Abstract
Purpose: Currently, clinical decisions regarding thermoradiotherapy treatments are based on clinical experience. Quantification of the radiosensitising effect of hyperthermia allows comparison of different treatment strategies, and can support clinical decision-making regarding the optimal treatment. The software presented here enables biological evaluation of thermoradiotherapy plans through calculation of equivalent 3D dose distributions.
Methods: Our in-house developed software (X-Term) uses an extended version of the linear-quadratic model to calculate equivalent radiation dose, i.e. the radiation dose yielding the same effect as the thermoradiotherapy treatment. Separate sets of model parameters can be assigned to each delineated structure, allowing tissue specific modelling of hyperthermic radiosensitisation. After calculation, the equivalent radiation dose can be evaluated according to conventional radiotherapy planning criteria. The procedure is illustrated using two realistic examples. First, for a previously irradiated patient, normal tissue dose for a radiotherapy and thermoradiotherapy plan (with equal predicted tumour control) is compared. Second, tumour control probability (TCP) is assessed for two (otherwise identical) thermoradiotherapy schedules with different time intervals between radiotherapy and hyperthermia.
Results: The examples demonstrate that our software can be used for individualised treatment decisions (first example) and treatment optimisation (second example) in thermoradiotherapy. In the first example, clinically acceptable doses to the bowel were exceeded for the conventional plan, and a substantial reduction of this excess was predicted for the thermoradiotherapy plan. In the second example, the thermoradiotherapy schedule with long time interval was shown to result in a substantially lower TCP.
Conclusions: Using biological modelling, our software can facilitate the evaluation of thermoradiotherapy plans and support individualised treatment decisions.
Introduction
Radiotherapy treatment of cancer patients is usually preceded by the design of a radiation treatment plan, in which the physical 3D dose distribution in the patient is simulated and optimised. It is well known that treatment outcome is not only determined by the physical dose, but also by the characteristics of the treatment schedule such as fraction dose, dose rate and overall treatment time. Based on experience, radiation oncologists can assess plan quality from a physical dose distribution. Additionally, a number of radiobiological models can assist in translating the effect of physical dose to clinical outcome, such as the linear-quadratic (LQ) model [Citation1,Citation2] and tumour control probability (TCP) models [Citation3–7]. Such radiobiological models are widely used for comparison and optimisation of treatment schedules [Citation8–10] and several software tools have been developed to support the use of these models [Citation6,Citation11,Citation12].
In thermoradiotherapy, i.e. the combined treatment with radiotherapy and mild hyperthermia, optimisation and evaluation of plan quality is a much greater challenge. The interplay between the radiation dose distribution and temperature distribution is complex, since the distributions may be heterogeneous, and the radiosensitising effect may be different within different tissues. Thus, thermoradiotherapy treatment plan evaluation based on clinical experience alone is difficult. Quantification of the radiosensitising effect using radiobiological modelling can help to assess treatment plan quality, which in turn allows comparison of different treatment strategies (e.g. radiotherapy with or without hyperthermia), and can support clinical decision-making regarding the optimal treatment.
Thermoradiotherapy treatments can be modelled using an extended version of the LQ model, in which the radiosensitivity parameters (α and β) are increased depending on the achieved temperature and the time interval between the radiotherapy and hyperthermia treatment [Citation13,Citation14]. This extended LQ model can be used to calculate the equivalent radiation dose, i.e. the radiation dose yielding the same effect as the thermoradiotherapy treatment. To account for heterogeneous radiation dose and temperature distributions, a voxel-based calculation of equivalent dose is essential. If such a voxel-based calculation of equivalent dose is performed, the problem of assessing the quality of a thermoradiotherapy plan has essentially been reduced to the (well-known) problem of assessing the quality of a conventional radiation dose distribution. Through this strategy, existing experience in radiotherapy plan assessment can also be utilised in thermoradiotherapy planning.
In this paper, we present an in-house developed software package named X-Term that simulates the eXTERMination of cancer. First, the workflow of the software and the main biological models used will be introduced. Then, two realistic examples are discussed to illustrate the potential clinical applications of the software.
Materials and methods
Program design and workflow
X-Term was designed for biological evaluation of thermoradiotherapy plans through calculation of 3D equivalent radiation dose distributions and subsequent evaluation using conventional tools for radiotherapy plan evaluation, such as dose–volume histogram (DVH) calculation and TCP models, to provide an integrated package for plan assessment. The workflow consists of several steps () described in detail in this section. References will be made to the four tabbed windows (DVH, Region of Interest (ROI) settings, Treatment Schedule and TCP) and the CT view of the graphical user interface ().
