Abstract
Background: Radiofrequency ablation (RFA) and percutaneous ethanol injection (PEI) are important treatments for patients with hepatocellular carcinoma (HCC) who are not eligible for resection and liver transplantation. Therefore, it is important to establish comparisons between RFA, PEI and the two therapies in combination.
Aims: To evaluate the clinical efficacy and safety of combined RFA-PEI versus monotherapy with either RFA or PEI for HCC to provide references for clinical practice and further research.
Methods: We searched all eligible studies published before September 2015 in the Cochrane Library, PubMed, Embase, Web of Science and Chinese databases, such as CBM, CNKI, VIP and WanFang and also retrieved papers from other sources. All relevant controlled trials were collected. Meta-analyses were performed using RevMan version 5.3 software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
Results: Thirteen trials with 1621 patients were identified. Compared with PEI, RFA was associated with significant improvement in overall survival (OS) rate at 1, 2, 3 and 4 years, cancer-free survival (CFS) rate at 1, 2 and 3 years and complete tumour necrosis. RFA was associated with a significant reduction in the local recurrence rate at 1, 2 and 3 years. However, RFA was also associated with a higher total risk of complications. Compared with RFA alone, combined RFA-PEI was associated with a significant improvement in the OS rate at 1.5, 2 and 3 years and a significant reduction in the local recurrence rate. However, combined RFA-PEI was also associated with a higher risk of fever.
Conclusion: The combination of RFA and PEI appears to be the optimal treatment strategy when considering combined RFA-PEI or either RFA or PEI alone. Combined RFA-PEI significantly improves OS and reduces the risk of local recurrence without increasing major complications. Further large-scale studies are needed to assess economic outcomes and quality of life.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and is a serious threat to human health as the second leading cause of cancer deaths worldwide [Citation1]. Furthermore, its incidence is increasing globally, especially in developing countries [Citation2]. HCC is characterised by insidious onset, rapid progress, poor prognosis and frequent relapse [Citation3]. The leading risk factors of HCC are chronic hepatitis B virus (HBV) infection in Africa and East Asia and chronic hepatitis C virus (HCV) infection in developed Western countries and Japan [Citation4–6].
Resection is currently the gold standard for HCC treatment, and liver transplantation is the first-line treatment for patients who are not suitable for resection [Citation7]. However, only a minority benefit from these therapies because of strict selection criteria and a shortage of donated organs, particularly for those with cirrhosis [Citation8]. Additionally, the postoperative recurrence rate is high [Citation4]. These factors favour the development of non-surgical treatment techniques to benefit these patients who are ineligible for surgery. Percutaneous ablation is currently the best choice among many non-surgical methods [Citation8,Citation9], including radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), percutaneous acetic acid injection (PAI), microwave ablation, cryoablation, laser-induced thermotherapy (LITT) and high-intensity focused ultrasound (HIFU) [Citation7].
RFA and PEI are the most generally evaluated and recognised percutaneous treatments [Citation10]. PEI as the original ablation method has been a standard treatment for HCC, and RFA has played an important role and is considered a very promising treatment [Citation11,Citation12]. Some published meta-analyses [Citation13–17] comparing RFA with PEI exist; however, there are no systematic reviews (SR) comparing combined RFA-PEI and monotherapy with either RFA or PEI alone to establish evidence for clinical practise. Accordingly, we performed the first SR to focus on the combination of RFA-PEI, and comprehensively compared the efficacy and safety between RFA-PEI, RFA alone and PEI alone. This SR about combination of RFA-PEI provides more important evidence for oncologists than previous SRs [Citation13–17] and can serve as more effective guidance for clinical treatment decisions and subsequent liver tumour research.
Materials and methods
Selection criteria
Study type: Only randomised clinical trials (RCTs) were included to compare RFA with PEI. Controlled clinical trials (CCT), cohort studies (CS) and case control studies (CCS) were all included to compare RFA-PEI in combination with either RFA or PEI alone on the basis of pre-retrieval. Studies were included regardless of allocation concealment and blinding, and irrespective of language.
Participants: Patients with HCC diagnosed by histological examination or clinical diagnostic criteria, regardless of Child–Pugh class, tumour size, or previous treatment for liver tumours. Patients with other previous or simultaneous serious organ dysfunctions were excluded.
Intervention and Comparison: Combined RFA-PEI vs. either RFA alone or PEI alone, and RFA vs. PEI.
Outcomes: The primary endpoint was the overall survival (OS) rate. The secondary endpoints included the cancer-free survival (CFS) rate, local recurrence rate and new HCC recurrence rate. The tertiary endpoints included assessments of complete tumour necrosis (complete tumour response), complications, quality of life and economic outcomes.
