Abstract
Purpose: To evaluate the impact of using monopolar thermal coagulation based on radiofrequency (RF) currents on intraoperative blood loss during liver resection.
Materials and methods: A prospective randomised controlled trial was planned. Patients undergoing hepatectomy were randomised into two groups. In the control group (n = 10), hemostasis was obtained with a combination of stitches, vessel-sealing bipolar RF systems, sutures or clips. In the monopolar radiofrequency coagulation (MRFC) group (n = 18), hemostasis was mainly obtained using an internally cooled monopolar RF electrode.
Results: No differences in demographic or clinical characteristics were found between groups. Mean blood loss during liver resection in the control group was more than twice that of the MRFC group (556 ± 471 ml vs. 225 ± 313 ml, p = .02). The adjusted mean bleeding/transection area was also significantly higher in the control group (7.0 ± 3.3 ml/cm2 vs. 2.8 ± 4.0 ml/cm2, p = .006). No significant differences were observed in the rate of complications between the groups.
Conclusions: The findings suggest that the monopolar electrocoagulation created with an internally cooled RF electrode considerably reduces intraoperative blood loss during liver resection.
Introduction
Intraoperative blood loss and perioperative transfusion increases mortality and morbidity and reduces long-term survival after hepatic resection in liver tumours [Citation1,Citation2]. Several methods of minimising intraoperative blood loss are currently available for hepatic resection, specifically during parenchymal transection [Citation3]. The most important resection techniques generally used today are finger fracture or the crush/clamp technique, Cavitron Ultrasonic Surgical Aspirator (CUSA, Cavitron, Stamford, CT), water-jet technology, stapler, and monopolar and bipolar electrosurgical electrodes [Citation4–7]. Different sealing devices such as harmonic scalpels, ultrasound scissors, radiofrequency-based monopolar and bipolar vessel-sealing systems have recently gained importance in liver surgery [Citation8–12]. Due to these continuous improvements in medical technology and post-operative care, reported perioperative mortality has dropped to 5% and morbidity rates vary between 20–40% [Citation6]. However, the optimal transection technique has still not been found and using different energy-based devices for different steps of the operation is also cumbersome in terms of the flow procedure and a certain degree of skill is required for their proper use [Citation9].
During surgical liver resection, the major amount of blood is lost during the parenchyma transection stage and during the final checking of the resection surface [Citation13]. The strategy based on pre-coagulating the parenchyma using radiofrequency (RF) energy by creating thick coagulation zones before division is interesting, since it allows vessels to be coagulated when they are still enclosed by tissue. This is obviously less complicated than managing bleeding caused by a vessel in the same transection plane. In any case, the underlying idea is always the same: vessels located in the coagulation zone are sealed due to the thermal denaturation of the collagen present on their walls and the flow of blood is subsequently stopped.
The idea of pre-coagulating tissue with RF energy before division was initially conducted with needle electrode arrays to allow small vessels to be sealed and subsequently dividing by surgical scalpel, theoretically without bleeding [Citation13]. It is also possible to create these coagulation zones prior to the division by using pencil-type RF electrodes working in monopolar soft coagulation mode (as illustrated in ), i.e. delivering high-power current (up to 2 A) through a good contact between electrode and tissue, and using a relatively low voltage (< 200 V). The principal drawback of the needle electrodes is that large vessels are often incompletely collapsed, which results in blood loss when transection is conducted, necessitating tedious suturing and additional clipping tasks [Citation13]. In contrast, pencil-type RF electrodes would allow surgeons to clearly identify bleeding points in the same transection plane caused by medium and large vessels and to manage them by creating a new deep coagulation zone as illustrated in . Finally, once the division has been accomplished, final applications of RF energy on the remaining resection surface would create new coagulation zones with preventive purposes to avoid late bleeding () and even enlarge resection margins to minimise local hepatic recurrence [Citation14–16].
