Abstract
Purpose: To determine the efficacy and safety of percutaneous hepatic wedge ablation in treating hepatic malignancies of the inferior margin.
Materials and methods: Seventy-seven patients with hepatic malignancies at the inferior margin underwent percutaneous radiofrequency ablation (RFA). Thirty-two patients underwent hepatic wedge ablation and 45 patients underwent conventional tumour ablation. Comparative analysis of the two groups was performed including gender, age, tumour size, number of ablation cycles, ablation duration and injected hydrodissection volume. The rate of technical success, local tumour progression, intrahepatic distant recurrence, major complications and overall survival were assessed and compared. Survival analysis was analysed using the Kaplan–Meier method. Differences in the survival rates were compared with log-rank test.
Results: The mean number of ablation cycles and ablation duration were significantly higher in the hepatic wedge ablation group than conventional tumour ablation (1.6 ± 0.9 vs. 1.2 ± 0.4, p = .042, and 30.2 ± 18.5 vs. 22.5 ± 8.5 min, p = .031, respectively). The local tumour progression rate was significantly lower in hepatic wedge ablation group (0% vs. 17.78%, p = .038) at median follow-up of 21 months. The rate of technical success, intrahepatic distant recurrence, major complications and overall survival did not differ between the two groups.
Conclusion: Hepatic wedge ablation appears to be a highly effective treatment for hepatic malignancies in the inferior margin and provides a better local control than conventional tumour ablation.
Introduction
Radiofrequency ablation (RFA), a local minimally invasive method, is a safe and effective treatment for liver tumour, which has clinically been widely used worldwide [Citation1–3]. For liver tumours with diameter less than 3 cm, ablation demonstrates similar efficacy as surgical resection [Citation4]. However, ablation still has its limitation for larger tumours or tumour located in particular anatomic locations [Citation5,Citation6]. For example, difficulty in treating tumour in thin (<5 cm) inferior margins of the liver has been noted due to the risk of thermal damage to adjacent structures such as the bowels [Citation7–9]. The surrounding liver tissue is limited for tumours in this anatomic location. This location is very close to critical structures such as the bowels which are sensitive to thermal injury and cause serious complication if damaged [Citation10–12]. Although hydrodissection techniques that instil fluid between the liver and adjacent critical structures have reduced the risks somewhat, adequate ablation of tumours in this region is still particularly challenging [Citation10,Citation13,Citation14]. To surmount these issues, our centre has proposed that bracketing the tumour site with two RF electrodes could potentially obtain a wedge ablation that might lead to a better or safer procedure for such cases. Specifically, the portion of tumour and surrounding liver tissues in closest proximity to the hepatic hilum was ablated first, then the whole tumour body was ablated up to the edge of the liver, resulting in a complete hepatic wedge ablation. Our study aimed to explore the efficacy and safety of this novel technique, compared to conventional ablation in the same period.
Materials and methods
Patients
From May 2008 to February 2014, 77 patients with 78 liver malignancies located at inferior margin were recruited in this study. There were 51 men and 26 women enrolled in this study. The mean age was 60.0 ± 12.1 years (range 35–86 years). The mean follow-up after RFA was 21.0 ± 12.8 months (range 3–56 months). Liver malignancies at the “inferior margin” were defined as those located in peripheral regions where the thickness of liver is less than 5 cm and the distance between tumour and hepatic surface is less than 5 mm (). These tumours were located at the hepatic segments of S6 (n = 52), S5 (n = 9), S4 (n = 5), S3 (n = 9) and S2 (n = 3). All the patients met the following criteria for RFA: (1) tumours visible on ultrasonography, (2) tumour size ≤5 cm, tumour number ≤3, (3) no direct invasion of adjacent organs, (4) the absence of portal venous thrombosis, (5) platelet count >50 × 103/mm3 and prothrombin activity >50% and (6) non-exophytic with protrusion of tumour beyond the liver surface limited to less than one third of the tumour volume. Patients were divided into two treatment groups: the hepatic wedge ablation group and a conventional tumour ablation group. All of the RFA treatments were performed by two radiologists who engaged in comprehensive discussion with the patients prior to the procedure and obtaining informed consent. The benefit and risks and other detailed information were explained by the radiologists. They specifically, noted that wedge ablation takes longer and induces greater damage to normal liver while obtaining larger ablation volumes, compared to conventional tumour ablation. Group selection was chosen by patients voluntarily after full explanation.