Search strategy
We searched for eligible studies from inception to Sept. 21st, 2015 in electronic databases including the Cochrane Library, PubMed, Embase, Web of Science and Chinese databases, such as the Chinese Biomedical Literature Database (CBM), the China National Knowledge Infrastructure (CNKI), the Chinese VIP Literature Database (VIP) and the Chinese WanFang Literature Database (WanFang). We used the following search terms: hepatocellular carcinoma, liver neoplasms, primary liver carcinoma, liver cell carcinoma, liver cell tumour, hepatic tumour, liver tumour, hepatic cancer, liver cancer, HCC, catheter ablation, radiofrequency thermal ablation, RFA, ablation techniques, chemical ablation, alcohol injection, ethanol ablation, PEI, randomised controlled trial, RCT and controlled clinical trial, among others.
There were no language restrictions. We also performed a manual search to retrieve sources that were not found in the computer search.
Study screening and data extraction
We screened studies according to the selection criteria. By reading study titles and abstracts, we excluded some papers that clearly failed to satisfy the inclusion criteria. We then retrieved and assessed the full text of the remaining studies. We contacted the authors whenever we found that important information was unavailable in the published work.
Data extraction included the author, country, publication date, study design, sample size, basic patient information, aetiology, tumour characteristics, treatment methods, treatment endpoints, treatment sessions, economic outcomes and follow-up duration. Some survival and recurrence data were extracted from graphs using DraftSight version 11.3 software (Dassault Systèmes, France).
Study screening and data extraction were performed independently by two investigators (Zheng Li and Kai Zhang). We carefully crosschecked and discussed inconsistencies afterwards. Discrepancies were resolved by the senior investigators (Denghai Mi and Shumei Lin).
Quality assessment
We performed a quality assessment in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0) [Citation18]. The assessment items included random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other biases. The two independent investigators (Zheng Li and Kai Zhang) assessed the study quality, and discrepancies were resolved by consensus.
Statistical methods
The meta-analysis was performed using RevMan version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) provided by the Cochrane organisation. Chi-square tests and I-square statistic were used to examine heterogeneity among included trials. When the homogeneity was considered adequate (p > .1, I2 < 50%), we used the fixed-effects model to perform the meta-analysis. Otherwise, the random effects model was conducted. When significant clinical heterogeneity existed, we performed subgroup analyses or qualitative synthetic analyses. The pooled odds ratios (OR) and 95% confidence intervals (CI) were computed for the outcomes. A p level less than 0.05 was adopted as the criterion for statistical significance.
Results
Selection and identification results
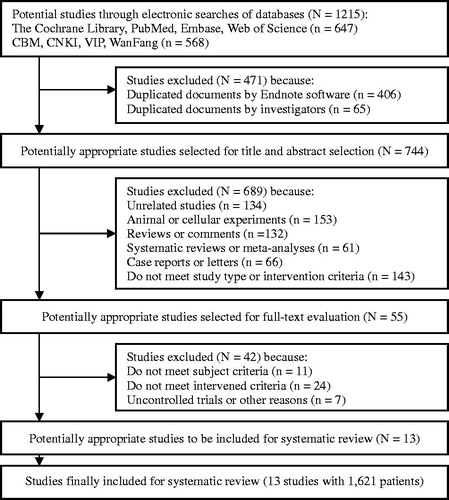
A total of 1215 references were retrieved after the initial search, and 471 references were excluded as duplicates by Endnote and manual assessment. Subsequently, 689 articles were excluded after careful screening of titles and abstracts. We examined the full texts of the 55 articles with potential value and excluded an additional 42 studies. One study with the largest sample size comparing RFA with PEI was excluded because of nonrandomised grouping. However, we referred to data from this study in our discussion [Citation19]. Finally, 13 studies [Citation20–32], including a total of 1621 patients, were deemed adequate for inclusion in this SR. All 13 studies [Citation20–32] were published in English, and all papers except for one [Citation27] were published in SCI journals [Citation20–26,Citation28–32]. There were no overlapping data among the 13 studies ().
Characteristics of the included studies
The baseline characteristics of the included studies are summarised in .
Table 1. Baseline characteristics of the studies included in the systematic review.
Seven studies [Citation20–26] compared RFA with PEI. Four of the studies were from Italy, two were from China and one was from Japan. Only one paper [Citation26] included all patients with a single tumour; additionally, 14 patients who were not eligible for RFA and shifted to PEI because of a segmental location were excluded from this paper. Only three papers [Citation22–24] mentioned tumour grade. In one study, Lin et al. [Citation22] created a conventional PEI group (2?5 ml/session) and a higher-dose PEI group (4?10 ml/session). However, the PEI doses in the other trials were 2?20 ml/session [Citation20,Citation21,Citation23–27]. Consequently, we combined the two groups from the Lin study into one PEI group to facilitate comparison with RFA. Another trial [Citation23] compared three treatment methods (RFA, PEI, PAI). We evaluated only the RFA and PEI data from this study according to the protocol. Livraghi et al. [Citation20] did not cover survival data. Only two studies [Citation25,Citation26] estimated economic outcomes. Three trials [Citation22–24] compared the duration of hospitalization between the two groups. Brunello et al. [Citation25] stopped enrolment early due to unexpected efficacy in the interim analysis.