Figure 1. Workflow for thermoradiotherapy plan evaluation in X-Term. Deformable image registration is not part of X-Term. Subscripts indicate the objects’ frame of reference: RT, radiotherapy planning CT; HT, hyperthermia planning CT. Dashed lines: DICOM import/export. Solid lines: internal links or non-standardised import/export.
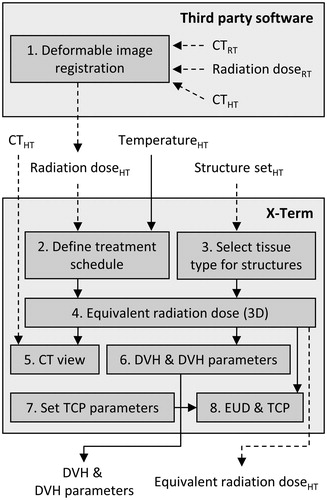
1. Deformable image registration. The radiation dose distribution is calculated using a commercial radiotherapy treatment planning system (Oncentra External Beam, Elekta AB, Stockholm, Sweden); the hyperthermia temperature distribution is calculated using dedicated in-house developed software [Citation15,Citation16]. To account for the different patient postures during hyperthermia and radiotherapy treatments, a dedicated CT scan is used for each of these simulations and deformable registration is used to resample the radiation dose in the frame of reference of the hyperthermia CT scan.
2. Define treatment schedule. The hyperthermia CT, associated structure set, simulated radiation dose distribution (all DICOM format) and temperature distribution (non-standardised format) are imported in X-Term. For each dose/temperature distribution, a treatment schedule is defined (). An overview of all treatment fractions is shown in the Treatment Schedule tab, and properties of individual fractions can be altered (). Each entry is associated with a 3D array containing the temperature/dose distribution for that treatment fraction. For easy comparison, two schedules can be evaluated simultaneously by including fractions in schedule 1 (S1) and schedule 2 (S2) using checkboxes. Physical or biological (i.e. equivalent) dose can be calculated for each schedule, where biological dose can be calculated according to any desired reference fraction size (typically 2 Gy/fraction).
Figure 2. Screen shots of several steps in the workflow. (A) Pop-up window to set the initial treatment schedule for an imported radiation dose or temperature distribution. (B) Treatment schedule tab, providing an overview of imported treatment fractions and settings for equivalent dose calculation. (C) ROI settings tab, where tissue types and priorities are assigned to each structure. (D) DVH parameter table, where a preset list of DVH parameters is selected and calculated. (E) TCP tab, where the target ROI, TCP model and TCP model parameters are selected. For a colour version of this figure, see the online version of this paper.
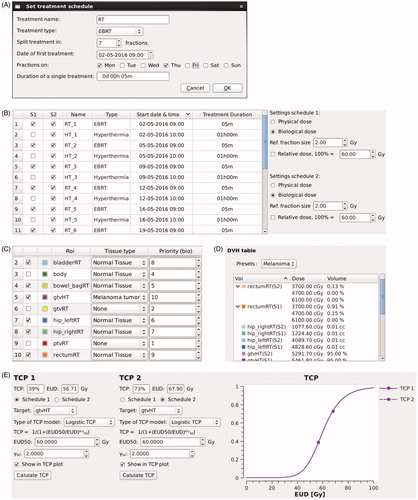
3. Select tissue type for structures. The tissue type and priorities for each structure are defined in the ROI settings tab (). When selecting a tissue type, a set of model parameters for the LQ model is read from an XML configuration file and assigned to the corresponding structure. For voxels located within multiple structures, the highest priority structure determines which tissue type is used for equivalent dose calculation. Checkboxes control visualisation of structures in the CT view and DVH tab.
4. Equivalent radiation dose (3D). The equivalent radiation dose distribution is calculated using EquationEquation (7)
(7) for each individual voxel and can be exported (DICOM) if desired.
5. CT view. The equivalent dose distribution can be visualised as an overlay in the CT view.
6. DVH and DVH parameters. DVHs can be evaluated in the DVH tab. Preset lists of DVH parameters, defined through configuration files, can be selected for calculation ().
7,8. Set TCP parameters; EUD and TCP. For either schedule, equivalent uniform dose (EUD) and TCP can be calculated (using EquationEquations (8)
(8) and Equation(9–11), respectively) by selecting the structure corresponding to the tumour, choosing a TCP model and the desired model parameters ().
Computational methods and theory
X-Term is written in C++, using the Qt (Qt, The Qt Company, Espoo, Finland) and Qwt libraries [Citation17]. In this section, we show how calculations of equivalent radiation dose, EUD, and TCP, have been implemented mathematically in X-Term.