Figure 1. Schematic diagram of tumour locations at the inferior margin of the liver including portions of segments 2, 3, 4 on the left, segments S5 and S6 on the right, where the thickness of liver is less than 5 cm. For tumours in this location, the distance between the tumour and hepatic surface is less than 5 mm.
In total, 32 patients (33 tumours) were treated by hepatic wedge ablation, including 18 primary hepatocellular carcinoma (HCC) (19 tumours) and 14 metastatic cancer (14 tumours). The average age was 57 ± 13 years old (range 35–80 years old) and tumour size was 2.9 ± 1.1 cm in diameter. Forty-five patients (45 tumours) were treated by conventional tumour ablation, including 23 HCC (23 tumours) and 22 metastatic malignancies (22 tumours). The average age was 62 ± 11 years old (range 38–86 years old) and tumour size was 2.9 ± 0.9 cm in diameter. In the hepatic wedge ablation group, metastatic cancer included four lung cancer, three colorectal cancer, two gastric cancer, two gastrointestinal stromal tumour, one cervical carcinoma, one ovarian cancer and one breast cancer. In the conventional tumour ablation group, metastatic cancer included 14 colorectal cancer, three lung cancer, three breast cancer and two gastric carcinoma. All patients subjected to ultrasound guided percutaneous RFA treatment were unwilling or not suitable for surgery due to senility, diabetes, severe cardiovascular disease and/or poor liver function, alone or in combination. There was no significant difference in age (p = .114), gender (p = .694) or tumour size (p = .985) between the two groups (). The protocol of this retrospective study was approved by the institutional review board of Beijing Cancer Hospital. Written informed consents (for clinical care consent for two procedures that were each considered standard of care, the hepatic wedge ablation was considered a novel procedure and still within art of practicee for RFA device) were obtained from all patients or legal guardians before RFA treatment.
Table 1. Patient characteristics (n = 77).
Ultrasound guided RFA procedure
GE Logiq E9 (New York, CT) and Aloka α-10 (Tokyo, Japan) ultrasound devices were used in this study. Real-time grey scale ultrasonography scanning was performed using 3.5–5.0-MHz small-sector and board-view convex probes equipped with attachments for biopsy and RFA electrode insertion. Colour ultrasound was used to avoid large vessels traversing the path of the applicator insertion. Contrast-enhanced ultrasound (CEUS) was regularly performed to confirm tumour coverage and tumour number prior to RFA. SonoVue (Bracco SpA, Milan, Italy) was used as the contrast agent in this study. RFA treatment was performed using the Celon RFA system (Teltow, Germany). These bipolar electrode needles were 16 G. All treatments were performed by two radiologists with more than eight years of experience of ultrasound-guided RFA each (C.M.H., Y.K.). The end point of ablation was determined by impendence and output power, as well as ultrasound/CEUS imaging showed safety margin was covered.
Hepatic wedge ablation
Two RF electrode needles were inserted under ultrasound guidance parallel to the tumour proximity to the hepatic hilum with the distance between two needles measured as less than 2.5 cm. The portion of tumour and surrounding liver tissues in closest proximity to the hepatic hilum was ablated first, in order to limit the blood supply to the tumour [Citation15] and the edge region of liver. Then the whole tumour body was ablated up to the edge of the liver, resulting in a complete hepatic wedge ablation. Intraperitoneal injection of distilled water was performed before and during the treatment to separate tumour from surrounding tissue and organs. We placed 20 G PTC needles (Hakko Medical Co., Ltd., Chikuma, Japan) between the liver and surrounding organs under ultrasound guidance. The target separation distance was more than 0.5 cm and injection volume of fluid was 100–500 ml ().
