Abstract
Purpose: To determine the feasibility of using radiofrequency hyperthermia (RFH) and to enhance the therapeutic effect of herpes simplex virus-thymidine kinase/ganciclovir (HSV-TK/GCV) for the treatment of hepatocellular carcinoma (HCC).
Materials and methods: Human HCC cells (HepG2) were first transfected with lentivirus/luciferase. For both in vitro confirmation and in vivo validation, luciferase-labeled HCC cells and HCC tumour xenografts on mice received different treatments: (i) combination therapy of intratumoral HSV-TK/GCV-mediated gene therapy plus magnetic resonance imaging heating guidewire (MRIHG)-mediated RFH; (ii) gene therapy only; (iii) RFH only; and (iv) phosphate-buffered saline (PBS) as control. Cell proliferation was quantified. Tumour changes were monitored by ultrasound imaging and bioluminescence optical imaging before and at days 7 and 14 after treatments, which were correlated with subsequent histology.
Results: In vitro, the lowest cell proliferation was seen in the combination therapy group compared with control groups (29 ± 6% vs. 56 ± 9%, 93 ± 4%, and 100 ± 5%, p < .05). Ultrasound imaging of treated animal xenografts showed smaller relative tumour volume in combination therapy group than those in three control groups (0.74 ± 0.19 vs. 1.79 ± 0.24, 3.14 ± 0.49 and 3.22 ± 0.52, p < .05). Optical imaging demonstrated significant decrease of bioluminescence signals of tumours in the combination therapy group, compared to those in three control groups (1.2 ± 0.1 vs. 1.9 ± 0.2% vs. 3.3 ± 0.6% vs. 3.5 ± 0.4%, p < .05). These imaging findings were correlated well with histologic confirmation.
Conclusion: RFH can enhance HSV-TK/GCV-mediated gene therapy of HepG2 cell line and mice human HCC xenografts, which may open new avenues for effective management of HCC using MR/RFH integrated interventional gene therapy.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver with an annual incidence of more than 1 million worldwide, and is the third leading cause of cancer-related death [Citation1–4]. Surgical resection or orthotopic liver transplantation has been considered as the first option for patients with very early stage HCCs [Citation2,Citation5,Citation6]. However, these therapies are often precluded, because of poor hepatic reserve due to liver cirrhosis, a shortage of donors or diseases at advanced tumour stages [Citation3,Citation6–8].
Radiofrequency ablation (RFA) is accepted as a safe and effective first-line treatment modality for the patients with early stages of primary HCC, who are not suitable for hepatic resection or liver transplantation [Citation2,Citation6,Citation8–11]. However, inadequate or sublethal RFA treatment may accelerate both malignant transformation and tumour progression in HCC [Citation9,Citation12], which may in part contribute to significantly higher incidence of local recurrence (up to 53%) than surgical resection [Citation13]. Large tumour size is also a major risk factor for local recurrence because of poorly defined ablative margins [Citation2,Citation14,Citation15]. It is necessary to apply sufficiently high temperatures and secure wide therapeutic margins, in an attempt to completely eradicate all residual HCC within the treated focal lesion.
However, a critical clinical problem with the current RFA techniques lies in its inconsistency in achieving complete response with large or irregular tumours, or when there is a “heat-sink” effect caused by peritumoral blood vessels [Citation16]. To overcome this problem, new strategies have been explored to extend the area of RFA-induced necrosis, such as RFA combined with transarterial chemoembolisation [Citation17], use of adjuvant sorafenib [Citation18] or temporary occlusion of hepatic vessels [Citation19,Citation20]. Compared with RFA alone, combination therapies may improve outcomes in patients with HCC, but tumour recurrence rates remain unacceptably high [Citation21].
Gene therapy is a frontier of modern medicine. To date, nearly 1000 gene therapy trials have been initiated or completed worldwide, with most of these trials focusing on gene-based treatments of cancers. Transfection of cancer cells with the herpes simplex virus-thymidine kinase gene followed by ganciclovir administration (HSV-TK/GCV) represents a promising example for treating malignancies. In comparison to conventional chemotherapies, tumour cells transfected with the HSV-TK gene can not only convert a prodrug into a toxic metabolite and thereby selectively eliminate tumour cells, but also produce a “bystander effect” that induces the death of neighbouring untransfected cancer cells [Citation22]. However, a critical weakness with systemic HSV-TK/GCV therapy is its low rate of gene transfection (less than 10%) and consequent low tumoricidal effect. Attempts to solve this problem have been made by increasing the concentration of HSV-TK genes, however this may lead to substantial toxicities to other organs via a systemic gene delivery approach.