One study [Citation27] from Egypt compared three groups (combined PEI-RFA, RFA and PEI), in which 91% of patients had a single tumour. Data from this study was used to compare both RFA vs. PEI and RFA vs. combined PEI-RFA.
Five studies [Citation27–32] compared RFA-PEI with RFA or PEI alone. Two of these studies were from China, one was from America, one was from Korea and one was from Japan. Shankar et al.’s study [Citation28] included patients with metastatic liver tumours, and Wong et al.’s study [Citation31] divided groups by the tumour location, however, all of these were irrespective to the results of the meta-analysis because of their qualitative synthetic evaluation.
Quality analysis
The quality analysis of the included studies is summarised in .
Table 2. Quality assessment of the studies included in the systematic review.
In the RCTs comparing RFA with PEI, no significant differences were observed in each study regarding patient age, gender, Child–Pugh class, proportion of cirrhotic patients, laboratory test results, number of tumours and tumour diameter. The baseline comparability had fine balance. The method of random sequence generation was unclear in one included RCT [Citation27], and one was randomly assigned by the location of the patient’s residence relative to the hospital [Citation20]. Other trials [Citation21–26] generated random sequences by computer or coded lists compiled from a random number generator. Six articles [Citation20–23,Citation25,Citation26] stated that allocations were concealed. Some studies reported that they did not adopt blinding because of the nature of the interventions [Citation20,Citation24,Citation25]. As the specificity of the two interventional therapies, blinding is not appropriate for physicians during the RFA and PEI procedures. Moreover, the primary effects are all objective indicators that are independent of blinding. Therefore, the evaluation for the assessment of blinding is considered as “Low risk of bias”. Three trials [Citation20,Citation23,Citation25] offered the data of loss to follow-up within the acceptable range. None of the included studies had selective outcome reporting.
There were six included papers [Citation27–32] that compared the combination group with the monotherapy group. Only two of them were RCTs [Citation27,Citation30]; the others were CCTs [Citation28,Citation29,Citation31] and a CCS [Citation32]. The baseline had fine balance in the two RCTs [Citation27,Citation30]. The study by Zhang et al. [Citation30] was confirmed to be a high quality RCT that generated its random sequence by computer and stated allocation concealment. Additionally, its patients were all blinded to the treatment assignment, and all analyses were performed on an intention-to-treat basis. There were not good baseline balance in the four non-RCT [Citation28,Citation29,Citation31,Citation32] studies, and quality assessment of them were low, but all of these were irrespective to the meta-analysis results because of their qualitative synthetic evaluation.
Meta-analysis of OS for RFA vs. PEI
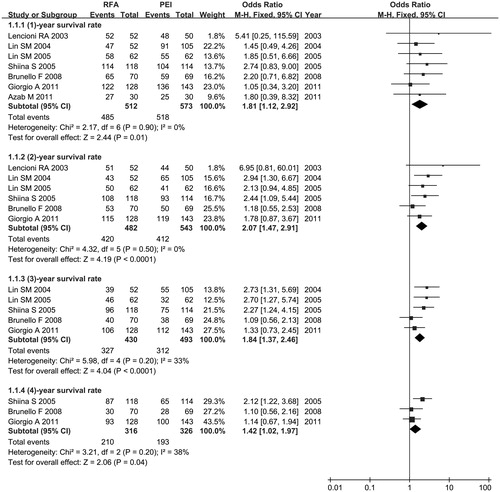
All studies [Citation21–27] except one [Citation20] reported OS data. Seven [Citation21–27], six [Citation21–26], five [Citation22–26] and three RCTs [Citation24–26] evaluated OS at 1, 2, 3 and 4 years, respectively. Five trials [Citation22–26] provided survival curves.
The meta-analysis showed that RFA significantly improved the OS rate over PEI at 1 year (OR 1.81, 95% CI =1.12–2.92, p = .01), 2 years (OR 2.07, 95% CI =1.47–2.91, p < .0001), 3 years (OR 1.84, 95% CI =1.37–2.46, p < .0001) and 4 years (OR 1.42, 95% CI =1.02–1.97, p = .04). Statistical homogeneity was adequate among all trials. Consequently, we used a fixed-effects model (p > .1, I2 < 50%) ().
Meta-analysis of CFS for RFA vs. PEI
Three RCTs [Citation21–23] reported CFS at 1 and 2 years. Two RCTs [Citation22,Citation23] reported CFS at 3 years.
