Abstract
Objective: This study aims to evaluate the local efficacy and long-term outcomes of microwave ablation (MWA) for treating large unresectable hepatocellular carcinomas (HCCs).
Methods: A total of 82 patients with 5-6 cm unresectable HCCs, who underwent a single MWA procedure during the period of January 2007 to July 2011, were retrospectively enrolled into this study. Percentages of technical success and complications of MWA and HCC local recurrence (LR) after MWA were determined. In addition, prognostic factors were screened and overall survival (OS) and recurrence-free survival (RFS) rates were estimated.
Results: One-, three- and five-year OS rates in this MWA-treated cohort were 92.7, 63.4 and 41.1%, respectively; and the corresponding RFS rates were 65.9, 31.7 and 23.0%, respectively. Primary technical efficacy was 89.0% after the first round of ablation, three (3.7%) patients developed major complications, and LR rate was 20.7%. Child-Pugh classification (p < .001), tumour location (p = .049) and LR (p = .002) were independent factors associated with OS, as determined by multivariate analysis.
Conclusions: MWA is safe and effective for the treatment of selected large HCCs, and provides an alternative treatment option for patients with unresectable HCCs. Furthermore, the favourable local efficacy of MWA could potentially improve long-term survival.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and results in the third highest tumour-related mortality, globally [Citation1]. HCC is common in China due to the high incidence of chronic hepatitis B virus (HBV) infection in the general population. Surgical resection (SR) remains the most effective method for the treatment of HCC. However, a portion of patients diagnosed with HCC are technically infeasible for SR; which are impeded by advanced cirrhosis, unfavourable location, or poor general conditions [Citation2]. Transcatheter arterial chemoembolisation (TACE) is one of the alternative options for treating unresectable HCCs, which improves survival rate [Citation3,Citation4]. However, TACE rarely necrotises the treated large lesion completely, which compromises its efficacy [Citation4]. Recently, local thermal ablations including radiofrequency ablation (RFA) and microwave ablation (MWA) have been widely used for HCC patients who are not suitable for resection.
Local thermal ablation could produce an extremely high percentage of extensive necrosis of HCC, which is ≤3 cm in size; leading to satisfactory long-term survival that is not inferior to SR [Citation5–8]. With the refinement of devices and techniques, percutaneous local thermal ablation has extended its treatment application to HCCs at sizes between >3 and <5 cm [Citation9,Citation10]. However, it remains non-consensual whether local thermal ablation is effective for treating >5 cm HCCs. The reported long-term prognosis was discouraging, and five-year survival rate was less than 20% for thermal ablation-treated patients with >5 cm HCCs [Citation9,Citation11].
The volume of a tumour expands exponentially with the increase in lesion diameter, and a higher energy is required to destroy a HCC with a large diameter. MWA potentially delivers higher intratumoral temperature and generates a larger ablation zone, but reduces the impact of the “heat sink effect” [Citation12,Citation13]. Therefore, we chose MWA for treating selected unresectable patients with a single 5–6 cm HCC in this study to establish local efficacy and long-term outcomes. The Eastern Hepatobiliary Surgery Hospital is the largest liver cancer centre in Asia. Our department is one of pioneers that carried out ablation treatments for liver cancer. Furthermore, our hospital is the largest ablation centre in Asia, and treats more than 3000 cases annually. Our results suggest that MWA is an effective treatment option for 5–6 cm unresectable HCCs.
Patients and methods
Patients
All treatments were approved by the Ethics Committee of the Eastern Hepatobiliary Surgery Hospital of the Second Military Medical University. Written informed consent was obtained from all patients prior to enrolment into this study.
From January 2007 to July 2011, 5692 liver cancer patients were admitted to the Department of Minimally Invasive Therapy for treatment with MWA. HCC diagnosis was established using the criteria listed by the American Association for the Study of Liver Diseases (AASLD) [Citation14]. Tumour size was determined by computed tomography (CT) or magnetic resonance imaging (MRI). Individual patients were evaluated by our multidisciplinary team including hepatologists, interventional radiologists and surgeons to select treatment options. Patients were excluded as a candidate of SR for the following reasons: (a) patients with deficiency of liver function reserve, in which SR could not tolerated; (b) aged patients with cardiac and pulmonary insufficiency, who cannot tolerate general anaesthesia; (c) patients with tumours located in unfavourable positions, which were difficult to remove by SR.