A previous study showed that radiofrequency (RF) heating can enhance lentivirus-mediated gene transfection and transduction efficiency in arterial atherosclerotic tissues, which involved the use of a magnetic resonance imaging heating guidewire (MRIHG) to induce a local hyperthermia in the target tissue [Citation7,Citation23]. Based on the concept of using RF energy to enhance gene transduction, the purpose of this study was to establish the feasibility of use of MRIHG-mediated radiofrequency hyperthermia (RFH) to augment the benefit of HSV-TK/GCV-mediated gene therapy on HCCs.
Materials and methods
Study design
This study was divided into two phases: (a) in vitro experiments to confirm RFH-enhanced therapeutic efficacy of HSV-TK/GCV on human HCC cells; and (b) in vivo experiments to validate the feasibility of using optical and ultrasound imaging to monitor RFH-enhanced HSV-TK/GCV gene therapeutic effect on mouse models with HCC xenografts.
Production of HSV-TK/lentivirus
HSV-TK lentiviral vector and third-generation lenti-combo packing mix (Applied Biological Materials Inc., Richmond, BC, Canada) and a HSV-TK plasmid vector, pORF9-HSV1tk (InvivoGen, San Diego, CA) were obtained commercially. All plasmids were amplified and extracted from E. coli and purified using the Qiagen Hispeed Plasmid Max Kit (Qiagen Inc., Valencia, CA). The final concentration of the plasmids in stock was adjusted to 2.0 mg/mL by dilution with phosphate-buffered saline (PBS).
Recombinant lentivirus-carrying HSV-TK was produced by transient transfection of T293 cells, according to the protocol provided by the manufacturer (Applied Biological Materials Inc.). Briefly, 10 μg of HSV-TK lentiviral expression vector, 10 μg of third-generation packaging system mix and 80 μL of transfection reagent were used to transfect T293 cells. The viral supernatant was concentrated using an Amicon Ultra-15 centrifugal filter units (Ultracel 100K, Millipore, Billerica, MA). Viral titre was determined based on the expression of green fluorescence protein (GFP) in transduced T293 cells as previously described [Citation24].
In vitro experiments
Cell culture and RFH-enhanced gene therapy
For the purpose of using molecular imaging to evaluate the therapeutic effect, human HCC cells (HepG2) were first transfected with luciferase/red fluorescence protein (Luc/RFP) gene/lentivirus, to create Luc/RFP-positive HepG2 cells according to the protocol provided by the manufacturer (GeneCopoeia Inc., Rockville, MD). Luc/RFP-positive cells were sorted out using fluorescence-activated cell sorting (Aria II, Becton Dickinson, Franklin Lakes, NJ). Cells were then cultured in Dulbecco’s modified Eagle’s medium (Hyclone, South Logan, UT) supplemented with 10% foetal bovine serum (Thermo Fisher Scientific Inc., Waltham, MA) and incubated with a humidified 5% CO2 atmosphere at 37 °C.
HepG2 cells were cultured in 4-chamber cell culture slides (Nalge Nunc International, Rochester, NY). RFH was performed by attaching a 0.022-inch MRIHG under the bottom of chamber 4 of the chamber slide. The MRIHG was connected to a custom RF generator for heating. A sterile 1.1-mm fibre optic temperature sensor was placed at the bottom of each chamber and connected to a temperature thermometer (PhotonControl, Burnaby BC, Canada) for real-time monitoring of the temperature change during RFH [Citation23,Citation25].
Cells in different groups were treated by (a) 30-min RFH at approximately 42 °C with HSV-TK/lentivirus (106 IU/well), followed by exposure to 50 μg/mL GCV one day after gene transduction; (b) 30-min RFH at approximately 42 °C with HSV-TK/plasmid, followed by exposure to 50 μg/mL GCV one day after gene transduction; (c) 30-min RFH-only; (d) HSV-TK/lentivirus + GCV therapy only (without RFH); (e) HSV-TK/plasmid + GCV therapy only (without RFH); and (f) PBS to serve as a control.