The CFS rates were significantly higher with RFA than PEI at 1 year (OR 1.75, 95% CI =1.08–2.83, p = .02), 2 years (OR 2.08, 95% CI =1.37–3.15, p = .0006) and 3 years (OR 2.66, 95% CI =1.55–4.58, p = .0004). A fixed-effects model was used because no statistical heterogeneity was present among all trials (p > .1, I2 < 50%) ().
Table 3. Meta-analysis of the efficacy and safety between RFA and PEI.
Meta-analysis of recurrence for RFA vs. PEI
A total of five RCTs [Citation21–24,Citation26] reported local recurrence at 1 and 2 years. Four RCTs [Citation22–24,Citation26] reported local recurrence at 3 years. The results demonstrated that RFA conferred a lower risk of local recurrence than PEI at 1 year (OR 0.40, 95% CI =0.23–0.67, p = .0006), 2 years (OR 0.31, 95% CI =0.20–0.48, p < .00001) and 3 years (OR 0.41, 95% CI =0.27–0.65, p < .0001). We used a fixed-effects model because the statistical homogeneity was adequate among all trials (p > .1, I2 < 50%) ().
A total of five RCTs [Citation21–25] reported new HCC recurrence rates. No significant difference was found between RFA and PEI with respect to new HCC recurrence (OR 0.92, 95% CI =0.68–1.25, p = .59). A fixed-effects model was used because no statistical heterogeneity was detected among all trials (p > .1, I2 < 50%) ().
Meta-analysis of complete tumour necrosis for RFA vs. PEI
Seven studies [Citation20–25,Citation27] reported complete tumour necrosis. A significant difference was observed between RFA and PEI with respect to complete tumour necrosis (OR 3.76, 95% CI =2.17–6.54, p < .00001). A fixed-effects model was performed because the statistical homogeneity was adequate among all trials (p > .1, I2 < 50%) ().
Meta-analysis of complications for RFA vs. PEI
Data for total complications were obtained from eight trials [Citation20–27] comparing RFA with PEI. Data for major complications were obtained from six [Citation20,Citation23–27] studies. RFA had a significantly higher risk than PEI regarding total complications (OR 2.02, 95% CI= 1.27–3.20, p = .003). Nevertheless, RFA did not differ significantly from PEI with regard to major complications (OR 2.12, 95% CI =0.97–4.65, p = .06). A fixed-effects model was used due to the statistical homogeneity was adequate (p > .1, I2 < 50%) ().
Complete details concerning complications in the eight studies [Citation20–27] comparing RFA with PEI are listed in .
Table 4. Comparison of complications between RFA and PEI.
Assessment of economic outcomes for RFA vs. PEI
We compared the economic outcomes of RFA and PEI for HCC treatment from three perspectives: costs of treatment, number of treatment sessions and length of hospitalisation. Only two studies [Citation25,Citation26] provided the costs of treatment; they stated that the costs of RFA (6540 Euros, 171 000 Euros, respectively) were higher than the costs of PEI (4097 Euros, 1359 Euros, respectively). Although both trials are from Italy, the significantly different costs are probably due to different methods of assessment. All trials except for one [Citation25] provided a mean number of treatment sessions () and suggested that the average treatment duration of RFA was shorter than the duration of PEI treatment. Three trials reported the length of hospitalisation [Citation22–24]. Two of them stated that the average length of stay for RFA treatment was longer, while the other trial stated the opposite result. This discrepancy is because patients in the studies with longer stays for RFA treatment were hospitalised for any complication, whereas the patients receiving PEI were treated as outpatients for minor complications. In the study reporting longer stays for PEI treatment, all patients were hospitalised for any complication.
Few studies have performed economic assessments of RFA and PEI. Furthermore, there are no uniform evaluation criteria. Consequently, we could not perform a meta-analysis of this issue in our study.
Comparison between combination and RFA monotherapy groups
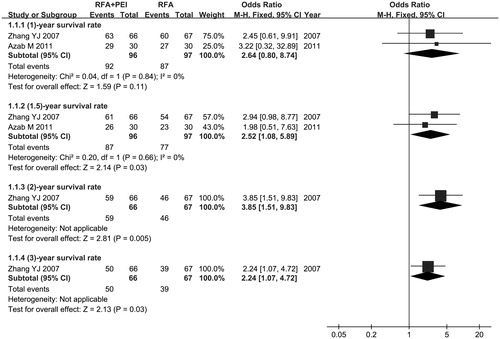
We performed a meta-analysis to evaluate OS of combined RFA-PEI. Compared with RFA alone, significant OS improvement was observed in favour of combination therapy, as demonstrated by the following results: 1.5-year OS rate (OR =2.52, 95% CI = 1.08–5.89, p = .03), 2-year OS rate (OR =3.85, 95% CI = 1.51–9.83, p = .005), 3-year OS rate (OR =2.24, 95% CI = 1.07–4.72, p = .03). However, there was no significant statistical difference in the 1-year OS rate (OR =2.64, 95% CI =0.80–8.74, p = .11). We did not synthesise the subgroups of different years because it was inapposite. The full details are shown in .
