were included, no significant changes in thyroid function or thyroid autoimmunity were observed [Citation21,Citation22]. In the retrospective study by Valcavi et al. [Citation24] among 122 patients, 2 developed hyperthyroidism and 2 hypothyroidism, but this finding looks unrelated to the LA procedure. In our series of patients, those positive for TPOAb and/or TgAb were not excluded, but did not develop any thyroid dysfunction as well as those who were thyroid antibody negative. Of those without thyroid antibodies, none developed thyroid autoimmunity. Therefore, our results confirmed that the LA procedure was not associated with increased risk of new onset of thyroid dysfunction and/or autoimmunity.
As a conclusion, the main finding of the present study was that LA performed better in spongiform than solid nodules, as the former showed a major, progressive and durable shrinkage while the latter showed a tendency to re-growth. Our results should be considered as suggestive and not conclusive, given the retrospective evaluation of data; another drawback may be represented by the fact that about 10% of the patients were lost to follow-up and that about 14% were not considered because of incomplete data. On the other hand, the exclusion of patients with incomplete data made the evaluated sample strictly selected, resulting in reliable results. Globally our results confirmed the efficacy of LA as a validated alternative to surgery, and evidence is so consistent that such treatment has been included in the guidelines for the diagnosis and management of thyroid nodules, released in 2016 by the American Association of Clinical Endocrinologists, American College of Endocrinology and Associazione Medici Endocrinologi [Citation33]. Of note, LA will hopefully open new perspectives in the treatment of thyroid malignant disease; this technique allowed a complete destruction of papillary thyroid cancer having a diameter less than 10 mm and a cytoreduction of cervical lymph node metastases from papillary thyroid cancer [Citation34–36]. Further prospective randomised trials are needed to assess which thyroid nodules benefit most from US-guided ablation techniques, then offering a tailored oriented approach to the patient.
Disclosure statement
The authors have nothing to disclose.
References
- Russ G, Leboulleux S, Leenhardt L, et al. (2014). Thyroid incidentalomas: epidemiology, risk stratification with ultrasound and workup. Eur Thyroid J 3:154–63.
- Paschke R, Hegedüs L, Alexander E, et al. (2011). Thyroid nodule guidelines: agreement, disagreement and need for future research. Nat Rev Endocrinol 7:354–61.
- Papini E, Guglielmi R, Bizzarri G, et al. (2007). Treatment of benign cold thyroid nodules: a randomized clinical trial of percutaneous laser ablation versus levothyroxine therapy or follow-up. Thyroid 17:229–35.
- Bandeira-Echtler E, Bergerhoff K, Richter B. (2014 Jun 18). Levothyroxine or minimally invasive therapies for benign thyroid nodules. Cochrane Database Syst Rev 6:CD004098.
- Bellantone R, Lombardi CP, Bossola M, et al. (2002). Total thyroidectomy for management of benign thyroid disease: review of 526 cases. World J Surg 26:1468–71.
- Papini E, Pacella CM, Hegedus L. (2014). Diagnosis of endocrine disease: thyroid ultrasound (US) and US-assisted procedures: from the shadows into an array of applications. Eur J Endocrinol 170:R133–46.
- Gharib H, Hegedüs L, Pacella CM, et al. (2013). Clinical review: nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab 98:3949–57.
- Valcavi R, Frasoldati A. (2004). Ultrasound-guided percutaneous ethanol injection therapy in thyroid cystic nodules. Endocr Pract 10:269–75.
- Bennedbaek FN, Hegedüs L. (2003). Treatment of recurrent thyroid cysts with ethanol: a randomized double-blind controlled trial. J Clin Endocrinol Metab 88:5773–7.
- Ha EJ, Baek JH, Kim KW, et al. (2015). Comparative efficacy of radiofrequency and laser ablation for the treatment of benign thyroid nodules: systematic review including traditional pooling and Bayesian network meta-analysis. J Clin Endocrinol Metab 100:1903–11.
- Baek JH, Lee JH, Valcavi R, et al. (2011). Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol 12:525–40.
- Deandrea M, Sung JY, Limone P, et al. (2015). Efficacy and safety of radiofrequency ablation versus observation for nonfunctioning benign thyroid nodules: a randomized controlled international collaborative trial. Thyroid 25:890–6.
- Pacella CM, Bizzarri G, Guglielmi R, et al. (2000). Thyroid tissue: US-guided percutaneous interstitial laser ablation – a feasibility study. Radiology 217:673–7.