To prepare the HSV-TK/plasmid gene transfection group cells, 0.020-μg/μL plasmid solution in sterile deionised water was combined with the addition of transfection reagent (FuGENE® HD, Promega, Madison, WI) with a FuGENE:DNA ratio of 4.5:1. Twenty-four hours after cell seeding, 0.50-μg HSV-TK/plasmid was added to the chamber. The cells were then exposed to 50 μg/mL GCV for 72 h [Citation26]. The HSV-TK/lentivirus gene transduction group cells were transfected with lentivirus supernatant at a dose of 106 IU/cell in the presence of 8 μg/mL hexadimethrine bromide solution (Polybrene, Abbott Laboratories, Chicago, IL). Cells were then incubated for 24 h, followed by an exposure of cells to 50 μg/mL GCV for 72 h.
Cell proliferation assay
Cell proliferation was evaluated by MTS assay 72 h after the GCV treatment. Briefly, MTS (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) agent was added to the cell chambers and incubated for 4 h. The absorbance was measured using a microplate reader at 490 nm. The relative cell proliferations of the different cell groups were evaluated using the equation Atreated−Ablank/Acontrol−Ablank, where A is the absorbance. Cells on slides were subsequently washed twice with PBS, fixed in 4% paraformaldehyde, counterstained with 4′,6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA), and then imaged with a laser confocal microscope (A1R; Nikon, Tokyo, Japan). The experiments for each cell group were repeated six times.
Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR)
The total RNA was extracted using All Prep DNA/RNA Mini kit (Qiagen Inc., Valencia, CA). Quantitative RT-PCR was performed using an Mx3005PTM real time PCR system (Stratagene, La Jolla, CA) with the TaqMan EZ RT-PCR kit, according to the manufacturer’s protocol (Applied Biosystems, Foster City, CA). We used forward primer 5′-GGTGATGACCTCTGCCCAGAT-3′, reverse primer 5′-TGTGAGGAGCCAGAACAGCAT-3′ and TaqMan(R) MGB Probe 6FAM-TGG GAA TGC CCT ATG C-MGBNFQ.
In vivo experiments
Animal model
The animal protocol was approved by the Institutional Animal Care and Use Committee. Thirty-six nu/nu mice (Charles River Laboratories, Wilmington, MA) aged 4–6 weeks were included in this study. 5 × 106 – 1 × 107 Luc/RFP-positive HCC (HepG2) cells in 100-μL Matrigel (Corning Life Sciences, Corning, NY) were subcutaneously injected into the left back of each mouse for tumour creation. Once the tumour size reached 5–8 mm in diameter, we initiated the treatment procedures.
DNA/liposome complex preparation
FuGENE/DNA complexes were prepared by adding a solution of 50-μg plasmid DNA and FuGENE® HD reagent in 50-μL sterile deionised water. This DNA–liposome mixture was then incubated for 45 min at room temperature before intratumoral injection. The mixture was directly injected into the subcutaneous HCC xenografts on mice under ultrasound guidance.
RF heating and gene therapy
The animals were anaesthetised with 1–3% isoflurane in 100% oxygen. Six mice in each of six groups were treated by (a) RFH plus HSV-TK/lentivirus therapy, which was carried out by intratumoral injections of 108 HSV-tk/lentivirus in a total of 60-μL PBS, followed by RFH at approximately 42 °C for 30 min; (b) RFH plus HSV-TK/plasmid therapy, which was carried out by intratumoral injection of 50-μg plasmid in a total of 50-μL sterile deionised water, followed by RFH at approximately 42 °C for 30 min; (c) 30-min RFH alone; (d) HSV-TK/lentivirus therapy without RFH; (e) HSV-TK/plasmid therapy without RFH; and (f) PBS to serve as a control. RFH was performed by inserting a 0.022-inch MRIHG into the tumour with its hot spot at the centre of each tumour, which was precisely positioned under real-time ultrasound imaging guidance. A 400-μm micro-fibre optical thermal sensor was subcutaneously placed in parallel to the MRIHG, so that the temperature at the RF-heated tumour could be measured instantaneously. One day after the gene injection, GCV at a daily dose of 5 mg/kg was intraperitoneally administered for 14 consecutive days.