The meta-analysis showed that combination therapy provided favourable results compared with RFA alone in terms of the local recurrence rate (OR = 0.29, 95% CI = 0.11–0.77, p = .01). The complete tumour necrosis rate in the combination group was also higher than that in the RFA group, but this difference was not significant (p > .05). The incidence of fever and pain in the combination group were higher than that in the RFA group, but there was a significant difference only in the incidence of fever (OR = 2.27, 95% CI = 1.20–4.28, p = .01). The complete details are listed in .
Table 5. Meta-analysis of the efficacy and safety between RFA-PEI and RFA alone.
There were five [Citation27–31] included papers comparing the combination group with the group receiving RFA alone. Quantitatively, synthetic research was performed with the RCTs of Azab et al. [Citation27] and Zhang et al. [Citation30] because of their high quality. However, for the remaining studies including the three CCTs, the high risk of bias and high clinical heterogeneity required that we use qualitative synthetic evaluation. Shankar et al. [Citation28], Kurokohchi et al. [Citation29] and Wong SN et al. [Citation31] reported that compared with RFA alone, consistently larger effects are achievable in fewer sessions using the combined method because RFA is potentiated by PEI [Citation28]. RFA-PEI enables the induction of comparable coagulative necrosis with a smaller energy requirement [Citation29]. RFA-PEI has slightly greater primary effectiveness for the management of HCC in high-risk anatomic locations [Citation31]. Additionally, the authors suggest that compared with RFA alone, RFA-PEI does not increase the incidence of complications [Citation28,Citation31] and is likely to be less invasive than RFA alone, irrespective of an enhanced induction of coagulative necrosis [Citation29].
Comparison between combination and PEI monotherapy groups
There were two [Citation27,Citation32] included papers comparing the combination group with the PEI monotherapy group; one is a RCT [Citation27] and the other is a CCS [Citation32]. The qualitative synthetic evaluation showed that combination treatment was superior to PEI alone. This conclusion could also easily be inferred from the above comparison of RFA vs. PEI and RFA vs. combination therapy.
Discussion
EASL–EORTC Clinical Practice Guidelines [Citation4] indicate that the incidence of HCC is increasing worldwide. Each year, new HCCs and HCC-related death rates are 749 000 cases and 692 000 cases respectively, representing a growing burden to global health. Hepatic resection is still the gold standard for HCC treatment. Surgical criteria are extremely strict for obtaining high survival and low-recurrence rates postoperatively. However, the majority of patients with HCC are diagnosed at late stages, at which time surgery may not be beneficial. Currently, the postoperative 5-year recurrence rate is as high as 70% [Citation4,Citation7,Citation8]. Not surprisingly, non-surgical methods have become a hot topic and are expected to replace surgery.
RFA produces its effect through thermal injury generated by electromagnetic energy delivered to the target tissues [Citation33,Citation34]. PEI kills tumours through dehydration and protein denaturation caused by ethanol diffusion into the tumour cells [Citation7]. RFA and PEI are the most broadly evaluated and recognised percutaneous treatments [Citation10]. We performed this SR and meta-analysis to comprehensively compare the efficacy and safety between RFA-PEI, RFA alone and PEI alone. Then oncologists can select the prior treatment based on individual patient characteristics.
Comparison between RFA and PEI
PEI as the first percutaneous ablation method has been a standard treatment for HCC, particularly in tumours smaller than 2 cm in diameter. It provides several advantages, including safety, easy implementation, low cost and few complications. However, unpredictable results and long treatment cycles restrict the use of PEI, mainly because of the heterogeneous distribution of injected ethanol within the tumours and the presence of intra-tumoural septation [Citation11]. RFA also has numerous advantages, including predictable efficacy, fewer treatment sessions, repeatable application and minimal invasion. Because of these characteristics and because it is more suitable for HCC greater than 2 cm, RFA may have an overall advantage over PEI for monotherapy [Citation35,Citation36].
This meta-analysis furnished evidence that RFA is superior to PEI in terms of OS at 1, 2, 3, and 4 years, CFS at 1, 2 and 3 years as well as local recurrence rates and complete tumour necrosis rates. Our results are similar to those of a cohort study from Taiwan [Citation19], which included 1036 cases with stage I–II HCC (RFA 658, PEI 378) and showed that RFA was better than PEI in improving survival rates. Recent studies [Citation37–40] revealed that RFA had similar or better efficacy than resection. Therefore, RFA may be a promising alternative to surgical resection.
RFA was associated with a higher risk of total complications than PEI, but there was no statistically significant difference between the two groups with regard to major complications. The major complication results were consistent with those reported by Bouza et al. [Citation16]. Numerous studies have verified that RFA is a safe and well-tolerated treatment method [Citation41,Citation42].