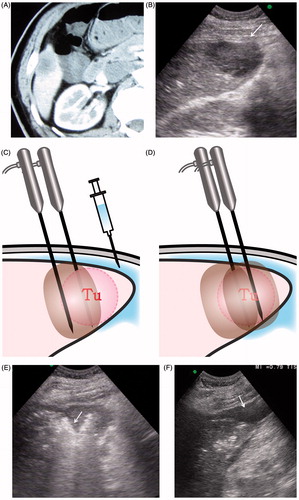
Figure 2. A 63-year old man with hepatocellular carcinoma at S6 was treated with percutaneous hepatic wedge ablation. (A) CT showed a slightly bulging tumour located at the inferior margin of Segment 6 with 3.5 cm in diameter. (B) Ultrasound showed a hypoechoic tumour (arrow) adjacent to surrounding digestive tract. (C) Two RF electrode needles were inserted parallel in the tumour side proximity to the hepatic hilum and the distance between two needles was less than 2.5 cm under ultrasound guidance. The portion of tumour (Tu) and surrounding liver tissues proximity to hepatic hilum was firstly ablated, in order to cut off blood supply to the tumour and the edge region of liver. (D) Then the whole tumour body was ablated. Distilled water was injected to separate tumour with surrounding digestive tract before and during ablation. (E) Hepatic wedge ablation: the hyperechoic area (arrow) was the portion of tumour and surrounding liver tissues proximity to hepatic hilum which was firstly ablated. (F) 160 ml distil water (arrow) was injected under ultrasound guidance to separate tumour with surrounding digestive tract. (G) Second, the portion of tumour (arrow) near to the edge of liver was ablated. (H) CEUS immediately after ablation showed no enhancement in tumour, and non-enhanced area was up to 5.0 cm, which suggested the tumour was completely coagulated. The arrow (arrow) showed proximal blood vessels to the tumour were cut off. (I) Enhanced CT one month after ablation: tumour and the surrounding tissue had no enhancement, suggesting the similar effect to limited segmental hepatectomy. CT also noted that the colon situated within 2 mm of the ablated zone was not injured.
Conventional tumour ablation
Conventional tumour ablation was performed using a conventional RFA ablation technique. Ablation was performed with the intention of coagulating the tumour itself plus an ablative margin of 0.5–1.0 cm, and thus 2–3 bipolar electrodes were inserted directly into the edges of the tumour at its maximum dimension. The distance between the needles was equal to or less than 2.5 cm. The tip of needle was placed approximately 0.5 cm deeper than tumour under ultrasound guidance. RF energy was simultaneously applied at 30–40 W. For tumours larger than 3 cm, a strategy of multiple overlapping ablations was used [Citation16]. Intraperitoneal injection of distilled water was also performed before and during the treatment.
Anaesthesia
Local infiltration anaesthesia was induced by 1% lidocaine (Liduokayin, Beijing, China) before the ablation operation. After accurate insertion of electrodes, sedative anaesthesia was induced by intravenous administration of 2.5–5.0 mg of midazolam (Roche, Basel, Switzerland) and 50–100 μg of fentanyl (Fentaini, Yi Chang, China). The patient’s vital signs, such as blood pressure, heart rate, respiration rate and oxygen saturation were continuously monitored during the procedure by an anaesthetist. These patients were moved to an inpatient’s room 1–2 h after treatment when they recovered conscious and there was no evidence of active bleeding seen on the ultrasound scans.
Assessment of efficacy and follow-up
Enhanced computed tomography (CT) or magnetic resonance image (MRI) and laboratory tests were performed one month after RFA, with follow-up every three months afterwards. CT or MRI results one month after the treatment were used to evaluate technical success. Based upon published guidelines [Citation7], the ablation was considered a success on the basis of all of the following findings at follow-up CT: (a) no contrast enhancement was detected within or around the tumour, (b) the margins of the ablation zone were clear and smooth and (c) the ablation zone extended beyond the tumour borders. Unsuccessful treatment was noted when abnormal enhancement was observed within or around the ablation zone. The presence of abnormal enhancement one month after RFA treatment was defined as technical failure. When no abnormal enhancement was found one month after RFA treatment, abnormal enhancement or wash out developed around the original tumour at follow-up, it was defined as local tumour progression. If new tumour was found in other regions of the liver, it was defined as intrahepatic distant recurrence. Serum alpha-fetoprotein testing was performed for HCC, while carcino-embryonic antigen, carbohydrate antigen 19-9 and other testing were performed for metastatic cancer, as appropriate. Enhanced CT or MRI results were blindly reviewed by other radiologists who were not familiar with the cases.