Optical imaging follow-up
Optical imaging was performed on an In-Vivo Optical Imaging Systems (In-vivo Extreme, Rheinstetten, Germany). Each animal was imaged the day before treatment and days 7 and 14 after the treatment. Animals received an intraperitoneal injection of d-luciferin 150 mg/kg (Interchim, France). Twenty minutes later, the bioluminescence images were acquired using the in vivo imaging system. Bioluminescent signal intensity of each tumour was quantified by summing detected photon counts using Bruker MI software (Brucker, Billerica, MA). Data were normalised to relative signal intensity (RSI) by using following equation: RSI = SIDn/SID0, where SI is signal intensity, Dn represents days after treatment and D0 is the day before treatment.
Ultrasound imaging follow-up
Ultrasound imaging of each tumour was performed the day before treatment and days 7 and 14 after the treatment using a 6–13 MHz linear transducer (Sonosite Inc., Bothell, WA). The longest dimensions of each tumour (in three orthogonal axes, X, Y and Z) were measured and used to calculate the volume of each tumour (volume = X*Y*Z*π/6). Data were normalised to relative tumour volume (RTV) by using the following equation: RTV = VDn/VD0, where V is tumour volume, Dn represents days after treatment, and D0 is the day before treatment.
Histologic correlation/confirmation
Tumours were harvested at day 14 after treatments. Tumour tissues were cryosectioned at 8-μm slices for apoptosis staining. Level of apoptosis was determined with a terminal deoxynucleotidyl transferase dUTP nick end labelling assay (TUNEL) using TACS XL Blue Label kit (Trevigen, Gaithersburg, MD). On one slide, six fields were randomly photographed using an Olympus DP72 digital camera. Apoptosis results were analysed as the apoptotic index, defined as the number of apoptotic cells/total number of cells ×100%.
RNA preparation and RT-qPCR
Tumour tissues from the HSV-TK/GCV-treated groups and the HSV-TK/GCV/RFH-treated group were collected. HSV-TK gene expression level in tumour tissues was quantified by qRT-PCR as described in the in vitro experiment.
Statistical analysis
Statistical software (SPSS, Version 19.0, SPSS Inc., Chicago, IL) was used for all data analyses. Non-parametric Mann–Whitney U test was used to compare (i) relative proliferation rates among different cell groups; (ii) RSIs measured by optical images; (iii) RTVs measured by ultrasound images; and (iv) HSV-TK gene expression rates at end points among various animal groups. A p value less than .05 was considered significantly different.
Results
Radiofrequency hyperthermia enhanced the therapeutic effect of HSV-TK/GCV on hepatocellular cancer cell line HepG2
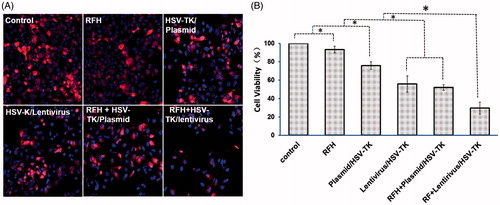
The in vitro experiments showed the lowest viability in the cell group treated with HSV-TK/lentivirus + GCV plus RFH, compared to those of groups with HSV-TK/plasmid + GCV plus RFH, HSV-TK/lentivirus + GCV alone, HSV-TK/plasmid + GCV alone, RFH alone and the control group with PBS (29.5 ± 6.6% vs. 52.1 ± 3.1% vs. 55.9 ± 8.9% vs. 75.9 ± 4.3% vs. 93.4 ± 3.6% vs. 100%, p < .05) (). The results of confocal microscopy are consistent with the MTS assay results, showing much less cells survived with the treatment of HSV-TK/lentivirus + GCV plus RFH than other groups ().
Figure 1. Therapeutic effect on hepatocellular cancer cells. (A) Confocal microscopy shows a decreased numbers of cells in the group with combination therapy of RFH plus HSV-TK/plasmid and HSV-TK/lentivirus, compared with the control, RFH alone and gene alone groups. (B) MTS assay was performed for six samples of each cell group, which demonstrated the lowest cell proliferation in the combination therapy group with RFH plus HSV-TK/lentivirus compared with those in other five groups (*p < .05).