Economic evaluations of RFA and PEI remain inconclusive. One retrospective study [Citation43] comparing costs of RFA and PEI showed that RFA is the more cost effective method, in contrast to the results of the two trials [Citation25,Citation26] included in this article. RFA requires much shorter treatment cycles than PEI. Accordingly, RFA treatment may be more cost-effective after the end of entire treatment period if no serious complications occur. More high-quality studies for economic evaluation of RFA and PEI are warranted.
Comparison between combination and monotherapy groups
The combination of RFA and PEI for HCC shows obvious synergy [Citation27,Citation30]. Some possible synergistic mechanisms include the following: (1) ethanol injected during PEI may be heated or even boiled by energy delivered during RFA, resulting in enhanced coagulation [Citation44]; (2) the destruction of intratumoural blood vessels by PEI may inhibit the cooling effect of circulating blood, which could attenuate the energy transfer during RFA [Citation45–47]; (3) the diffusion of ethanol into the non-coagulated area after RFA may contribute to the wider spread of tumour coagulation effects [Citation48,Citation49]; (4) ethanol diffusion beyond the margin of HCC destroyed by RFA may achieve a safety margin to improve efficacy [Citation48]; and (5) RFA is not feasible when tumours are located near larger blood vessels and adjacent organs [Citation50,Citation51] because of a high risk of treatment failure and complications due to their location [Citation31,Citation52]. In these cases, PEI plays a role when used either alone or in combination with RFA, according to the size or location of the tumours [Citation49].
The combination of RFA and PEI for HCC may be a promising therapeutic strategy because of their mutual complementarity. Until now, substantial research has demonstrated that combining RFA and PEI can overcome the limitations of each treatment as a monotherapy, resulting in improved efficacy and fewer treatment sessions and complications [Citation48,Citation53–56].
The meta-analysis results demonstrated that compared with RFA alone, combined RFA-PEI was associated with a significant improvement in OS at 1.5, 2 and 3 years and a significant reduction in the local recurrence rate. However, combined RFA-PEI treatment was associated with a higher incidence of fever. Qualitative synthetic evaluation shows that combined RFA-PEI was significantly superior to either RFA or PEI alone, with good clinical safety and tolerability. Several other studies [Citation48,Citation53,Citation55,Citation57] support this conclusion.
The combination of RFA and PEI seems to be the optimal treatment strategy when considering the options of combined RFA-PEI or either RFA or PEI as a monotherapy. Combined RFA-PEI significantly improves OS and reduces the risk of local recurrence without increasing the rates of major complications.
Study limitations
There are some limitations of this SR and the included studies. First, the results might be affected by the non-uniformity of the included studies, as well as the diverse intervention and safety evaluation standards among studies. Second, a complete evaluation was not finished because of inadequate clinical data about economic assessment and quality of life after treatment. Third, most included studies of this SR did not implement blinding, but this did not cause significant biases in this special intervention evaluation. Finally, although there were six included papers comparing combination with monotherapy, only two of them were RCTs. More RCTs are needed to reach a definitive conclusion on the superiority of combination of RFA and PEI.
There were no consistent definitions of complications or uniform standards in all included studies for symptoms such as fever and pain. Accordingly, inconsistent reporting may have influenced the accuracy of the results to an extent.
Further large, high-quality studies are needed to assess economic impact and quality of life measures. Complete evaluations of RFA and PEI treatment should also be performed in the future to help clinicians establish optimal treatment protocols based on tumour location, tumour size, costs and other factors.
So far, this article is the first SR to analyse the combination of RFA-PEI. It addresses the largest sample size and the most number of included studies, most importantly, it comprehensively compares the efficacy and safety between combined RFA-PEI, RFA alone and PEI alone, which is superior to other similar research [Citation13–17]. Therefore, this SR provides a more important evidence for oncologists to help guide their treatment decisions.
Conclusions and future prospects
In this study, we have demonstrated that combined RFA-PEI is a more effective therapy for HCC than either RFA or PEI alone, without an increase in major complications.
The study included 13 trials with 1621 patients and comprehensively compared the efficacy and safety between combined RFA-PEI, RFA alone and PEI alone. However, there are still some deficiencies, as discussed in the study limitations above. We are hopeful that more high quality, large-scale, long-term multi-centre trials will be conducted in the future. In clinical practice, we should be able to offer the most appropriate treatment strategy for each individual patient according to the tumour size, location, patient condition, economic value and other factors.
Acknowledgements
We thank all authors for their contributions to this systematic review.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Torre LA, Bray F, Siegel RL, et al. (2015). Global cancer statistics, 2012. CA Cancer J Clin 65:87–108.
- Meza-Junco J, Montano-Loza AJ, Liu DM, et al. (2012). Locoregional radiological treatment for hepatocellular carcinoma; Which, when and how? Cancer Treat Rev 38:54–62.
- Ryder SD. (2003). Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut 52:1–8.