Safety assessment
Complications were recorded for both groups during follow-up. Major complications were referred to events threatening the lives of patients, causing disability, requiring hospitalisation or prolonging hospitalisation [Citation7]. We used ultrasound to monitor the entire ablation procedure and detect early complications such as bleeding. Generally, the patients were hospitalised for 1–2 days after the RFA procedure. Patients were monitored by means of inquiry, physical examination, complete blood cell counts, and renal and hepatic function tests during their hospital stay.
Statistical analyses
SPSS 19.0 statistical analysis software was employed. Continuous variables were presented as mean ± standard deviation (x±s), and were compared using independent samples t-test. Categorical variables were compared using chi-square test or Fisher's exact test. Survival analysis was analysed by Kaplan–Meier method. Differences in the survival rates were compared with log-rank test. p < .05 was considered as statistically significant.
Results
Ablation data
The number of ablation cycles was significantly higher in hepatic wedge ablation group (1.6 ± 0.9 vs. 1.2 ± 0.4, p = .042) than conventional tumour ablation group. The mean ablation duration was significantly longer in hepatic wedge ablation group (30.2 ± 18.5 vs. 22.5 ± 8.5 min of electrode activation time, p= .031). The mean injected hydrodissection fluid volume was significantly larger in the hepatic wedge ablation group (240.3 ± 170.5 ml vs. 164.2 ± 143.2 ml, p = .036). Based upon enhanced CT one month after RFA, the ablation area diameter was larger in wedge ablation group than conventional ablation group (4.2 ± 0.9 cm vs. 3.7 ± 1.2 cm, p = .032).
Local tumour progression and intrahepatic distant recurrence
The comparison of the treatment effects between hepatic wedge ablation group and conventional tumour ablation group is shown in . The local tumour progression rate of hepatic wedge ablation was significantly lower than conventional tumour ablation (0% vs. 17.8%, p = .038). There was no significant difference in technical success rate and intrahepatic distant recurrence rate between the two groups (p = .232, p = .656). The local tumour progression was found in eight cases (five tumours >3 cm, three tumours in S4, two in S5, two in S2, one in S6, ablation time was 22.3 ± 8.9 min) in conventional ablation group () and then they were treated with TACE (n = 4), re-ablation (n = 2) or surgical resection (n = 2). The contrast imaging findings of local tumour progression included nodular enhancement at the margin of ablation zone at arterial phase (n = 4), irregular enhancement at one side of ablation zone (n = 2), ring like enhancement (n = 2), and all had wash out on late phase images.
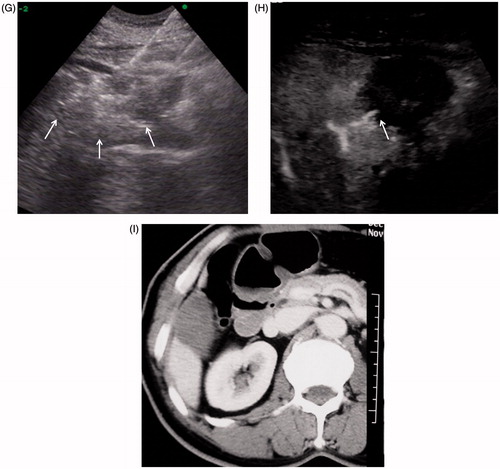
Figure 3. A 77-year old man with liver metastasis of colon cancer received percutaneous conventional tumour RFA. Local tumour progression occurred one year after tumour ablation. (A) Arterial phase in enhanced CT before RFA: the hypo-enhanced metastasis tumour was located the inferior margin of Segment 6. (B) The tumour was directly coagulated by simultaneous RF application with three electrodes. (C) Enhanced CT one month after RFA showed no tumour residue was found in the coagulated area. (D) Enhanced MRI (T1WI in arterial phase) one year after treatment showed tumour local progression at the inferior of coagulated area (arrow).
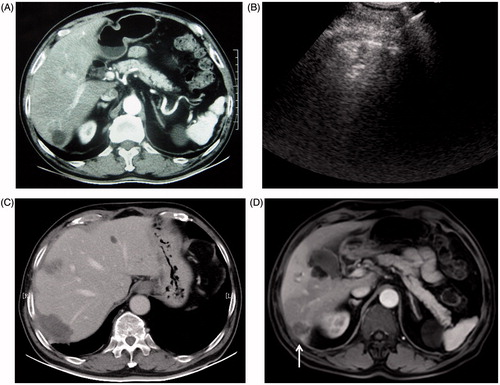
Table 2. Comparison of clinical outcomes after RFA.