The synergistic therapeutic effect of RFH combined with HSV-TK/GCV on mice subcutaneous hepatocellular cancers
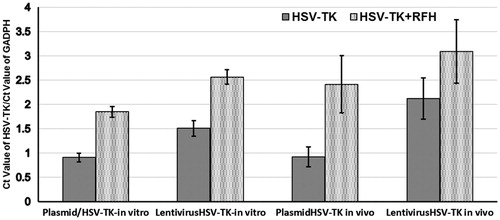
Hepatocellular cancers were transfected with luciferase/m-cherry/lentivirus gene before being subcutaneously inoculated into the back of the mouse for creating tumour xenografts. All the mice tumours carrying the luciferase gene have the capability of catalysing the oxidation of luciferin to emit bioluminescence (). The signal intensity is well correlated with the bioactivities of the tumours. This therapeutic effect was well mirrored with in vivo follow-up optical imaging that showed that only in the tumour group treated with HSV-TK/lentivirus + GCV plus RFH demonstrated decreased relative photon counts after the treatment. Compared with the control group, tumours in the combination therapy group have more than 80% decrease of bioluminescence signal intensity (). Corresponding to the change of the bioluminescence signal intensity, follow-up ultrasound imaging also demonstrated that the treatment of HSV-TK/lentivirus + GCV plus RFH significantly inhibited the growth of tumours manifesting as the lowest average RTV. Compared with the control group, the average RTV decreased 79.6% in the group with combination therapy (). qRT-PCR quantitative analysis of HSV-TK gene expression showed that RFH could enhance HSV-TK gene expression level in both HCC cells and mice tumours with 1-fold increase of HSV-TK gene expression in cells transfected with HSV-TK/plasmid and 71% increase of gene expression in the cells HSV-TK/lentivirus. The increase of gene expression was consistent with the lowest relative photons and tumour volumes in the group treated with HSV-TK/lentivirus + GCV plus RFH, compared with the group treated by HSV-TK/GCV and RFH alone ().
Figure 2. Optical/x-ray images of tumours in six animal groups at days 0, 7 and 14 after different treatments. (A) The optical images demonstrate a significant decrease of photon signals of tumours in the animal group treated by combined therapy (gene + RFH), compared with RFH alone, gene alone and control animal groups, with the lentivirus/HSV-TK + RFH group showing the lowest signal. (B) Quantitative analysis of relative photon counts in each group shows that the lowest relative photon counts were seen in the group treated with the combination therapy of lentivirus/HSV-TK + GCV plus RFH (*p < .05).
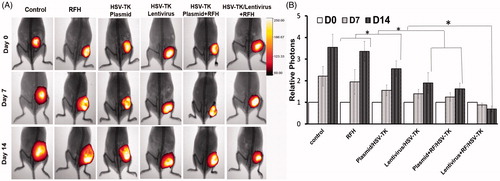
Figure 3. (A) Ultrasound images of tumours in six animal groups (arrows). The tumour size in the group with combination therapy of HSV-TK/lentivirus + GCV plus RFH is the smallest, compared with other six groups. (B) Quantitative analysis of tumour volumes in six mice of each group shows that the lowest relative tumour volumes were seen in the group treated with the combination therapy of lentivirus/HSV-TK + GCV plus RFH (*p < .05).
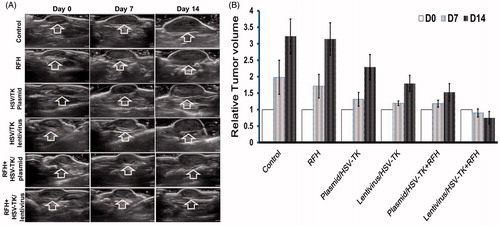
Figure 4. qRT-PCR quantitative analysis of HSV-TK gene expression in cells and tumour tissues. RFH could enhance HSV-TK gene expression level in both HCC cells and mice tumours.
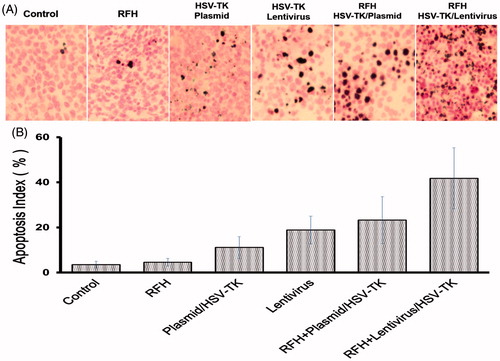
Measurements of the harvested tumours show that the tumour size in the combination therapy group is the smallest, compared with the other three groups (). Histologic analysis of tumour apoptosis further displayed a higher apoptotic index in the group treated with HSV-TK/lentivirus + GCV plus RFH than in the groups treated with HSV-TK/plasmid + GCV plus RFH or RFH alone (41.77% ± 13.5% vs. 23.3% ± 10.4%, p < .05; and 41.77% ± 13.5% vs. 4.57% ± 1.71%, p < .05) ().