Figure 1. Creation of large coagulation zones using monopolar thermal coagulation could minimise intraoperative blood loss throughout different stages of liver resection: initial coagulation of the vessels located in zone to be transected (A), coagulation of vessels on the margin of resection during the transection (B), and preventive coagulation of the remaining resection surface (C).
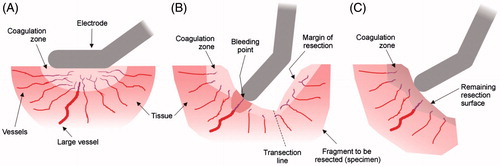
We hypothesised that the creation of all these coagulation zones throughout liver resection (prior to the division to coagulate vessels located in zone to be transected, at the bleeding points on the transection surface during the division, and finally on the remaining resection surface) using a pencil-like RF electrode in monopolar soft coagulation mode would minimise intraoperative blood loss. We planned a prospective randomised controlled trial in order to test the hypothesis.
Materials and Methods
Patients
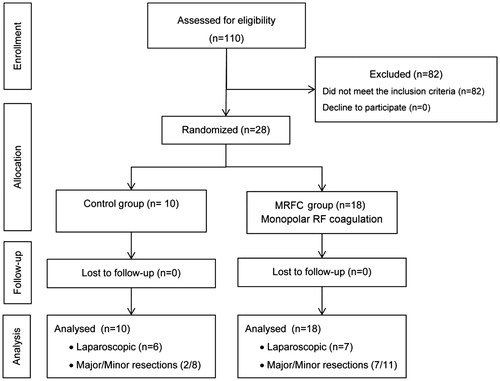
From July 2010 to February 2014, all the patients who underwent partial hepatectomy at the Hospital del Mar (Barcelona, Spain) were considered for inclusion in the study. The inclusion criteria were colorectal liver metastases to be resected by any type of hepatectomy and American Anaesthesia Association (ASA) ranging from I to III via open or laparoscopic surgery. With these criteria, 28 patients were enrolled in the study, and were randomly allocated to the control group or the monopolar RF coagulation (MRFC) group. The allocation schedule was generated by computer-generated random numbers, with an allocation ratio of 1:2 of assignment to each group (). All the patients signed an informed consent form before surgery, and underwent a careful pre-operative assessment of their disease, including spiral computed tomography or magnetic resonance imaging, and showed no evidence of unresectable extrahepatic disease. The study was approved by the local ethical committee and by the Spanish Agency of Medicines and Medical Devices (AEMPS) and was registered and audited as a randomised-clinical trial (AGEMED 312/08 EC) according to the European Directive for Clinical Trials with Medical Devices (Directive 93/42/EEC).
Surgical procedure
All the procedures were performed by the same surgeons (FB and IP). For open surgery, the procedure was similar to that described in [Citation17]. For laparoscopic surgery, after the pneumoperitoneum was established and exposure obtained, laparoscopic ultrasound was used to identify the tumour. In both groups, the dissection was carried out with standard devices such as ultrasonic dissectors, bipolar forceps and Ligasure device (Valleylab, Boulder, CO). Hemostasis was obtained in the control group with a combination of stitches, bipolar forceps and Ligasure (Medtronic, Minneapolis, MN), including sutures or clips. In contrast, hemostasis in the MRFC group was mainly obtained with the Coolinside device (Apeiron Medical, Valencia, Spain) which has shown a short learning curve [Citation17]. This device works in coagulation soft mode, delivering RF power through an internally cooled electrode, and creating large coagulation zones 6 − 9 mm in depth [Citation18,Citation19]. Additionally, it has a built-in blade which allows cutting the previously coagulated tissue, and thus avoids the need for an additional dissecting device. In the laparoscopic approach, the Coolinside device was introduced through a 12-mm trocar.
Note that although the Ligasure device (which is really an RF-based device) was employed at some point in the procedure in both groups, essentially in hepatectomies next to large vessels, the creation of RF-induced deep coagulation zones was exclusively limited to the MRFC group, as bipolar devices (as Ligasure) exhibit a significantly smaller thermal spread (i.e. coagulation depth) [Citation20].