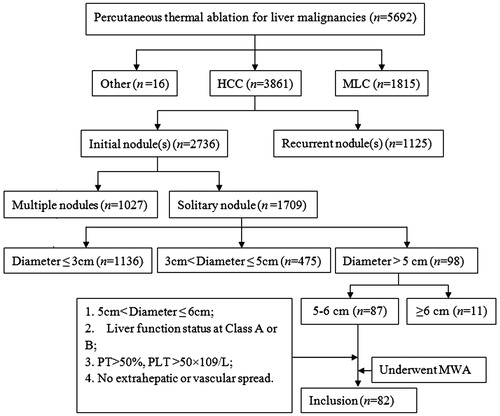
A cohort of 82 patients was retrospectively enrolled into this study. Inclusion criteria were: (a) initial solitary HCC nodule 5–6 cm in size; (b) Child-Pugh class A or B liver function; (c) prothrombin activity (PT) > 50% and platelet (PLT) count >50 × 109/L; (d) the absence of extrahepatic or vascular spreading. The flowchart of patient selection is demonstrated in . Clinical characteristics of the enrolled patients are summarised in .
Table 1. Clinical characteristics of the 82 enrolled patients.
Unfavourable locations for SR were indicated when HCC nodules were located ≤5 mm from major vessels that consists of the first or second branch of the portal veins, the inferior vena cava, or main hepatic veins [Citation15]. Portal hypertension was considered when oesophageal varicose and splenomegaly with a platelet count <100 × 109/L were detected [Citation16].
The primary endpoint was five-years overall survival (OS), the secondary endpoint was local recurrence, and additional endpoints included technical success rate, recurrence free survival (RFS) and distant recurrence.
Microwave ablation
A FORSEATM microwave delivery system (Qinghai Microwave Electronic Institute, Nanjing, China) was used as the MWA device in this study. This system is composed of a MTC-3 MW generator with 2450 MHz frequency and 1–100 W power output, a flexible low-loss coaxial cable, and a 14-gauge cooled shaft antenna. The antenna consists of an 18 cm-long shaft coated with Teflon to prevent tissue adhesion, and a 3 cm-long antenna exposed at its terminus with a 1.5 cm-long active tip coated with polytetrafluoroethylene.
All MWA procedures were performed percutaneously by three experienced interventional radiologists (G.J.Q., Z.H.L and Y.C.). Each radiologist has more than 20 years of experience in percutaneous interventional therapy. For local ablation, the patient’s posture and optimal insertion site were adjusted, according to the tumour localisation detected under real-time ultrasound (SSI-5500; Kaili Technology, Shenzhen, China). This ultrasound was also used for the guidance and monitoring of ablation procedures. All patients were under local anaesthesia with 1% lidocaine in combination of intravenous 0.1 mg of Tramadol. According to our previous study, a single cycle of ablation with a 3-cm long antenna could generate a 3-cm diameter ablation zone [Citation13]. An overlapping ablative technique with multiple antenna insertions was applied to ensure the complete ablation of the lesion and maintain a 0.5–1 cm safe margin beyond the tumour. These procedures could be divided into five steps: (1) After local anaesthesia at the puncture sites, under the guidance of real-time ultrasound, two parallel antennas were spaced at approximately 4 cm and were initially placed approximately 0.5 cm apart from the tumour margin at the level of the maximum diameter of the tumour. The tip of the antenna was placed at the deepest, and microwave energy was simultaneously delivered [Citation17]. (2) Afterwards, the antennas were withdrawn for approximately 2 cm and 4 cm successively, and another two rounds of simultaneous ablation were applied. Then, the antennas were drawn back. (3) After local anaesthesia at the puncture sites at another intercostal space, under the guidance of real-time ultrasound, two parallel antennas were spaced at approximately 2 cm, and were initially placed approximately 1 cm apart from the tumour centre at the level of the maximum diameter. The tip of the antenna was placed at the deepest part of the tumour, and microwave energy was simultaneously delivered. (4) Then, the antennas were withdrawn for approximately 2, 4 and 6 cm successively; and another three rounds of simultaneous ablation were applied. (5) Finally, additional one or two antenna insertions and ablations were used to target the hypoechoic areas that have not been treated. The ablation of each nodule usually required 5–7 insertions. All ablations were completed through one treatment session. Each microwave (MW) energy output was set at 80–100 W for 5–10 min. After each ablation procedure, probe tracks were coagulated for 10–20 s to prevent potential tumour seeding and bleeding. A single procedural duration lasted for 30–50 min according to the size and location of the tumour, as well as the real-time ultrasound images.