Figure 5. Histology of tumours. Representative tumours harvested from six different animal groups, showing the smallest tumour size treated by combination therapy (lentivirus/HSV-TK + GCV plus RFH), compared with other treatments.
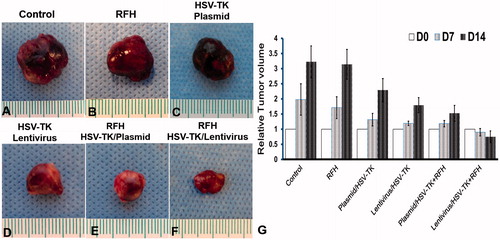
Figure 6. Apoptosis with TUNEL staining of tumour tissues. (A) Apoptotic staining shows more apoptotic cells in the combination therapy group (gene + RFH) in comparison to the control groups. (B) Quantitative analysis of apoptosis in tumour tissues for six mice in each group shows that RFH combined with either plasmid/HSV-TK + GCV or lentivirus/HSV-TK + GCV could induce significantly more apoptosis than the therapy of plasmid/HSV-TK + GCV, lentivirus/HSV-TK or RFH alone (*p < .05).
Discussion
RFA is widely accepted as the first alternative for patients with early-stage HCC who are not suitable for surgical resection or liver transplantation [Citation9,Citation27]. RFA can effectively induce the necrosis of HCC measuring 3 cm or smaller because the ablative hyperthermia can yield a necrotic area of 5 cm or larger in diameter in one session, which can secure the complete ablation of a 3-cm HCC with a margin of at least 0.5 cm. However, routine HCC screening is not frequently carried out in detection of early-stage HCC for patients with underlying high risks of developing HCC, such as chronic hepatitis B and hepatic cirrhosis. This leads to the reality that a vast majority of HCCs are diagnosed at advanced stage, often with tumour sizes exceeding 5 cm. With the recent technical advances, such as the cluster electrode and the use of a generator that can deliver more thermal energy to tissues, attempts has been made to treat HCCs larger than 3–5 cm in diameter [Citation2], but the local recurrence rate from such attempts is still much higher than that from RFA of the tumour lesions with a diameter less than 3 cm. Studies reported that tumour size is the only significant predictor for local tumour progression [Citation2,Citation18]. For the larger tumours, the tumour cells in the tumour margin cannot be eradicated by the insufficient hyperthermia due to heat sink effect of large vessels around the tumour margins [Citation2,Citation18].
In this study, we investigated the possibility of using non-ablative hyperthermia at a temperature of 42 °C to enhance intratumoral HSV-TK/GCV-mediated gene therapy of HCCs. Findings of the study demonstrated that MRIHG-induced RFH can significantly enhance HSV-TK gene expression in HCC cells and HCC tumours; which thereby resulted in a low survival of HCC cells in in vitro experiments as well as shrunken tumour volumes and decreased optical signal intensities of treated tumours in the in vivo experiments. In addition, our study provides evidence that both optical imaging and ultrasound imaging offer useful tools to assess the response of HCC to gene therapeutic regimens for such basic studies.