Outcome measures
The outcomes were: 1) transection time: total transection time, including time for achieving complete hemostasis; 2) blood loss: total blood loss during transection (from suction device and blood-soaked gauzes); 3) transection area: obtained by delineation of the transection plane (digitally photographed) using 3D Doctor software (Able Software Corp., Lexington, MA); 4) transection speed: the ratio of transection area to transection time; 5) blood loss per transection area; and 6) the complications described in [Citation17]. Additionally, the biochemical analysis of ferritin, iron and transferrin was measured in urine previously and immediately after transection to check its association with the hepatic coagulative necrosis of the transection margin, given that a considerable amount of the body’s ferritin is stored in the hepatocytes [Citation21].
Statistical analysis
The sample size of the study in each group was calculated by means of the formula proposed by Lehr [Citation22]: N = 16/SMD2, where SMD is the standardised mean difference between the two means being compared. The difference between groups was calculated from the mean blood loss per transection area for each group [Citation17,Citation23] and the minimum sample size per group was N = 9. All the statistical tests were two-sided and a p values <.05 was considered to indicate statistical power. The analysis was performed on SPSS Statistics software (SPSS Inc., Chicago, IL). Data are given as the mean ± standard deviation or by a confidence interval, while complications are described.
Results
During the study period, 110 patients were assessed for eligibility for this study. Eighty-two patients were subjected to liver resection but were excluded for the following reasons: primary liver tumours (n = 54), cyst tumour (n = 16) and others (n = 12). No patients refused to take part in the study. Twenty-eight patients suffering from hepatic metastases underwent hepatic resection and were randomly allocated to either the control (n = 10) or the MRFC group (n = 18) (). No primary disease of the liver was observed in any patient. There were no deviations, no crossovers or withdrawals after randomisation. summarises the baseline and operative characteristics. No statistically significant difference was noted between the groups regarding pre- and intra-operative patient characteristics.
Table 1. Demographic and clinical characteristics of the patients involved in the study.
No differences were observed either in the weight of the surgical specimen or in the mean area of the transection between the control and MRFC groups, respectively. The use of temporary vascular occlusion (Pringle manoeuvre) was similar in both groups (40 and 38%, respectively) but, when required, its duration in the control group was more than double that of the MRFC group ().
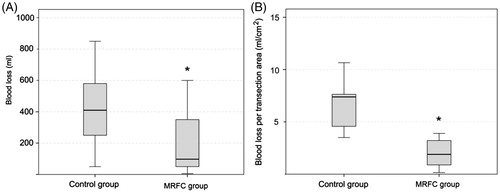
No significant differences were observed either in transection time (77.7 ± 38.9 min and 64.2 ± 44.5 min) or in median transection speed (1.3 ± 1.2 cm2/min and 1.4 ± 0.7 cm2/min) for the control and the MRFC group, respectively. However, mean blood loss during liver resection in the control group was more than twice that of the MRFC group (556 ± 471 ml and 225 ± 313 ml, respectively, p = .021). The differences between the groups were also greater when considering the adjusted variable of the blood loss (mean blood loss per transection area): 7.0 ± 3.3 ml/cm2 vs. 2.8 ± 4.0 ml/cm2 for the control and the MRFC group, respectively (p = .006) ().
Figure 3. Box plots depicting blood loss during the transection (A) and blood loss per transection area (B) for control and monopolar RF coagulation (MRFC) groups. The boxes represent the interquartile range which contains 50% of the values. The whiskers are lines that extend from the box to the highest and lowest values, excluding outliers. The line across the box indicates the median. Both outcomes were statistically significant (*p < .05).
As shown in , no differences were observed in the rate of complications between the groups, nor were there significant differences in mortality and morbidity. The only exitus took place in the MRFC group because of several post-operative complications associated with a combined non-hepatic procedure (related to intestinal reconstruction). Four patients subjected to major hepatectomies in the MRFC group required blood transfusions (see for details). Finally, no significant differences were observed in either ferritin, iron or transferrin urine levels between the groups, though a post-transection increase in ferritin was observed in many cases in the MRFC group ().