Assessment of therapeutic efficacy and follow-up
Contrast-enhanced CT/MRI scan was performed four weeks after treatment. Technical success was defined as a target tumour completely covered by the ablated zone with no enhancement at the artery phrase in the ablated zone. Residual unablated tumour was defined as the appearance of tumour foci at the edge of the ablation zone. Primary technical efficacy was defined as technical success achieved after initial ablation. Residual unablated tumours were treated by a second session of MWA or combined with percutaneous ethanol injection (PEI) [Citation18]. Secondary efficacy was defined as technical success achieved after a complementary session of ablation [Citation18]. For tumours located at edge of the liver, 5–10 mL of ethanol was injected at the edge. If the tumour remains unablated after complementary ablation, the treatment was considered a failure and the patient was recommended for TACE or radiotherapy. Major complication was defined as an event that led to substantial morbidity and disability, which increased the level of care, or induced a substantially prolonged hospital stay [Citation18]. This included any case where blood transfusion or interventional drainage procedure was required. All other complications were considered minor [Citation18].
Follow-up was conducted for patients with technical success. Contrast enhanced CT/MRI was regularly performed every 2–3 months in the first two years and every six months thereafter, in order to determine the local recurrence (LR) and distant recurrence (DR). Chest radiography was taken every six months to detect extrahepatic metastasis. LR was defined as a new tumour foci connected with the completely ablated tumour, and DR was defined as a new nodule in the untreated liver or extrahepatic regions [Citation18]. Recurrence-free survival was defined as the period between the date of ablation and the date of LR or DR detection. The treatment for the recurrent tumour was determined by the characteristics of the recurrent tumour, recommendations of our multidisciplinary team, and requests of patients. The last follow-up date was 30 August 2015.
Analysis of prognosis factors
All available variables including gender, age, aetiology, Hepatic B virus (HBV)-DNA, total bilirubin (TBIL), direct bilirubin (DBIL), alanine aminotransferase (ALT), albumin (ALB), gamma-glutamyl transpeptidase (GGT), alpha-fetoprotein (AFP), platelet (PLT), prothrombin time (PT), Child-Pugh classification, portal hypertension, tumour location, local efficacy (primary technical efficacy vs. residual unablated tumour), LR, DR and treatment for recurrence were assessed to analyse possible associations with OS and LR.
Statistical analysis
SPSS version 17.0 (SPSS, Chicago, IL) was used for statistical analysis. Continuous results were expressed as mean ± standard deviation (SD). OS and RFS were evaluated by the Kaplan-Meier method. Potential risk factors for OS, RFS, LR and DR were screened by log-rank test analyses. Any variable found to be significant at p < .05 in log-rank test analyses were subjected to further analysis through the multivariate Cox proportional hazards model. All statistical tests were two sided. A p value <.050 was considered statistically significant.
Results
Local efficacy
Primary technical efficacy () was achieved in 73 (89.0%) patients. Nine patients with residual unabated tumour received complementary ablations and achieved secondary efficacy. Among these nine patients, seven patients underwent a second MWA, and the remaining two patients received MWA combined with PEI. Overall technical success rate was 100% (82/82) in this study. Mean interval between the first and second treatments was 28 days.
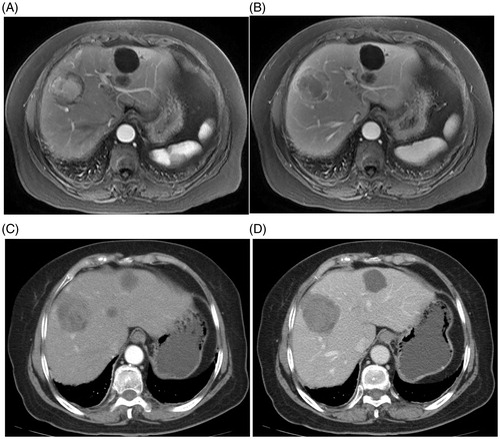
Figure 2. Imaging document of the presence and disappearance of HCC lesions. The MWA-treated subject was a 52-year-old male HCC patient who had HBV-related liver cirrhosis. (A) Arterial phase; (B) Portalvenous phase. Pre-treatment CT images shows a tumour as a 5.4-cm hyperintense nodule on an intense arterial enhancement (A), with an enhancement recession (B). (C) Arterial and (D) portalvenous phase. MRI images obtained at four weeks after treatment show no enhancements inside or beside the ablation zone.