Survival
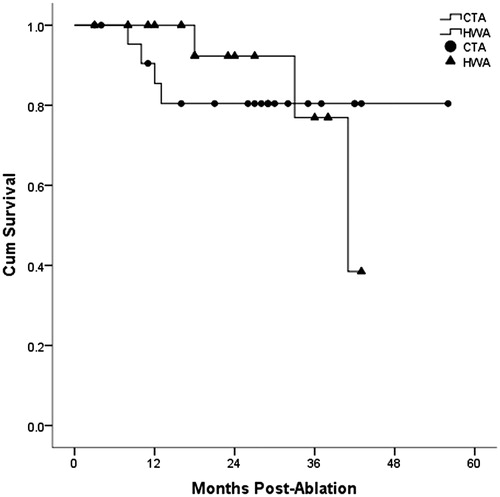
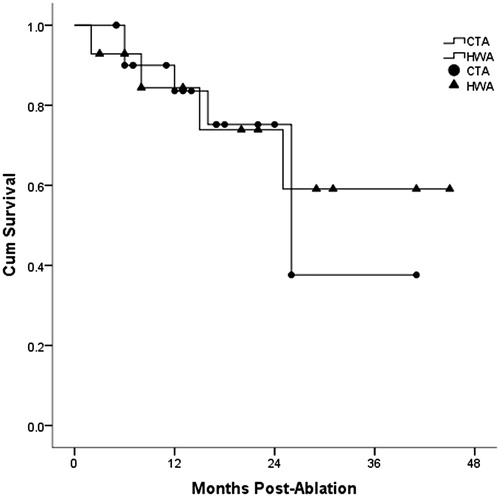
Comparison of overall survival for HCC and liver metastasis is shown in and . The one, two and three-year overall survival for HCC in hepatic wedge ablation group and conventional tumour ablation group was 100.0%, 92.3%, 76.9% and 85.4%, 80.4%, 80.4%, respectively. However, there was no significant difference between the two groups (p = .942). One, two and three-year overall survival for liver metastasis in hepatic wedge ablation group and conventional tumour ablation group was 84.4%, 73.9%, 59.1% vs. 83.6%, 75.2%, 37.6%, respectively. There was no significant difference between the two groups (p = .811).
Major complication
No major complication was found in the hepatic wedge ablation group. In the conventional tumour ablation group, one patient suffered massive haemorrhagic pleural effusion, which resolved after drainage. There was no significant difference in major complications rates between the two groups (p = .584).
Discussion
Surgical resection has been conventionally considered the most effective cure for HCC. Given the removal of relatively larger volumes of normal liver tissue, most patients with liver cirrhosis or poor liver function require very careful consideration when choosing anatomical hepatectomy. Limited segmental hepatectomy, a common surgical resection which requires tumour resection margin distance of at least 0.5 cm [Citation17,Citation18], can retain more normal liver tissue, and reduce the incidence of postoperative liver failure. Inspired by limited segmental hepatectomy surgery, wedge ablation was applied when tumour is near the inferior margin of liver in the current study. The HCC blood supply commonly come from hepatic artery system and the direction was from hepatic hilum to peripheral liver. The main blood supply of tumour in inferior margin was cut off or decreased when the liver tissue proximity to the hepatic hilum was ablated first. Tumour and surrounding liver tissues in proximity to the hepatic hilum was ablated, which essentially recreated an image-guided minimally invasive limited segmental hepatectomy. This method further allowed ablation of the tumour blood supply, reducing the risk of local residue. Expanding ablation area can also simultaneously ablate the satellite focus, which hide in the periphery of tumour, thus reducing the local tumour progression. This study showed that the local tumour progression rate of hepatic wedge ablation group was significantly lower than conventional tumour ablation group, which confirmed that hepatic wedge ablation could help to improve local treatment effects.
Previous studies showed that when HCC were treated with RFA, ablative margin was an important factor for local therapeutic efficacy [Citation19]. When periablational margins were greater than 1 cm, the local tumour progression rate was lower than those less than 1 cm. Yet, it must be acknowledged that for tumours located on the edge of liver, there is no possibility of creating a safety margin to the visceral surface of liver. Indeed, larger treatment zones can potentially cause excessive damage to the surrounding anatomic structures. In this study, the extent of ablation at the more central hilar side of the tumour was increased, which facilitated achieving a true wedge ablation when the thickness of liver is less than 5 cm. By using this method, the local tumour progression could be effectively reduced.