LQ model
Calculation of the equivalent radiation dose is based on the generalised LQ model [Citation1,Citation2]
(1)
where D (Gy) is the total dose, α (Gy−1) and β (Gy−2) are the radiosensitivity parameters and G is the Lea–Catcheside protraction factor that accounts for repair during irradiation. The protraction factor
(2)
depends on dose rate R (Gy/h) and repair rate μ (h−1) [Citation18]. For piecewise constant dose rates, a closed form of this expression can be derived [Citation19],
(3)
where di and dj are the doses delivered in fractions i and j, respectively, and where fi,j and hj are given by
(4)
(5)
with subscript s/f indicating the start/end time of a fraction.
The effect of hyperthermia is included by using radiosensitivity parameters α and β that depend on the temperature T (°C) achieved during hyperthermia and the time interval tint (h) between corresponding hyperthermia and radiotherapy treatment fractions,
(6)
similar to Kok et al. [Citation13]. Several functional forms for α(T, tint) and β(T, tint) can be selected through a configuration file.
Equivalent radiation dose
The external beam radiotherapy (EBRT) dose that is equivalent to a thermoradiotherapy treatment is calculated using
(7)
where α37 and β37 are the radiosensitivity parameters at 37 °C, G is given by EquationEquations (3)–(5) and dref (Gy) is the fraction size of the reference EBRT treatment (for a derivation, see Supplementary Appendix A). X-Term uses EquationEquations (3)–(5) and Equation(7)
(7) to calculate the equivalent radiation dose for each voxel. Since fi,j and gj only depend on the treatment schedule, these factors are pre-calculated for efficiency.
Equivalent uniform dose
EUD is calculated according to the method proposed by Niemierko [Citation20] as
(8)
where EQDRT,k (Gy) is the equivalent radiation dose in the kth voxel of the structure (for a derivation, see Supplementary Appendix B).
Tumour control probability
Three TCP models were implemented in X-Term. First, a Poisson TCP model
(9)
where VT (cm3) is the tumour volume and ρ0 (cm−3) is the density of clonogenic cells within the tumour [Citation3–5].
Second, a logistic model used by Gay and Niemierko [Citation6]
(10)
where EUD50 (Gy) is the EUD resulting in TCP = 50% and γ50 is the normalised slope at EUD = EUD50.
Third, a model used by Okunieff et al. [Citation7] that is based on the inverse logit function
(11)
with
(12)
Clinical application: two realistic examples
Two realistic examples are presented to illustrate the use and potential clinical applications of X-Term. The parameters and relations assumed in these examples are discussed in this section. Where possible, parameter values will be reasonable estimates based on current literature, though few data are available. Although real patient CT scans are used, the examples do not otherwise correspond to real patient cases. Thus, no clinical decisions were made based on these evaluations. The CT scans used in these examples were made as part of the standard workflow in our institute, and the Medical Ethics Committee waived the requirement for informed consent regarding the use of these CT scans.
The LQ parameters were assumed to increase exponentially with temperature (T) and decrease exponentially with the time interval between the end of radiotherapy and the start of hyperthermia treatment (tint), such that they are given by
(13)
(14)
where α37 = α(37,0), α41 = α(41,0), β37 = β(37,0), β41 = β(41,0) and τ (h) is the time constant of the exponential decay. The exponential decay as function of time is in accordance with the effect of time interval reported by Overgaard [Citation21].
Equivalent radiation dose (EquationEquation (7)(7) ) only depends on the ratios α37/β37 (Gy), α41/α37 and β41/β37. For tumours, α41/α37 = 1.5 (1–2), β41/β37 = 2.5 (1.5–4) was assumed, with α37/β37 = 3 Gy for melanoma tumours (Example 1) and α37/β37 = 10 Gy for cervical tumours (Example 2) [Citation22]. Since randomised trials have shown no significant increase in radiation toxicity [Citation23–26], less radiosensitisation was assumed for normal tissue: α37/β37 = 3 Gy, α41/α37 = 1.5 (1–2), β41/β37 = 2 (1.5–2.5). To model the uncertainty in the hyperthermic radiosensitisation, the ranges in α41/α37 and β41/β37 are used to determine a confidence interval in the equivalent dose calculation, i.e. for tumours, equivalent dose calculations with α41/α37 = 1, β41/β37 = 1.5 and α41/α37 = 2, β41/β37 = 4 were performed to provide, respectively, the lower and upper limits of a confidence interval. Similarly, for normal tissue, equivalent dose calculations with α41/α37 = 1, β41/β37 = 1.5 and α41/α37 = 2, β41/β37 = 2.5 provided lower and upper limits of a confidence interval. Furthermore, τ = 1.5 h was assumed for tumour tissue and τ = 1 h for normal tissue, since animal studies suggest radiosensitisation to disappear more rapidly for normal tissues [Citation21]. A repair rate of μ = 0.67 h−1 was used for both tumour [Citation27–29] and normal tissue [Citation30], but since high dose rates are used in both examples, results are insensitive to this assumption. All equivalent doses were calculated with dref = 2 Gy (commonly denoted as EQD2).