Complications
Minor complications of mild to moderate pain occurred in 56 (68.3%) patients, and fever occurred in 51 (62.2%) patients. Major complications developed in three (3/82, 3.7%) patients including liver abscess, massive ascites and persistent jaundice, respectively. Liver abscess was treated with drainage and anti-infection treatment. Massive ascites was treated with albumin infusion, diuretic and drainage. Persistent jaundice was treated with T-tube drainage and liver-protecting medicine. All three patients with major complications were all successfully treated, and no treatment-related death occurred in this study.
Local recurrence
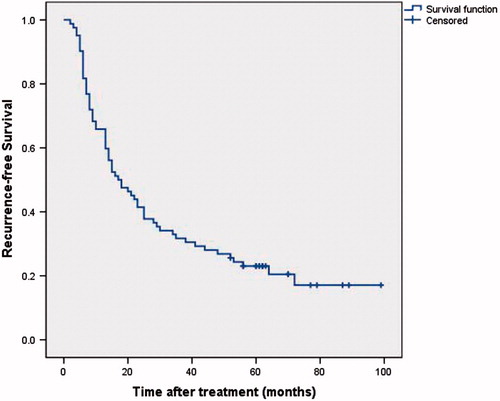
Local recurrence (LR) was observed in 17 (17/82, 20.7%) patients during the follow-up period. Mean time for LR emergence was 9.5 ± 5.6 months (range, 4.0–25.0 months), and median time was 7.0 ± 4.2 months. One-, two-, three- and five-year LR rates were 14.6, 19.5, 20.7 and 20.7%, respectively. Among these 17 patients with LR, 12 patients were treated with repeat MWA, and five patients underwent TACE. Among the 16 variables examined by log-rank test, age, PLT and Child-Pugh classification were factors significantly associated with LR. After Cox multivariate analysis, age and PLT emerged as independent factors indicating possible LR ().
Table 2. Analysis of factors associated with local recurrence.
Distant recurrence
Distant recurrence (DR) occurred in 47 (47/82, 57.3%) patients, in which 44 patients had intrahepatic new lesions and three patients had lung metastases during the follow-up period. Mean time for the detection of DR was 22.0 ± 17.4 months (range, 2.0–72.0 months), and median time was 20.0 ± 14.7 months. Among the 47 patients with DR, 26 patients underwent repeat MWA, 15 patients underwent TACE, and six patients received radiation therapy.
Recurrence-free survival
No patient was lost of follow-up. One-, three- and five-year RFS rate was 65.9, 31.7 and 23.0%, respectively (.
Overall survival
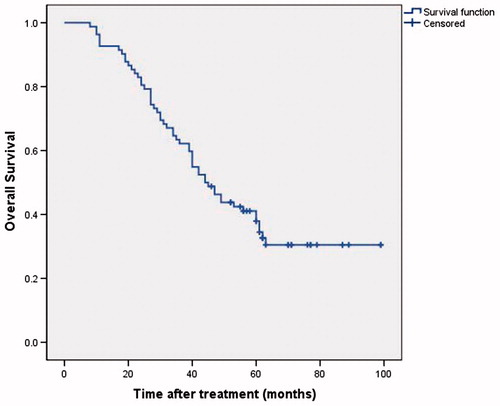
Among the 82 patients, 27 patients were still alive at the end of the last follow-up date; and the mean follow-up period was 42.0 months. One-, three- and five-year OS rate was 92.7, 63.4 and 41.1%, respectively (. Univariate analysis demonstrated that age, AFP, Child-Pugh classification, tumour location and LR were significant factors associated with OS. Child-Pugh classification, tumour location and LR were independent predictors for OS, as identified by multivariate analysis ().