- Chianelli M, Bizzarri G, Todino V, et al. (2014). Laser ablation and 131-iodine: a 24-month pilot study of combined treatment for large toxic nodular goiter. J Clin Endocrinol Metab 99:E1283–6.
- Rotondi M, Amabile G, Leporati P, et al. (2009). Repeated laser thermal ablation of a large functioning thyroid nodule restores euthyroidism and ameliorates constrictive symptoms. J Clin Endocrinol Metab 94:382–3.
- Døssing H, Bennedbaek FN, Bonnema SJ, et al. (2007). Randomized prospective study comparing a single radioiodine dose and a single laser therapy session in autonomously functioning thyroid nodules. Eur J Endocrinol 157:95–100.
- Døssing H, Bennedbaek FN, Hegedüs L. (2006). Beneficial effect of combined aspiration and interstitial laser therapy in patients with benign cystic thyroid nodules: a pilot study. Br J Radiol 79:943–7.
- Døssing H, Bennedbæk FN, Hegedüs L. (2013). Interstitial laser photocoagulation (ILP) of benign cystic thyroid nodules – a prospective randomized trial. J Clin Endocrinol Metab 98:E1213–17.
- Bernardi S, Dobrinja C, Fabris B, et al. (2014). Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol 2014:934595.
- Gambelunghe G, Fatone C, Ranchelli A, et al. (2006). Randomized controlled trial to evaluate the efficacy of ultrasound-guided laser photocoagulation for treatment of benign thyroid nodules. J Endocrinol Invest 29:RC23–6.
- Papini E, Rago T, Gambelunghe G, et al. (2014). Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab 99:3653–9.
- Døssing H, Bennedbaek FN, Hegedüs L. (2005). Effect of ultrasound-guided interstitial laser photocoagulation on benign solitary solid cold thyroid nodules – a randomised study. Eur J Endocrinol 152:341–5.
- Døssing H, Bennedbæk FN, Hegedüs L. (2011). Long-term outcome following interstitial laser photocoagulation of benign cold thyroid nodules. Eur J Endocrinol 165:123–8.
- Valcavi R, Riganti F, Bertani A, et al. (2010). Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid 20:1253–61.
- Gambelunghe G, Bini V, Stefanetti E, et al. (2014). Thyroid nodule morphology affects the efficacy of ultrasound-guided interstitial laser ablation: a nested case–control study. Int J Hyperthermia 30:486–9.
- Ritz JP, Lehmann KS, Zurbuchen U, et al. (2009). Ex vivo and in vivo evaluation of laser-induced thermotherapy for nodular thyroid disease. Lasers Surg Med 41:479–86.
- Mertyna P, Hines-Peralta A, Liu ZJ, et al. (2007). Radiofrequency ablation: variability in heat sensitivity in tumors and tissues. J Vasc Interv Radiol 18:647–54.
- Cakir B, Gul K, Ersoy R, et al. (2008). Subcapsular hematoma complication during percutaneous laser ablation to a hypoactive benign solitary thyroid nodule. Thyroid 18:917–18.
- Bernardi S, Lanzilotti V, Papa G, et al. (2016). Full-thickness skin burn caused by radiofrequency ablation of a benign thyroid nodule. Thyroid 26:183–4.
- Di Rienzo G, Surrente C, Lopez C, et al. (2012). Tracheal laceration after laser ablation of nodular goitre. Interact Cardiovasc Thorac Surg 14:115–16.
- Pacella CM, Mauri G, Achille G, et al. (2015). Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab 100:3903–10.
- Bernardi S, Dobrinja C, Fabris B, et al. (2014). Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol 2014:934595.
- Gharib H, Papini E, Garber JR, et al. (2016). American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules – 2016 update. Endocr Pract 22:622–39.
- Valcavi R, Piana S, Bortolani GS, et al. (2013). Ultrasound-guided percutaneous laser ablation of papillary thyroid microcarcinoma: a feasibility study on three cases with pathological and immunohistochemical evaluation. Thyroid 23:1578–82.
- Mauri G, Cova L, Ierace T, et al. (2016). Treatment of metastatic lymph nodes in the neck from papillary thyroid carcinoma with percutaneous laser ablation. Cardiovasc Intervent Radiol 39:1023–30.
- Papini E, Bizzarri G, Bianchini A, et al. (2013). Percutaneous ultrasound-guided laser ablation is effective for treating selected nodal metastases in papillary thyroid cancer. J Clin Endocrinol Metab 98:E92–7.