Example 1: Radiotherapy compared to thermoradiotherapy in a previously irradiated area
For the first example, we consider a patient with a solitary lymph node metastasis of a melanoma in the left para-iliacal region. Suppose that the patient received adjuvant radiotherapy (13 × 2 Gy) for stage I seminoma testis at the right side 10 years earlier. In this example, risk of toxicity for irradiation of the nodal metastasis is considered substantial because of considerable overlap between the two radiation plans.
To evaluate potential gain of thermoradiotherapy, a conventional 7 × 5 Gy radiotherapy plan (2 fractions per week) was compared to a thermoradiotherapy plan. The thermoradiotherapy plan consists of 7 radiotherapy fractions, combined with 4 weekly hyperthermia sessions of 1 h each. Hyperthermia is assumed to be applied after radiotherapy, with a time interval of 30 min. Using X-Term, the thermoradiotherapy plan was designed to yield an equal EUD (and therefore TCP) by iteratively scaling the radiation fraction dose for that plan and calculating the EUD until it matched the EUD of the conventional 7 × 5 Gy radiotherapy-only plan. Normal tissue effects were assessed by comparing DVHs and DVH parameters. Previous irradiation was accounted for assuming 50% long-term recovery. Thus, conventional DVH parameters (and limits thereon) of organs located centrally and at the right side were reduced by half of the previously received dose (13 Gy).
Example 2: 30 min compared to 4 h time interval between radiotherapy and hyperthermia
In the second example, we consider a stage IIIb cervical cancer patient who has a contraindication for chemotherapy. According to Dutch guidelines, such a patient would be treated with thermoradiotherapy: 23 × 2 Gy daily radiotherapy + weekly hyperthermia (for 1 h), where hyperthermia is delivered after radiotherapy. At the end of the treatment period, a brachytherapy boost would be scheduled (EQD2 = 24 Gy).
Suppose that the patient lives close to a radiotherapy centre, but the nearest centre specialised in hyperthermia is 3.5 h away. Thus, the patient prefers to receive radiotherapy treatments at the nearby hospital and only travel once a week for the hyperthermia treatment. However, this will increase the time interval between radiotherapy and hyperthermia to 4 h (compared to 30 min), which could negatively affect local control. To address this issue, both scenarios were simulated using X-Term.
The tumour DVH and EUD was calculated for both treatment schedules. The brachytherapy dose distribution could not be included in the equivalent dose calculation, since small geometric errors in the registration between the brachytherapy MR and hyperthermia CT result in large errors in DVH and EUD calculations as a result of the strong gradients in the brachytherapy dose distribution. Formal TCP modelling was thus impossible. Therefore, we translated the difference in EUD into an expected increase/decrease of TCP assuming a TCP change of 2%/Gy. This is the typical slope of TCP models at 50% local control [Citation7], which is the approximate local control in advanced stage cervical cancer patients [Citation31].
Results
Example 1: Radiotherapy compared to thermoradiotherapy in previously irradiated area
The radiotherapy dose distribution was imported twice; one of these was combined with the hyperthermia treatment. After iterative scaling of the radiation fractions of the thermoradiotherapy treatment, 7 × 4.34 Gy + 4 × hyperthermia was established to yield a similar TCP as the conventional 7 × 5 Gy radiotherapy plan. However, the EQD2 in normal tissues for the thermoradiotherapy plan was considerably lower, as is apparent by comparison of the isodose lines to the colour wash in the CT view (), the DVHs () and the DVH parameters (). The bowel bag V47Gy is of particular interest, since at 146 cm3 this parameter substantially exceeded clinically acceptable limits for the conventional plan. For the combined thermoradiotherapy plan, the V47Gy is considerably reduced to 99 (38–128) cm3, though it still exceeds conventional clinical limits.
Figure 3. Treatment schedule tab and CT view (A) and DVHs (B) for Example 1. Colour wash (CT view) and dashed lines (DVHs) show the equivalent dose (EQD2) for the conventional 7 × 5 Gy radiotherapy schedule; isodose lines (CT view), solid lines and shaded areas (DVHs) show the equivalent dose (EQD2) for the 7 × 4.34 Gy + 4 × hyperthermia schedule. Shaded areas represent the confidence intervals resulting from uncertainty in biological parameters. For a colour version of this figure, see the online version of this paper.