Table 2. Mortality and morbidity in patients included in the study.
Table 3. Clinical details of the patients which required blood transfusion after surgical resection.
Table 4. Biochemical levels of ferritin, iron and transferrin in urine previously and after the surgical transection.
Discussion
Intra-operative blood loss and peri-operative transfusion not only increase the risk of surgical morbidity and mortality [Citation1,Citation2,Citation24] but also jeopardise long-term survival because they actually increase the recurrence rate of the tumour being resected [Citation24,Citation25]. One of the goals in liver operations is therefore to reduce blood loss, and several RF-assisted devices have been developed for this purpose. Our hypothesis was that the creation of large coagulation zones in different stages of the liver resection could reduce intraoperative blood loss. Our findings confirmed that there was a reduction from 556 ± 471 ml to 225 ± 312 ml and these differences were even greater when we took into account the adjusted variable (7.0 ± 3.3 ml/cm2 vs. 2.8 ± 4.0 ml/cm2). These values are in agreement with the previously reported experience using the Coolinside device [Citation17–19], and with reported median values from other specialised institutions (ranging from 155 ml to more than 750 ml) [Citation1,Citation9,Citation26–29]. Importantly, the blood loss in our MRFC group was relatively close to the value reported in a clinical study (355 ml) in which monopolar RF coagulation was conducted using TissueLink (Medtronic, Minneapolis, MN) combined with a CUSA device [Citation8].
Overall, our results are comparable or even better than current published data from leading liver units. Furthermore, these differences are greater when the adjusted blood loss is evaluated in the transection area (7.0 ± 3.3 ml/cm2 vs. 2.8 ± 4.0 ml/cm2 for control and MRFC groups, respectively, see ). This compares favourably with other leading techniques, such as the water jet (10.6 ± 15.3 ml/cm2) [Citation7] and even other RF-assisted devices (5.5 ml/cm2) [Citation29]. In comparison with other RF electrodes for monopolar coagulation (such as TissueLink) [Citation8], it must be acknowledged that the Coolinside device does not speed up the transection, though it is no slower than others in either the open or laparoscopic approaches.
Both groups showed similar rates of post-operative morbidity (∼30%), comparable to other published studies [Citation8,Citation30,Citation31]. As detailed in , the patients that required blood transfusions in the MRFC group possessed risk factors that could have played a role in the decision to transfuse, but the transfusion was not a direct consequence of blood loss. These results are also comparable to currently published data from leading liver units [Citation1,Citation8,Citation9,Citation26–28].
Although there were no significant differences, the biochemical findings suggest that the creation of large thermal coagulation zones could be associated with an occasional increase in the urine levels of ferritin, iron and transferrin after the hepatic procedure (). As it is known that most ferritin is stored in the parenchyma of the hepatocytes [Citation20], the temporary elevation of urinary ferritin after transection could be associated with the thick band of coagulated tissue in the transaction margin of the MRFC group (.
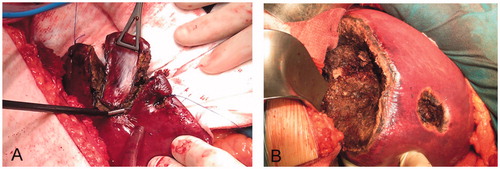
Figure 4. Examples of RF-induced large coagulation zones in the monopolar RF coagulation (MRFC) group. (A) Limited resection in which the monopolar electrode with built-in blade was used as sole sealing and dissecting device. (B) Segmentectomy of segments 5–8, 4a, and two limited resections. Note the thickness of the coagulation zones created on the resection surfaces, which were possibly responsible for the minimal intraoperative bleeding in the MRFC group.