Given the paucity of systemic therapies available, gene therapy may become an attractive therapeutic alternative for HCC. Among the strategies, transfer of the HSV-TK gene, followed by GCV therapy has been extensively studied [Citation28–31]. GCV is phosphorylated by HSV-TK to be di- and tri-phosphorylated molecules by endogenous enzymes in the target cell, which then induces death of the tumour cell by inhibiting cellular DNA polymerase activity [Citation22]. Gene transfer relies on the use of genetically engineered viral or non-viral vectors that promote incorporation into cells and allows its transient or stable expression. Sufficient transduction efficiency and expression of the transgene are two key components to be considered when promoting a therapeutic effect on genetically manipulated cells [Citation31]. Among various vectors, lentiviral vectors exhibit the unique advantages over adenoviral vectors and adeno-associated viral (AAV) vectors. Lentiviral vector can yield the much higher transduction and transfection efficiency than plasmid vector and AAV vector but less hepatic toxicity and systemic immunologic response than adenoviral vectors [Citation29–31]. However, one study revealed that after intratumoral injection of lentivirus/LacZ gene, only 5 and 20% of tumour cells exhibited the LacZ gene expression [Citation22], which hints the necessity of developing strategies to increase the gene transduction and transfection in tumour cells. This study establishes the proof of principle that RFH can enhance the gene expression in HCC cells and boost the therapeutic effect of HSV-TK/GCV on both HCC cells in vitro and HCC tumours in vivo. Although unknown, the mechanism of hyperthermia-enhanced gene therapy may be associated with the increased permeability of cell membranes, which allows for more influx of genetic material. Ultimately, one application of this effect would be to take advantage of the RFH at the margin of RF ablate tumours, which can specifically enhance HSV-TK/GCV-mediated gene therapy, and eradicate potential residual non-ablated tumour cells at the tumour margin. The current study has confirmed the feasibility that along with the session of RF ablation, HSV-TK/lentivirus genetic material can be directly delivered into the margins of the tumours through a transarterial catheter or a multichannel needle for direct intratumoral administration. Targeted vector introduction into the tumour via hyperthermia may prove beneficial in avoiding the toxicity of systemic exposure while achieving higher rates of gene expression and an improved therapeutic benefit.
In this study, we used a hyperthermia temperature of 42.0 °C only, additional experiments are warranted to select the optimal temperature and to maximise the enhancing effect of RFH on gene therapy. Because our study focused on establishing the proof of principle of the technique, we only explored the feasibility of this technique on mouse tumours. A further study of technique validation should be carried out in middle-to-large animals with orthotopic liver cancers, such as rabbits bearing hepatic VX2 tumours, which is a necessary step for clinical translation of the technique. In addition, in the present study we had to limit our follow-up time up to two weeks after the treatments. This was because longer follow-up period would result in the xenograft tumour masses, especially in the control animal group, becoming more than 10% of the body weight, which was not approved by our Institutional Animal Care and Use Committee. Thus, as a limitation, this study did not allow us to evaluate the long-term therapeutic effects with follow-up optical imaging and ultrasound imaging.
In conclusion, local non-ablative RFH can enhance HSV-TK/GCV-mediated therapeutic effect on human HCC cell line (HepG2) and mice HCC xenografts, which may help to decrease marginal tumour recurrence in RF-ablated hepatic malignancies by innovative integration of RF technology, interventional oncology, and direct intratumoral gene therapy, rather than systemic gene therapy.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article
References
- Kalva SP, Thabet A, Wicky S. (2008). Recent advances in transarterial therapy of primary and secondary liver malignancies. Radiographics 28:101–17.
- Lee DH, Lee JM, Lee JY, et al. (2014). Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 270:900–9.
- Nishikawa H, Kimura T, Kita R, Osaki Y. (2013). Radiofrequency ablation for hepatocellular carcinoma. Int J Hyperthermia 29:558–68.
- Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. (2012). Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992-2008. Hepatology 55:476–82.
- Lin SM. (2009). Recent advances in radiofrequency ablation in the treatment of hepatocellular carcinoma and metastatic liver cancers. Chang Gung Med J 32:22–32.
- Kim JW, Kim JH, Sung KB, et al. (2014). Transarterial chemoembolization vs. radiofrequency ablation for the treatment of single hepatocellular carcinoma 2 cm or smaller. Am J Gastroenterol 2014;109:1234–40.
- Gillams ARLW. (2008). Five-year survival following radiofrequency ablation of small, solitary, hepatic colorectal metastases. J Vasc Interv Radiol 19:712–7.
- Hocquelet A, Balageas P, Laurent C, et al. (2015). Radiofrequency ablation versus surgical resection for hepatocellular carcinoma within the Milan criteria: a study of 281 Western patients. Int J Hyperthermia 31:749–57.
- Yoshida S, Kornek M, Ikenaga N, et al. (2013). Sublethal heat treatment promotes epithelial-mesenchymal transition and enhances the malignant potential of hepatocellular carcinoma. Hepatology 58:1667–80.