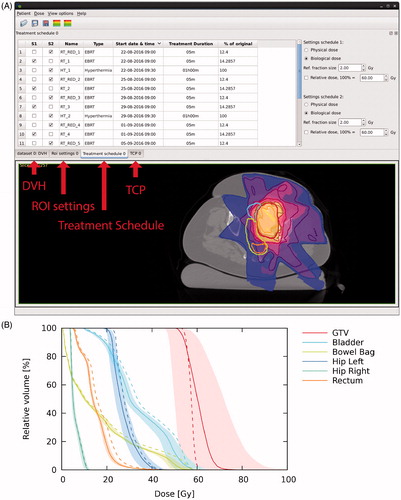
Table 1. DVH parameter table (Example 1).
The heterogeneous temperature in the hyperthermia treatment resulted in a more heterogeneous EQD2 in the tumour for the thermoradiotherapy plan, resulting in a slightly lower D95% (). By design, the net effect was an equal EUD (56.7 Gy), but due to uncertainty in radiobiological parameters, EUD could range from 49.2 to 63 Gy. In the end, it is up to the radiation oncologist to decide whether the risk of underdosing the tumour due to this uncertainty is acceptable in view of the potential sparing of the bowel bag.
Example 2: 30 min compared to 4 h time interval between radiotherapy and hyperthermia
For the second example, the hyperthermia temperature distribution was imported twice: first with 30 min between radiotherapy and hyperthermia, and next with a delay of 4 h between radiotherapy and hyperthermia (see ).
Figure 4. (A) Defining the 30-min time-interval schedule (S1) and 4-h time-interval schedule (S2) (Example 2). (B) DVHs (EQD2) for the 30-min and 4-h time-interval schedules (Example 2). Shaded areas represent the confidence intervals resulting from uncertainty in biological parameters.
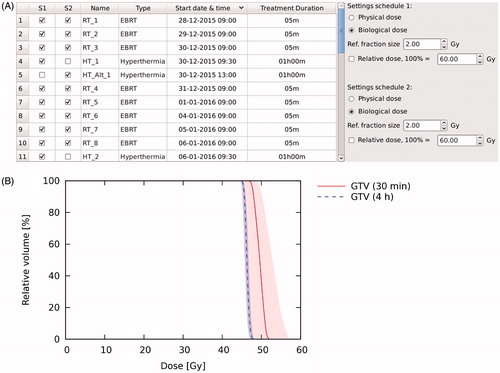
EQD2 was higher throughout the whole tumour for the 30 min time-interval schedule (). For the GTV, the D95% was higher in the 30 min time-interval schedule by 2.3 (0.4–3.9) Gy. Similarly, EUD in the 30 min time-interval schedule was higher by 3.1 (0.4–5.6) Gy. With an estimated TCP increase of 2% per Gy, TCP would be 6% (1–10%) higher for the 30 min time-interval schedule. Evaluation of both plans showed that in terms of tumour control, it was preferable to give both hyperthermia and radiotherapy treatments within the same institute.
Discussion
Radiobiological modelling is widely used in radiotherapy and a number of software applications are available to facilitate such modelling [Citation6,Citation11,Citation12]. In this paper, we have presented software that allows for 3D radiobiological modelling to be extended to the field of thermoradiotherapy. Using the concept of equivalent radiation dose for thermoradiotherapy treatments, the complexity of modelling thermoradiotherapy treatments is reduced to the more familiar problem of modelling outcome for conventional radiotherapy treatments. This allows evaluation of thermoradiotherapy plans using conventional methods (DVHs, DVH parameters and TCP), which have also been implemented in the current software, and against well-known clinical limits (e.g. limits on DVH parameters).
The two examples were presented to illustrate the value of biological evaluation of thermoradiotherapy plans. In the first example, the potential benefit of thermoradiotherapy compared to conventional radiotherapy for normal tissue sparing was assessed for a patient who was previously irradiated to the same area. This example demonstrates that biological evaluation of thermoradiotherapy plans can help to support complex, individual clinical decisions. It is well known that the temperature achieved in hyperthermia treatments depends on the exact location of the tumour, as well as the patient anatomy [Citation32,Citation33]. Similarly, the dose to organs at risk depends on their localisation relative to the tumour. Thus, because of the complex interplay between the ability to heat the patient and the localisation of the tumour and organs at risk, clinical reasoning alone is not sufficient to assess potential gain of thermoradiotherapy and software-based biological evaluation is essential.