- European Association For The Study Of The Liver, European Organisationi For Research And Treatment Of Cancer. (2012). EASL–EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56:908–43.
- Omata M, Lesmana LA, Tateishi R, et al. (2010). Asian Pacific association for the study of the liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 4:439–74.
- McClune AC, Tong MJ. (2010). Chronic hepatitis B and hepatocellular carcinoma. Clin Liver Dis 14:461–76.
- Ansari D, Andersson R. (2012). Radiofrequency ablation or percutaneous ethanol injection for the treatment of liver tumours. World J Gastroenterol 18:1003–8.
- Bruix J, Sherman M. (2011). Management of hepatocellular carcinoma: an update. Hepatology 53:1020–2.
- Forner A, Bruix J. (2010). Ablation for hepatocellular carcinoma: is there need to have a winning technique? J Hepatol 52:310–2.
- Gervais DA, Arellano RS. (2011). Percutaneous tumor ablation for hepatocellular carcinoma. AJR Am J Roentgenol 197:789–94.
- Lencioni R. (2010). Loco-regional treatment of hepatocellular carcinoma. Hepatology 52:762–73.
- Lau WY, Lai EC. (2009). The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg 249:20–5.
- Cho YK, Kim JK, Kim MY, et al. (2009). Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology 49:453–9.
- Germani G, Pleguezuelo M, Gurusamy K, et al. (2010). Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol 52:380–8.
- Orlando A, Leandro G, Olivo M, et al. (2009). Radiofrequency thermal ablation vs. percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomised controlled trials. Am J Gastroenterol 104:514–24.
- Bouza C, Lopez-Cuadrado T, Alcazar R, et al. (2009). Meta-analysis of percutaneous radiofrequency ablation versus ethanol injection in hepatocellular carcinoma. BMC Gastroenterol 9:31.
- Shen A, Zhang H, Tang C, et al. (2013). Systematic review of radiofrequency ablation versus percutaneous ethanol injection for small hepatocellular carcinoma up to 3 cm. J Gastroenterol Hepatol 28:793–800.
- Higgins JPT, Green S (eds). (2011). Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration, Available from www.cochrane-handbook.org
- Lin ZZ, Shau WY, Hsu C, et al. (2013). Radiofrequency ablation is superior to ethanol injection in early-stage hepatocellular carcinoma irrespective of tumour size. PLoS One 8:e80276.
- Livraghi T, Goldberg SN, Lazzaroni S, et al. (1999). Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology 210:655–61.
- Lencioni RA, Allgaier HP, Cioni D, et al. (2003). Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 228:235–40.
- Lin SM, Lin CJ, Lin CC, et al. (2004). Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology 127:1714–23.
- Lin SM, Lin CJ, Lin CC, et al. (2005). Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 54:1151–6.
- Shiina S, Teratani T, Obi S, et al. (2005). A randomised controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 129:122–30.
- Brunello F, Veltri A, Carucci P, et al. (2008). Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: a randomised controlled trial. Scand J Gastroenterol 43:727–35.
- Giorgio A, Di Sarno A, De Stefano G, et al. (2011). Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomised controlled trial. Anticancer Res 31:2291–5.
- Azab M, Zaki S, El-Shetey AG, et al. (2011). Radiofrequency ablation combined with percutaneous ethanol injection in patients with hepatocellular carcinoma. Arab J Gastroenterol 12:113–18.
- Shankar S, vanSonnenberg E, Morrison PR, et al. (2004). Combined radiofrequency and alcohol injection for percutaneous hepatic tumor ablation. AJR Am J Roentgenol 183:1425–9.
- Kurokohchi K, Watanabe S, Masaki T, et al. (2005). Comparison between combination therapy of percutaneous ethanol injection and radiofrequency ablation and radiofrequency ablation alone for patients with hepatocellular carcinoma. World J Gastroenterol 11:1426–32.
- Zhang YJ, Liang HH, Chen MS, et al. (2007). Hepatocellular carcinoma treated with radiofrequency ablation with or without ethanol injection: a prospective randomized trial. Radiology 244:599–607.
- Wong SN, Lin CJ, Lin CC, et al. (2008). Combined percutaneous radiofrequency ablation and ethanol injection for hepatocellular carcinoma in high-risk locations. AJR Am J Roentgenol 190:W187–95.
- Cha DI, Lee MW, Rhim H, et al. (2013). Therapeutic efficacy and safety of percutaneous ethanol injection with or without combined radiofrequency ablation for hepatocellular carcinomas in high risk locations. Korean J Radiol 14:240–7.
- Goldberg SN. (2001). Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound 13:129–47.
- Mulier S, Jiang Y, Jamart J, et al. (2015). Bipolar radiofrequency ablation with 2 x 2 electrodes as a building block for matrix radiofrequency ablation: Ex vivo liver experiments and finite element method modelling. Int J Hyperthermia 31:649–65.