- Tohme S, Geller DA, Cardinal JS, et al. (2013). Radiofrequency ablation compared to resection in early-stage hepatocellular carcinoma. HPB 15:210–7.
- Pompili M, Saviano A, de Matthaeis N, et al. (2013). Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma </=3 cm. Results of a multicenter Italian survey. J Hepatol 59:89–97.
- Masuda T, Beppu T, Ishiko T, et al. (2008). Intrahepatic dissemination of hepatocellular carcinoma after local ablation therapy. J Hepatobiliary Pancreat Surg 15:589–95.
- Mori K, Fukuda K, Asaoka H, et al. (2009). Radiofrequency ablation of the liver: determination of ablative margin at MR imaging with impaired clearance of ferucarbotran-feasibility study. Radiology 251:557–65.
- Kang TW, Lim HK, Lee MW, et al. (2015). Aggressive intrasegmental recurrence of hepatocellular carcinoma after radiofrequency ablation: risk factors and clinical significance. Radiology 276:274–85.
- Facciorusso A, Di Maso M, Muscatiello N. (2016). Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia 32:339–44.
- Lu DS, Raman SS, Limanond P, et al. (2003). Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol 14:1267–74.
- Takuma Y, Takabatake H, Morimoto Y, et al. (2013). Comparison of combined transcatheter arterial chemoembolization and radiofrequency ablation with surgical resection by using propensity score matching in patients with hepatocellular carcinoma within Milan criteria. Radiology 269:927–37.
- Fukuda H, Numata K, Moriya S, et al. (2014). Hepatocellular carcinoma: concomitant sorafenib promotes necrosis after radiofrequency ablation-propensity score matching analysis. Radiology 272:598–604.
- Yamasaki T, Kurokawa F, Shirahashi H, et al. (2002). Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow. Comparison with standard percutaneous radiofrequency ablation therapy. Cancer 95:2353–60.
- de Baere T, Deschamps F, Briggs P, et al. (2008). Hepatic malignancies: percutaneous radiofrequency ablation during percutaneous portal or hepatic vein occlusion. Radiology 248:1056–66.
- Chen L, Sun J, Yang X. (2016). Radiofrequency ablation-combined multimodel therapies for hepatocellular carcinoma: current status. Cancer Lett 370:78–84.
- Gerolami R, Uch R, Faivre J, et al. (2004). Herpes simplex virus thymidine kinase-mediated suicide gene therapy for hepatocellular carcinoma using HIV-1-derived lentiviral vectors. J Hepatol 40:291–7.
- Du X, Qiu B, Zhan X, et al. (2005). Radiofrequency-enhanced vascular gene transduction and expression for intravascular MR imaging-guided therapy: feasibility study in pigs. Radiology 236:939–44.
- Tiscornia G, Singer O, Verma IM. (2006). Production and purification of lentiviral vectors. Nat Prot 1:241–5.
- Zhang T, Zhang F, Meng Y, et al. (2013). Diffusion-weighted MRI monitoring of pancreatic cancer response to radiofrequency heat-enhanced intratumor chemotherapy. NMR Biomed 26:1762–7.
- Chen L, Guo G, Liu T, et al. (2011). Radiochemotherapy of hepatocarcinoma via lentivirus-mediated transfer of human sodium iodide symporter gene and herpes simplex virus thymidine kinase gene. Nucl Med Biol 38:757–63.
- Lee TY, Lin JT, Ho HJ, et al. (2015). Evaluation of the effect of cumulative operator experience on hepatocellular carcinoma recurrence after primary treatment with radiofrequency ablation. Radiology 276:294–301.
- Lee KH, Piao H, Son BR, et al. (2004). Herpes simplex virus thymidine kinase and granulocyte macrophage colony-stimulating factor combination gene therapy in a murine CT26 cell colon cancer model. Cancer Gene Ther 11:570–6.
- Palmer DH, Hussain SA, Johnson PJ. (2005). Gene- and immunotherapy for hepatocellular carcinoma. Exp Opin Biol Ther 2005;5:507–23.
- Schuster MJ, Wu GY. (1997). Gene therapy for hepatocellular carcinoma: progress but many stones yet unturned! Gastroenterology 112:656–9.
- Gerolami R, Uch R, Brechot C, et al. (2003). Gene therapy of hepatocarcinoma: a long way from the concept to the therapeutical impact. Cancer Gene Ther 10:649–60.