The ability to support individual clinical decisions may also prove useful for clinical trials. Inclusion criteria in clinical trials generally aim to include patients for whom the potential benefit of the experimental treatment is highest. The ability to assess potential gain of thermoradiotherapy treatments for individual patients enables inclusion in thermoradiotherapy trials based on planning-based inclusion criteria, such as a minimum expected TCP increase or minimum decrease in dose to organs at risk. This is beneficial both for demonstrating the effectiveness of thermoradiotherapy, and for optimisation of thermoradiotherapy treatment schedules (e.g. by studying the effect of the number of hyperthermia treatments), since smaller patient groups are needed to obtain significant results.
The second example demonstrates the value of biological evaluation for thermoradiotherapy planning studies. Currently, planning studies are usually based on the separate hyperthermia and radiotherapy treatment plans (e.g. [Citation34–36]), not taking into account the interaction between the two modalities. However, by taking the interaction between both treatments into account, the effect of parameters which change the interaction, such as the effect of time interval between the radiotherapy and hyperthermia treatments, can be studied. This topic is particularly interesting since there is little consensus on the optimal order and time interval between radiation and hyperthermia treatments [Citation21,Citation37,Citation38]. Additionally, planning studies may help to identify new tumour sites that could benefit from thermoradiotherapy, as it is known that thermoradiotherapy is more effective in certain tumour sites (e.g. cervix, recurrent breast) than in others (e.g. rectum) [Citation23,Citation25]. Furthermore, potential gain of new treatment strategies may be assessed, such as combining hyperthermia with brachytherapy [Citation39] or with proton irradiation [Citation40].
Finally, software like X-Term can also be used to model conventional radiotherapy treatments, since this is simply the limiting case, where α(T, tint) = α37 and β(T, tint) = β37. Much of the existing software focusses on equivalent dose calculation of a single point (e.g. prescription dose) or a number of points (e.g. calculation of equivalent DVHs), implicitly assuming identical dose distributions for each treatment fraction. Full 3D radiobiological evaluation enables radiobiological modelling of doses that vary per fraction, for example to study the radiobiological effects of patient setup errors or adaptive radiotherapy strategies.
One of the major challenges in radiobiological modelling of thermoradiotherapy plans is the lack of accurate model parameters. In conventional radiobiological modelling, parameter uncertainty is commonly resolved by exploring a range of possible parameter values. A similar strategy is essential in biological modelling of thermoradiotherapy treatments, and has been employed in the examples in this study. Although the confidence intervals in this study represent the extreme (and improbable) scenarios where either both α41/α37 and β41/β37 are low or both are high, it is clear that the current uncertainty in model parameters leads to substantial uncertainty in EQD2 calculations (particularly in Example 1), limiting the overall accuracy of the biological evaluation. Moreover, we have assumed a mathematical relationship between the radiosensitivity parameters, time and temperature (α(T, tint) and β(T, tint)) to illustrate the use of the software, but since literature data is scarce on this topic, the exact relationship has yet to be established. More accurate determination of these parameters and their relationship with time and temperature are essential to advance the field of biological modelling for thermoradiotherapy treatments.
Another challenge is the simulation of temperature distributions in hyperthermia treatment planning. The quantitative accuracy of such simulations is known to be limited, as literature-based tissue properties are often assumed for calculations [Citation41]. This field, however, is progressing towards more individualised planning, for example by using functional MRI scans to determine dielectric properties [Citation42] and advanced thermal modelling including angiogram-based discrete vasculature [Citation43].
Our software focusses on modelling the radiosensitising effect of hyperthermia, since it is this interaction that is ignored in conventional thermoradiotherapy planning, where radiotherapy and hyperthermia plans are made and optimised separately. However, hyperthermia is also known to have a direct cytotoxic effect [Citation44–46] and an indirect radiosensitisation effect through reoxygenation/perfusion [Citation47–50]. Additionally, the amount of direct toxicity is known to be subject to thermotolerance if multiple hyperthermia sessions per week are given [Citation44–46,Citation51]. Modelling of direct cytotoxicity and thermotolerance can be done using a stochastic model, as described by Rosner et al. [Citation52], but this was not incorporated in the software presented here and will be subject of future research.
While incorporating direct cytotoxicity and thermotolerance would add to the realism of the biological model, it poses several challenges regarding the determination of model parameters. To determine which fraction of the hyperthermic effect is due to radiosensitisation and which part is due to direct cytotoxicity (and derive model parameters accordingly), clinical data with variation in total radiation dose is needed. Moreover, to determine model parameters incorporating thermotolerance, different heating times and/or different times between hyperthermia sessions are needed. Such variation is typically not present in clinical data, as patients are generally treated according to a fixed protocol. Incorporation of reoxygenation effects is even more challenging, as these require information about the oxygenation status of tumours in vivo. Our software therefore initially only accounts for direct radiosensitisation, limiting the number of degrees of freedom in the biological model. Then, as new clinical schedules are explored and model predictions start to deviate from clinical observations, factors for direct cytotoxicity and thermotolerance can be added. The advantage is that when clinical observations start to deviate from the model, by definition, sufficient variation is present in the clinical data to train the additional model parameters. Following this procedure, an iterative refinement of the biological evaluation can be obtained, gradually incorporating DNA repair inhibition, direct cell kill and thermotolerance, and reoxygenation mechanisms [Citation14].