Table 3. Analysis of factors associated with overall survival.
Subgroup analysis
One-, three- and five-year OS rates in patients with unfavourable tumour location were 92.9, 78.6 and 53.6%, respectively; which were significantly better than OS rates (92.6, 55.6 and 34.7%, respectively) in patients with favourable tumour location (p = .025).
One-, three- and five-year OS rates in patients with liver function status at Child-Pugh Class A were 98.4, 79.4 and 50.3%, respectively; which were significantly better than OS rates (73.7, 10.5 and 10.5%, respectively) in patients with liver function at Child-Pugh Class B (p < .001).
One-, three- and five-year OS rates in patients ≤65 years old were 94.8, 72.4 and 46.5%, respectively; which were significantly better than OS rates (87.5, 41.7 and 27.3%, respectively) in patients >65 years old (p = .049).
One-, three- and five-year OS rates in patients with LR were 82.4%, 29.4% and 0.0%, respectively; which were significantly poorer than OS rates (95.4%, 72.3% and 52.0%, respectively) in patients without developing LR (p ≤ .001).
Discussion
In this study, we retrospectively analysed local efficacy and long-term OS in a cohort of HCC patients who had 5–6 cm unresectable tumours, and these patients were treated with MWA. We found that the HCC lesion was 100% ablated after initial or an additional complementary round of MWA. Furthermore, five-year OS reached more than 40%, which was a significant improvement, compared to the reported 17.3% [Citation9] and 13.1% [Citation11]. Our results suggest that 5–6 cm HCCs can be treated with MWA without compromising the OS of patients.
A sufficient “safe margin” is usually referred to 1 cm beyond the lesion when a tumour is resected, which is aimed to eliminate potential peritumoral satellite nodules and micro-vascular invasion, and is required for a curable HCC therapy. TACE is an alternative treatment for unresectable HCCs. However, the power of chemoembolisation is limited and barely necrotises nodules [Citation3,Citation4]. The combination of TACE with ablation for treating larger unresectable HCCs (>5 cm) has been reported [Citation19–21]. Iezzi et al. [Citation20] first reported the comparison of an additional ablation treatment following TACE, with TACE alone for large unresectable HCCs. These results revealed better local efficacy and longer survival in the combination group. Recently, a comparison was made between TACE and RFA, and the combination treatment revealed similar results [Citation21]. In our institution, this combination treatment is recommended for HCCs that are size ranged between 7 and 10 cm. In this study, we selected 5–6 cm HCCs to establish whether a single MWA modality could achieve similar local efficacy as the combination therapy for this category of HCC. Moreover, this combination therapy not only expands the procedure, but also increases the difficulty of the procedure. Moreover, TACE would certainly cause additional liver injury, elevating the risk for complications. Tumour volume expands exponentially with the increase in lesion diameter. Much higher energy is required to destroy longer diameters of HCC. MWA has the potential to deliver significantly more power that generates sufficiently high temperatures to necrotise the cancerous tissue and create a larger ablation zone. Furthermore, it also reduces the impact of the "heat sink effect" [Citation12,Citation13]. Moreover, overlapping ablation techniques that target multi-sites in multi-layers can maximise the ablation zone and leave no sanctuary for the cancer tissue.
Yin et al. [Citation9] reported a primary technical efficacy rate of 65.0% for treating 5.0 to 7.0 cm tumours by RFA and MWA. Other recent literature reported primary technical efficacy rates between 75.0 and 81.8% for the treatment of large HCCs by local thermal ablation [Citation11,Citation22] and 53.0–87.5% by combination treatment [Citation19,Citation20]. In our study, primary technical efficacy rate was 89.0%, which was significantly higher than the above cited studies. After complementary ablation of residual tumours, all patients achieved technical success. Our results provide evidence for the high effectiveness of the local ablation of large HCCs with MWA.