- Shiina S, Tateishi R, Yoshida H, et al. (2007). Local ablation therapy for hepatocellular carcinoma. From ethanol injection to radiofrequency ablation. Saudi Med J 28:831–7.
- Lee DH, Lee JM, Lee JY, et al. (2014). Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 270:900–9.
- Park EK, Kim HJ, Kim CY, et al. (2014). A comparison between surgical resection and radiofrequency ablation in the treatment of hepatocellular carcinoma. Ann Surg Treat Res 87:72–80.
- Lee YH, Hsu CY, Chu CW, et al. (2015). Radiofrequency ablation is better than surgical resection in patients with hepatocellular carcinoma within the Milan criteria and preserved liver function: a retrospective study using propensity score analyses. J Clin Gastroenterol 49:242–9.
- Fang Y, Chen W, Liang X, et al. (2014). Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J Gastroenterol Hepatol 29:193–200.
- Hocquelet A, Balageas P, Laurent C, et al. (2015). Radiofrequency ablation versus surgical resection for hepatocellular carcinoma within the Milan criteria: a study of 281 Western patients. Int J Hyperthermia 31:749–57.
- Weis S, Franke A, Mossner J, et al. (2013). Radiofrequency (thermal) ablation versus no intervention or other interventions for hepatocellular carcinoma. Cochrane Database Syst Rev 12:11–54.
- de Baere T, Risse O, Kuoch V, et al. (2003). Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol 181:695–700.
- Seror O, N'Kontchou G, Tin Tin Htar M, et al. (2006). Ethanol versus radiofrequency ablation for the treatment of small hepatocellular carcinoma in patients with cirrhosis: a retrospective study of efficacy and cost. Gastroenterol Clin Biol 30:1265–73.
- Nakai M, Sato M, Yamada K, et al. (2001). [Percutaneous hot ethanol injection therapy (PHEIT) for hepatocellular carcinoma]. Gan to Kagaku Ryoho 28:1633–7.
- Goldberg SN, Kruskal JB, Oliver BS, et al. (2000). Percutaneous tumour ablation: increased coagulation by combining radio-frequency ablation and ethanol instillation in a rat breast tumour model. Radiology 217:827–31.
- Hall SK, Ooi EH, Payne SJ. (2015). Cell death, perfusion and electrical parameters are critical in models of hepatic radiofrequency ablation. Int J Hyperthermia 31:538–50.
- Poch FG, Rieder C, Ballhausen H, et al. (2016). The vascular cooling effect in hepatic multipolar radiofrequency ablation leads to incomplete ablation ex vivo. Int J Hyperthermia 32:749–56.
- Kurokohchi K, Watanabe S, Masaki T, et al. (2002). Combined use of percutaneous ethanol injection and radiofrequency ablation for the effective treatment of hepatocelluar carcinoma. Int J Oncol 21:841–6.
- Kurokohchi K, Watanabe S, Masaki T, et al. (2002). Combination therapy of percutaneous ethanol injection and radiofrequency ablation against hepatocellular carcinomas difficult to treat. Int J Oncol 21:611–5.
- Jiang K, Dong J, Zhang W, et al. (2014). Effect of one-off complete tumour radiofrequency ablation on liver function and postoperative complication in small hepatocellular carcinoma. Eur J Surg Oncol 40:576–83.
- Seror O, N'Kontchou G, Muhammad M, et al. (2007). [The impact of large vessel proximity on effectiveness of radiofrequency ablation of hepatocellular carcinoma: a controlled study]. J Radiol 88:1157–64.
- Teratani T, Yoshida H, Shiina S, et al. (2006). Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology 43:1101–8.
- Sakr AA, Saleh AA, Moeaty AA. (2005). The combined effect of radiofrequency and ethanol ablation in the management of large hepatocellular carcinoma. Eur J Radiol 54:418–25.
- Huang GL, Lin MX, Xie XY, et al. (2014). Combined radiofrequency ablation and ethanol injection with a multipronged needle for the treatment of medium and large hepatocellular carcinoma. Eur Radiol 24:1565–71.
- Lin JW, Lin CC, Chen WT, Lin SM. (2014). Combining radiofrequency ablation and ethanol injection may achieve comparable long-term outcomes in larger hepatocellular carcinoma (3.1–4 cm) and in high-risk locations. Kaohsiung J Med Sci 30:396–401.
- Huang H, Liang P, Yu XL, et al. (2015). Safety assessment and therapeutic efficacy of percutaneous microwave ablation therapy combined with percutaneous ethanol injection for hepatocellular carcinoma adjacent to the gallbladder. Int J Hyperthermia 31:40–7.
- Vallone P, Catalano O, Izzo F, Siani A. (2006). Combined ethanol injection therapy and radiofrequency ablation therapy in percutaneous treatment of hepatocellular carcinoma larger than 4 cm. Cardiovasc Intervent Radiol 29:544–51.