Finally, in the future, as combination therapies become more complicated (with e.g. triple modality treatments like hyperthermia + chemotherapy + radiotherapy or hyperthermia + radiotherapy + radiosensitising agents such as halogenated pyrimidines [Citation53]), there will be an increasing need to account for the individual and synergistic effects of each of these therapies in the treatment planning stage. This could be done in the same framework introduced here, by combining simulated temperature, radiation dose and drug dose distributions and expressing them as one equivalent dose distribution.
Conclusion
Up to now, clinical decisions regarding treatment schedules of combined radiotherapy and hyperthermia treatments were based on clinical experience. Here, we have presented an application that can model the biological effects of thermoradiotherapy, thereby facilitating the evaluation of thermoradiotherapy plans for individual patients.
Supplementary File
Download MS Word (25 KB)Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Douglas BG, Fowler JF. (1976). The effect of multiple small doses of X rays on skin reactions in the mouse and a basic interpretation. Radiat Res 66:401–26.
- Barendsen G. (1982). Dose fractionation, dose rate and iso-effect relationships for normal tissue responses. Int J Radiat Oncol Biol Phys 8:1981–97.
- Munro TR, Gilbert CW. (1961). The relation between tumour lethal doses and the radiosensitivity of tumour cells. Br J Radiol 34:246–51.
- Porter EH. (1980). The statistics of dose/cure relationships for irradiated tumours. Part I. Br J Radiol 53:210–27.
- Porter EH. (1980). The statistics of dose/cure relationships for irradiated tumours. Part II. Br J Radiol 53:336–45.
- Gay HA, Niemierko A. (2007). A free program for calculating EUD-based NTCP and TCP in external beam radiotherapy. Phys Med 23:115–25.
- Okunieff P, Morgan D, Niemierko A, Suit HD. (1995). Radiation dose–response of human tumors. Int J Radiat Oncol Biol Phys 32:1227–37.
- De Leeuw AAC, Van de Kamer JB, Moerland MA, et al. (2011). The effect of alternative biological modelling parameters (α/β and half time of repair T½) on reported EQD2 values in the treatment of advanced cervical cancer. Radiother Oncol 101:337–42.
- Avanzo M, Trovo M, Stancanello J, et al. (2015). Hypofractionation of partial breast irradiation using radiobiological models. Phys Med 31:1022–8.
- Uzan J, Nahum AE. (2012). Radiobiologically guided optimisation of the prescription dose and fractionation scheme in radiotherapy using BioSuite. Br J Radiol 85:1279–86.
- Sanchez-Nieto B, Nahum AE. (2000). Bioplan: software for the biological evaluation of radiotherapy treatment plans. Med Dosim 25:71–6.
- Warkentin BJ, Stavrev P, Stavreva N, et al. (2004). A TCP-NTCP estimation module using DVHs and known radiobiological models and parameter sets. J Appl Clin Med Phys 5:50–63.
- Kok HP, Crezee J, Franken NAP, et al. (2014). Quantifying the combined effect of radiation therapy and hyperthermia in terms of equivalent dose distributions. Int J Radiat Oncol Biol Phys 88:739–45.
- Crezee H, van Leeuwen CM, Oei AL, et al. (2016). Thermoradiotherapy planning: integration in routine clinical practice. Int J Hyperthermia 32:41–9.
- Kok HP, Van Haaren PMA, Van de Kamer JB, et al. (2005). High-resolution temperature-based optimization for hyperthermia treatment planning. Phys Med Biol 50:3127–41.
- van Haaren PMA, Kok HP, van den Berg CAT, et al. (2007). On verification of hyperthermia treatment planning for cervical carcinoma patients. Int J Hyperthermia 23:303–14.
- Rathmann U, Wilgen J. The Qwt user’s guide. [Internet]. Available from: http://qwt.sourceforge.net/ [Accessed 14 July 2016].
- Lea DE, Catcheside DG. (1942). The mechanism of the induction by radiation of chromosome aberrations in Tradescantia. J Genet 44:216–45.
- Brenner DJ, Hall EJ. (1991). Conditions for the equivalence of continuous to pulsed low dose rate brachytherapy. Int J Radiat Oncol Biol Phys 20:181–90.
- Niemierko A. (1997). Reporting and analysing dose distributions: a concept of equivalent uniform dose. Med Phys 24:103–10.