One of the challenges in treating HCC is LR, which is mainly associated with tumour size [Citation23]. The larger the tumour, the more likely LR would occur. The aggressiveness of a tumour is aggravated by its increase in size. For instance, HCCs >3 cm tend to exhibit more invasive behaviours, and HCCs >5 cm have a higher possibility for capsular invasion, tumour thrombi and satellite nodules [Citation24]. On the other hand, the creation of a safe margin that is not less than 0.5–1.0 cm for larger nodules by an overlapping ablation technique remains a challenge. In a previous study, LR incidence was 31.2-40.9% in MWA-treated large HCCs [Citation9,Citation11]. The present study revealed a 20.7% LR rate, which was much lower than in previous reports. A relatively smaller size (5–6 cm) may partially account for the difference in LR rate. However, it is important to note that ablation skill also contributes to lower LR rate. Our experience emphasises that an ablative margin of 0.5–1.0 cm should be produced around the tumour to ensure the elimination of potential peritumoral satellite lesions or microscopic invasion.
Most LRs were detected within the first and second year after ablation, which contributed to the shortening RFS. A short period for the emergence of LR (within primary 1–2 years) was a significant risk factor of poor OS in HCC patients treated with RFA [Citation25]. Our results yielded similar conclusions that patients with LR had undesirable long-term outcome.
Distant recurrence of HCC after treatment is very common. Five-year DR of medium and large HCCs treated with local thermal ablation could reach as high as 53.8–54.7% [Citation9–11]. DR rate in this cohort was 57.3%, which was comparable but undesirable. A high DR rate reflects the invasive property of larger tumours [Citation24]. In addition, the multi-site occurrence of HCC in the liver could trigger metastasis to other organs via the portal vein, leading to a high DR rate.
In addition to AFP and LR, other factors associated with OS and RFS include age, Child-Pugh classification and tumour location. Aged patients and Child-Pugh Class B were negative predictors for long-term prognosis [Citation9,Citation10,Citation26]. Interestingly, the unfavourable location in young patients with unresectable HCC was a positive factor in our study, because these patients had sufficient liver function reserve when there was no active necroinflammation or decompensated cirrhosis. An encouraging 50.3% OS was noted with MWA in this category, and MWA could be a first-choice of treatment for these patients.
Local thermal ablation is notably safer compared with SR [Citation5–7,Citation27]. Liang et al. [Citation27] reported an incidence of 2.6% for major complications after MWA. Furthermore, a major complication rate of 9.2% was reported for medium and large HCCs (3–7 cm) treated with RFA and MWA [Citation9], and 14.3% for large HCCs (5–8 cm) treated with MWA [Citation28]. In our study, only three (3.7%) patients developed major complications, which was a significant improvement over previous reports.
Appropriate selection of patients is the key to safety, local efficacy and better long-term outcomes. The inclusion criteria for treating large HCCs with MWA should be stringent. It is crucial to evaluate the tumour burden, liver function reserve and basic status of patients before selection [Citation29]. All patients in this cohort were initially diagnosed with a single HCC limited to 5–6 cm in size. Furthermore, the liver function status of patients was at Class A or B; and they did not have extrahepatic, vascular spread or poor general conditions. The inclusion criteria adopted by our study could be regarded as proposed indications for the MWA treatment of large HCCs.
Our hospital is the largest hepatobiliary surgery centre in Asia, and we perform more than 3000 HCC cases with ablations every year. A large patient source allowed us to present this study with a relatively large sample size in treating large HCCs with MWA. We believe that this is the first study to investigate the long-term prognosis of MWA-based treatment of 5–6 cm HCCs. There were a few limitations in this study. First is the absence of control groups with other alternative treatments. Second, it was difficult to directly compare the prognosis of hepatic resection (HR)-treated HCC patients, since all of our cases were unresectable. Nevertheless, our results offer a promising hint, but require further validation through prospective, well-controlled and multicenter based large cohort studies.
Conclusion
In summary, this study suggests that MWA can be safely used for treating large unresectable HCCs. We have shown that MWA treatment delivers promising local efficacy and substantially improves OS in this cohort when patients with large HCCs are carefully selected.
Disclosure statement
The authors report no declarations of interest.
References
- Jemal A, Bray F, Centre MM, et al. (2011). Global cancer statistics. CA Cancer J Clin 61:69–90.
- Kim YS, Lim HK, Rhim H, Lee MW. (2014). Ablation of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol 28:897–908.
- Lo CM, Ngan H, Tso WK, et al. (2002). Randomised controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 35:1164–71.
- Biolato M, Marrone G, Racco S, et al. (2010). Transarterial chemoembolization (TACE) for unresectable HCC: a new life begins? Eur Rev Med Pharmacol Sci 14:356–62.
- Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321–8.
- Hocquelet A, Balageas P, Laurent C, et al. (2015). Radiofrequency ablation versus surgical resection for hepatocellular carcinoma within the Milan criteria: A study of 281 Western patients. Int J Hyperthermia 31:749–57.
- Pompili M, Saviano A, de Matthaeis N, et al. (2013). Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma </=3 cm. Results of a multicenter Italian survey. J Hepatol 59:89–97.
- Shi J, Sun Q, Wang Y, et al. (2014). Comparison of microwave ablation and surgical resection for treatment of hepatocellular carcinomas conforming to Milan criteria. J Gastroenterol Hepatol 29:1500–7.
- Yin XY, Xie XY, Lu MD, et al. (2009). Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer 115:1914–23.
- Sun AXE, Cheng ZL, Wu PP, et al. (2015). Clinical outcome of medium-sized hepatocellular carcinoma treated with microwave ablation. World J Gastroenterol 21:2997–3004.
- Liu Y, Zheng Y, Li S, et al. (2013). Percutaneous microwave ablation of larger hepatocellular carcinoma. Clin Radiol 68:21–6.
- Andreano A, Huang Y, Meloni MF, et al. (2010). Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys 37:2967–73.
- Qian GJ, Wang N, Shen Q, et al. (2012). Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol 22:1983–90.
- Bruix J, Sherman M. (2011). Management of hepatocellular carcinoma: an update. Hepatology 53:1020–2.
- Teratani T, Yoshida H, Shiina S, et al. (2006). Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology 43:1101–8.
- Kuang M, Xie XY, Huang C, et al. (2011). Long-term outcome of percutaneous ablation in very early-stage hepatocellular carcinoma. J Gastrointest Surg 15:2165–71.
- Kuang M, Lu MD, Xie XY, et al. (2007). Liver cancer: increased microwave delivery to ablation zone with cooled-shaft antenna-experimental and clinical studies. Radiology 242:914–24.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. Radiology 273:241–60.
- Liu C, Liang P, Liu F, et al. (2011). MWA combined with TACE as a combined therapy for unresectable large-sized hepotocellular carcinoma. Int J Hyperthermia 27:654–62.
- Iezzi R, Pompili M, La Torre MF, et al. (2015). Radiofrequency ablation plus drug-eluting beads transcatheter arterial chemoembolization for the treatment of single large hepatocellular carcinoma. Dig Liver Dis 47:242–8.
- Tang C, Shen J, Feng W, et al. (2016). Combination therapy of radiofrequency ablation and transarterial chemoembolization for unresectable hepatocellular carcinoma: a retrospective study. Medicine (Baltimore) 95:e3754.
- Seror O, N'Kontchou G, Ibraheem M, et al. (2008). Large (>or =5.0-cm) HCCs: multipolar RF ablation with three internally cooled bipolar electrodes–initial experience in 26 patients. Radiology 248:288–96.
- Chinnaratha MA, Sathananthan D, Pateria P, et al. (2015). High local recurrence of early-stage hepatocellular carcinoma after percutaneous thermal ablation in routine clinical practise. Eur J Gastroenterol Hepatol 27:349–54.
- Lu XY, Xi T, Lau WY, et al. (2011). Pathobiological features of small hepatocellular carcinoma: correlation between tumour size and biological behaviour. J Cancer Res Clin Oncol 137:567–75.
- Liang HH, Chen MS, Peng ZW, et al. (2008). Percutaneous radiofrequency ablation versus repeat hepatectomy for recurrent hepatocellular carcinoma: a retrospective study. Ann Surg Oncol 15:3484–93.
- Lee DH, Lee JM, Lee JY, et al. (2014). Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 270:900–9.
- Liang P, Wang Y, Yu X, Dong B. (2009). Malignant liver tumors: treatment with percutaneous microwave ablation-complications among cohort of 1136 patients. Radiology 251:933–40.
- Liang PC, Lai HS, Shih TT, et al. (2015). Initial institutional experience of uncooled single-antenna microwave ablation for large hepatocellular carcinoma. Clin Radiol 70:e35–40.
- Lencioni R, Chen XP, Dagher L, Venook AP. (2010). Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: how can outcomes be improved?. Oncologist 15:42–52.