Some limitations of this study must be pointed out. First, the sample size, even though small, was specifically designed to show up differences in the adjusted blood loss according to previous clinical results [Citation17]. In fact, we found that this sample was big enough to point out significant differences between the groups. Second, some authors have suggested that the use of RF-assisted transection devices may facilitate abscess formation, biliary leakage or damage to vessels or main hepatic ducts. In this respect, we did not find significant differences between the groups which could be linked directly to the device used (). This use of the RF electrode to create large thermal coagulation zones was associated with similar rates of post-operative morbidity (30%) as the conventional techniques used with the control group and other published studies [Citation8,Citation30,Citation31]. This large coagulation did not impede correct evaluation of the margin either. As detailed in , the patients in the MRFC group that required blood transfusion presented risk factors that compromised the transfusion, but the transfusion was not a direct consequence of blood loss during transection. Third, although the results about positive margin and local recurrence in the MRFC group suggest that the coagulation zones created with RF could be treating residual tumour at the margin, the numbers are still too small to obtain solid conclusions, and hence this issue warrants further evaluation. Fourth, the cost of both techniques was not evaluated in this study.
In conclusion, the creation of extensive coagulation zones using a monopolar RF electrode during different stages of liver resection (in both the open and laparoscopic approaches) reduces intraoperative blood loss.
Acknowledgements
We would like to thank the Statistical Department at the Hospital del Mar (Barcelona) for their methodological support and allocation of patients.
Disclosure statement
RQ, EB, and FB declare a stock ownership in Apeiron Medical S.L., a company that has a licence for US Patent 8,303 584 on the Coolinside device employed in the study. The other authors have no conflict of interests or financial ties to disclose.
Funding
This work was supported by a medical research grant from the Spanish Government (FIS PI080934) and by Spanish “Programa Estatal de Investigación, Desarrollo e Innovación Orientada a los Retos de la Sociedad” under Grant TEC2014–52383-C3-R (TEC2014–52383-C3–1-R and TEC2014–52383-C3–3-R).
References
- Poon RT, Fan ST, Lo CM, et al. (2004). Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg 240:698–708.
- Sima CS, Jarnagin WR, Fong Y, et al. (2009). Predicting the risk of perioperative transfusion for patients undergoing elective hepatectomy. Ann Surg 250:914–21.
- Romano F, Franciosi C, Caprotti R, et al. (2005). Hepatic surgery using the Ligasure vessel sealing system. World J Surg 29:110–12.
- Sun HC, Qin LX, Lu L, et al. (2006). Randomized clinical trial of the effects of abdominal drainage after elective hepatectomy using the crushing clamp method. Br J Surg 93:422–6.
- Nagano Y, Matsuo K, Kunisaki C, et al. (2005). Practical usefulness of ultrasonic surgical aspirator with argon beam coagulation for hepatic parenchymal transection. World J Surg 29:899–902.
- Riediger C, Mueller MW, Geismann F, et al. (2013). Comparative analysis of different transection techniques in minor and major hepatic resections: a prospective cohort study. Int J Surg 11:826–33.
- Rau HG, Duessel AP, Wurzbacher S. (2008). The use of water-jet dissection in open and laparoscopic liver resection. HPB (Oxford) 10:275–80.
- Felekouras E, Petrou A, Neofytou K, et al. (2014). Combined ultrasonic aspiration and saline-linked radiofrequency precoagulation: a step toward bloodless liver resection without the need of liver inflow occlusion: analysis of 313 consecutive patients. World J Surg Oncol 12:357. doi: 10.1186/1477-7819-12-357.
- Berber E, Akyuz M, Aucejo F, et al. (2014). Initial experience with a new articulating energy device for laparoscopic liver resection. Surg Endosc 28:974–8.
- Kaibori M, Matsui K, Ishizaki M, et al. (2013). A prospective randomized controlled trial of hemostasis with a bipolar sealer during hepatic transection for liver resection. Surgery 154:1046–52.
- Gotohda N, Yamanaka T, Saiura A, et al. (2015). Impact of energy devices during liver parenchymal transection: a multicenter randomised controlled trial. World J Surg 39:1543–9.