In the conventional tumour ablation group, only tumours with a 0.5–1.0 cm margin of normal liver tissue around the tumour were ablated. There were four cases of tumour residual (two cases of HCC and two cases of metastases) and eight cases of local tumour progression (two cases of HCC and six cases of metastases) in this group. These results were likely caused by incomplete ablation, leaving the local tumour residual to grow and leading to local tumour progression. Previous studies have shown that local tumour progression usually occurred within six months after local treatment, especially for larger tumours and metastatic cancer with tumour size being the most significant factor affecting local tumour progression rate [Citation20].
Tumours located at the subcapsular, inferior margin with potential protrusion from the liver were most often located abutting surrounding organs, such as the stomach and colon. In these settings, percutaneous ablation can therefore potentially harm surrounding tissue and cause complications. Previous studies showed that percutaneous ablation can lead to digestive tract perforation when the tumour is located less than 0.5 cm from digestive tract [Citation21]. Yet, in order to avoid complications such as digestive tract perforation, margin distance is often insufficient during percutaneous ablation therapy, which can lead to residual tumour. To solve this problem, we applied intraperitoneal hydrodissection to achieve the separation of the digestive tract and liver tumours [Citation22]. Ablation was only performed when the injected distilled water could separate tumour (or ablation zone) and the digestive tract by more than 0.5 cm. In this study, compared with conventional tumour ablation group, larger amount of fluid was injected to completely separate liver tumour at inferior margin from the digestive tract in the hepatic wedge ablation group. Thus, although expanded liver tissues were ablated in hepatic wedge ablation group, there was no significant difference in major complications between the two groups. Therefore, as long as appropriate protective measures are taken, hepatic wedge ablation of tumour at inferior margin could be a safe and feasible method.
In this study, overall survival did not differ between the two groups. The possible reasons include that: (1) as a local therapy method, tumour ablation cannot prevent intrahepatic distant recurrences that impact upon survival; (2) local tumour progression in the conventional tumour ablation group was treated with further therapies, which might compensate and equalise survival between the two groups; and 3) follow-up was not long enough to detect the difference, suggesting that further longer follow-up is needed. Although the new ablation technique may not improve overall survival, nevertheless, the dramatic reduction in the number of patients requiring additional invasive and costly procedures may make wedge ablation particularly appealing for appropriately selected patients.
Other clinical investigators have likewise attempted to modify conventional tumour ablation techniques in an attempt to improve outcomes. For example, Seror et al. have most recently reported a “no-touch”, multibipolar RFA strategy for HCC tumours and report a high local tumour progression-free survival rate [Citation23]. It should be noted though that in our series more than 80% patients had HCCs located in proximity to large vessels or nearby structures to the extent that it was not possible to achieve the safety margins required for the described no-touch ablation. Hence, in comparison to the simultaneous RF application reported, our technique was to sequentially ablate the more central portions of the liver first to decrease tumour supply and then ablate tumour itself – a novel modification especially fit for tumours located at the inferior margin. Additionally, this technique is feasible for all kinds of RFA electrodes while multiple RFA needles placement simultaneously like celon biopolar needles would be better candidate equipment to finish this technique.
This study was limited by a possible selection bias resulting from the comparison of these nonrandomised groups and retrospective profile. Other clinical and tumour characteristics such as year of accrual, tumour type, isolated vs. other metastasis disease might influence the outcome comparison. The prospective multicenter randomised trial will be required to further clarify the role of hepatic wedge ablation in the therapeutic strategy in the future. Second, the survival result between the two groups did not show significant difference. However, the local control benefit in the wedge ablation group was achieved with a larger ablation zone, and this fact should serve to strengthen the conclusion that the new technique is an improvement over conventional RFA in these difficult-treated cases.
In conclusion, hepatic wedge ablation appears to be a highly effective treatment for tumours in the hepatic inferior margin and provides a better local control than conventional tumour ablation. In clinical practice, it can potentially be recommended for the patients with liver malignancies at inferior margin.