- Hanyong S, Wanyee L, Siyuan F, et al. (2015). A prospective randomized controlled trial: comparison of two different methods of hepatectomy. Eur J Surg Oncol 41:243–8.
- Romano F, Garancini M, Uggeri F, et al. (2013). The aim of technology during liver resection — a strategy to minimise blood loss during liver surgery. In: H Abdeldayem, ed. Hepatic surgery. New York: InTech, 167–205.
- Itano O, Ikoma N, Takei H, et al. (2015). The superficial precoagulation, sealing, and transection method: a “bloodless” and “ecofriendly” laparoscopic liver transection. Technique. Surg Laparosc Endosc Percutan Tech 25:e33–6.
- Kianmanesh R, Ogata S, Paradis V, et al. (2008). Heat-zone effect after surface application of dissecting sealer on the “in situ margin”; after tumorectomy for liver tumors. J Am Coll Surg 206:1122–8.
- Ogata S, Kianmanesh R, Varma D, et al. (2005). Improvement of surgical margin with a coupled saline-radio-frequency device for multiple colorectal liver metastases. J Hepatobiliary Pancreat Surg 12:498–501.
- Burdío F, Grande L, Berjano E, et al. (2010). A new single-instrument technique for parenchyma division and hemostasis in liver resection: a clinical feasibility study. Am J Surg 200:e75–80.
- Burdío F, Navarro A, Berjano E, et al. (2008). A radiofrequency-assisted device for bloodless rapid transection of the liver: a comparative study in a pig liver model. Eur J Surg Oncol 34:599–605.
- Navarro A, Burdio F, Berjano EJ, et al. (2008). Laparoscopic blood-saving liver resection using a new radiofrequency-assisted device: preliminary report of an in vivo study with pig liver. Surg Endosc 22:1384–91.
- Hefermehl LJ, Largo RA, Hermanns T, et al. (2014). Lateral temperature spread of monopolar, bipolar and ultrasonic instruments for robot-assisted laparoscopic surgery. BJU Int 114:245–52.
- Lieu PT, Heiskala M, Peterson PA, et al. (2001). The roles of iron in health and disease. Mol Aspects Med 22:1–87.
- Lehr R. (1992). Sixteen S-squared over D-squared: a relation for crude sample size estimates. Stat Med 11:1099–102.
- Lesurtel M, Selzner M, Petrowsky H, et al. (2005). How should transection of the liver be performed?: a prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann Surg 242:814–22.
- Sitzmann JV, Greene PS. (1994). Perioperative predictors of morbidity following hepatic resection for neoplasm. A multivariate analysis of a single surgeon experience with 105 patients. Ann Surg 219:13–7.
- Kooby DA, Stockman J, Ben-Porat L, et al. (2003). Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg 237:860–9.
- Huang ZQ, Xu LN, Yang T, et al. (2009). Hepatic resection: an analysis of the impact of operative and perioperative factors on morbidity and mortality rates in 2008 consecutive hepatectomy cases. Chin Med J (Engl) 122:2268–77.
- Pai M, Frampton AE, Mikhail S, et al. (2012). Radiofrequency assisted liver resection: analysis of 604 consecutive cases. Eur J Surg Oncol 38:274–80.
- Ercolani G, Ravaioli M, Grazi GL, et al. (2008). Use of vascular clamping in hepatic surgery: lessons learned from 1260 liver resections. Arch Surg 143:380–7.
- Richter S, Kollmar O, Schuld J, et al. (2009). Randomized clinical trial of efficacy and costs of three dissection devices in liver resection. Br J Surg 96:593–601.
- Polignano FM, Quyn AJ, de Figueiredo RS, et al. (2008). Laparoscopic versus open liver segmentectomy: prospective, case-matched, intention-to-treat analysis of clinical outcomes and cost effectiveness. Surg Endosc 22:2564–70.
- Dagher I, Di Giuro G, Dubrez J, et al. (2009). Laparoscopic versus open right hepatectomy: a comparative study. Am J Surg 198:173–7.