Disclosure statement
Goldberg S.N. sponsored research and consulting Angiodynamics (Marlborough, MA) and Cosman Company (Burlington, MA). The other authors stated no financial relationship to disclose.
References
- Peng ZW, Liu FR, Ye S, et al. (2013). Radiofrequency ablation versus open hepatic resection for elderly patients (>65 years) with very early or early hepatocellular carcinoma. Cancer 119:3812–20.
- Yan K, Chen MH, Yang W, et al. (2008). Radiofrequency ablation of hepatocellular carcinoma: long-term outcome and prognostic factors. Eur J Radiol 67:336–7.
- Facciorusso A, Di Maso M, Muscatiello N. (2016) Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia 32:339–44.
- Bruix J, Sherman M. (2005). Management of hepatocellular carcinoma. Hepatology 42:1208–36.
- Teratani T, Yoshida H, Shiina S, et al. (2006). Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology 43:1101–8.
- Chen MH, Yang W, Yan K, et al. (2008). Radiofrequency ablation of problematically located hepatocellular carcinoma: tailored approach. Abdom Imaging 33:428–36.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. Radiology 273:241–60.
- Kong WT, Zhang WW, Qiu YD, et al. (2009). Major complications after radiofrequency ablation for liver tumours: analysis of 255 patients. World J Gastroenterol 15:2651–6.
- Chen TM, Huang PT, Lin LF, Tung JN. (2008). Major complications of ultrasound-guided percutaneous radiofrequency ablations for liver malignancies: single centre experience. J Gastroenterol Hepatol 23:445–50.
- Song I, Rhim H, Lim HK, et al. (2009). Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol 19:2630–40.
- Kim YS, Rhim H, Paik SS. (2006). Radiofrequency ablation of the liver in a rabbit model: creation of artificial ascites to minimise collateral thermal injury to the diaphragm and stomach. J Vasc Interv Radiol 17:541–7.
- Kitchin D, Lubner M, Ziemlewicz T, et al. (2014). Microwave ablation of malignant hepatic tumours: intraperitoneal fluid instillation prevents collateral damage and allows more aggressive case selection. Int J Hyperthermia 30:299–305.
- Zhang M, Liang P, Cheng ZG, et al. (2014). Efficacy and safety of artificial ascites in assisting percutaneous microwave ablation of hepatic tumours adjacent to the gastrointestinal tract. Int J Hyperthermia 30:134–41.
- Kondo Y, Yoshida H, Shiina S, et al. (2006). Artificial ascites technique for percutaneous radiofrequency ablation of liver cancer adjacent to the gastrointestinal tract. Br J Surg 93:1277–82.
- Dodd GD III, Dodd NA, Lanctot AC, Glueck DA. (2013). Effect of variation of portal venous blood flow on radiofrequency and microwave ablations in a blood-perfused bovine liver model. Radiology 267:129–36.
- Chen MH, Yang W, Yan K, et al. (2004). Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients-mathematic model, overlapping mode, and electrode placement process. Radiology 232:260–71.
- Eltawil KM, Kidd M, Giovinazzo F, et al. (2010). Differentiating the impact of anatomic and non-anatomic liver resection on early recurrence in patients with hepatocellular carcinoma. World J Surg Oncol 8:43–50.
- Poon RT, Fan ST, Ng IO, Wong J. (2000). Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg 231:544–51.
- Kono M, Inoue T, Kudo M, et al. (2014). Radiofrequency ablation for hepatocellular carcinoma measuring 2 cm or smaller: results and risk factors for local recurrence. Dig Dis 32:670–7.
- Bleicher RJ, Allegra D, Nora DT, et al. (2003). Radiofrequency ablation in 447 complex unresectable liver tumors: lessons learned. Ann Surg Oncol 10:52–8.
- Chen MH, Yang W, Yan K, et al. (2006). Treatment Strategy to optimize radiofrequency ablation for liver malignancies. J Vasc Interv Radiol 17:671–83.
- Chen MH, Goldberg SN. (2009). Radiofrequency ablation of liver tumour: basic and clinical studies. Beijing, China: People?s Medical Publishing House, 241–70.
- Seror O, N'Kontchou G, Nault JC, et al. (2016). Hepatocellular carcinoma within Milan Criteria: no-touch multibipolar radiofrequency ablation for treatment-long-term results. Radiology 280:611–21.