- Overgaard J. (1980). Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumor and its surrounding normal tissue in vivo. Int J Radiat Oncol Biol Phys 6:1507–17.
- Franken NAP, Oei AL, Kok HP, et al. (2013). Cell survival and radiosensitisation: modulation of the linear and quadratic parameters of the LQ model (Review). Int J Oncol 42:1501–15.
- van der Zee J, González D, van Rhoon GC, et al. (2000). Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Lancet 355:1119–25.
- Huilgol NG, Gupta S, Sridhar CR. (2010). Hyperthermia with radiation in the treatment of locally advanced head and neck cancer: a report of randomized trial. J Cancer Res Ther 6:492–6.
- Vernon CC, Hand JW, Field SB, et al. (1996). Radiotherapy with or without hyperthermia in the treatment of superficial localised breast cancer: results from five randomised controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys 35:731–44.
- Overgaard J, Bentzen SM, Overgaard J, et al. (1995). Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet 345:540–3.
- Kal HB, Van Gellekom MPR. (2003). How low is the alpha/beta ratio for prostate cancer? Int J Radiat Oncol Biol Phys 57:1116–21.
- Fowler J, Chappell R, Ritter M. (2001). Is alpha/beta for prostate tumors really low? Int J Radiat Oncol Biol Phys 50:1021–31.
- Roberts SA, Hendry JH, Swindell R, et al. (2004). Compensation for changes in dose-rate in radical low-dose-rate brachytherapy: a radiobiological analysis of a randomised clinical trial. Radiother Oncol 70:63–74.
- Joiner MC, Bentzen SM. (2009). Fractionation: the linear-quadratic approach. In: Joiner MC, van der Kogel AJ, eds. Basic clinical radiobiology. 4th ed. London: Hodder Arnold, 102–19.
- Van Der ZJ, González DG. (2002). The Dutch Deep Hyperthermia Trial: results in cervical cancer. Int J Hyperthermia 18:1–12.
- van Haaren PMA, Hulshof MCCM, Kok HP, et al. (2008). Relation between body size and temperatures during locoregional hyperthermia of oesophageal cancer patients. Int J Hyperthermia 24:663–74.
- Sreenivasa G, Gellermann J, Rau B, et al. (2003). Clinical use of the hyperthermia treatment planning system HyperPlan to predict effectiveness and toxicity. Int J Radiat Oncol Biol Phys 55:407–19.
- De Greef M, Kok HP, Bel A, Crezee J. (2011). 3D versus 2D steering in patient anatomies: a comparison using hyperthermia treatment planning. Int J Hyperthermia 27:74–85.
- Kok HP, de Greef M, Borsboom PP, et al. (2011). Improved power steering with double and triple ring waveguide systems: the impact of the operating frequency. Int J Hyperthermia 27:224–39.
- Dobler B, Khemissi A, Obermeier T, et al. (2016). Re-irradiating spinal column metastases using IMRT and VMAT with and without flattening filter – a treatment planning study. Radiat Oncol 11:33.
- Stauffer P, Schlorff J, Taschereau R, et al. (2004). Combination applicator for simultaneous heat and radiation. Conf Proc IEEE Eng Med Biol Soc 4:2514–17.
- Stewart FA, Denekamp J. (1978). The therapeutic advantage of combined heat and X rays on a mouse fibrosarcoma. Br J Radiol 51:307–16.
- Crezee J, van Leeuwen CM, Oei AL, et al. (2016). Biological modelling of the radiation dose escalation effect of regional hyperthermia in cervical cancer. Radiat Oncol 11:14.
- Datta NR, Puric E, Schneider R, et al. (2014). Could hyperthermia with proton therapy mimic carbon ion therapy? Exploring a thermo-radiobiological rationale. Int J Hyperthermia 30:524–30.
- Kok HP, Wust P, Stauffer PR, et al. (2015). Current state of the art of regional hyperthermia treatment planning: a review. Radiat Oncol 10:196.
- Balidemaj E, Kok HP, Schooneveldt G, et al. (2016). Hyperthermia treatment planning for cervical cancer patients based on electrical conductivity tissue properties acquired in vivo with EPT at 3 T MRI. Int J Hyperthermia 32:558–68.
- Kok HP, Gellermann J, van den Berg CAT, et al. (2013). Thermal modelling using discrete vasculature for thermal therapy: a review. Int J Hyperthermia 29:336–45.
- Yarmolenko PS, Moon EJ, Landon C, et al. (2011). Thresholds for thermal damage to normal tissues: an update. Int J Hyperthermia 27:320–43.
- Dewhirst MW, Viglianti BL, Lora-Michiels M, et al. (2003). Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia 19:267–94.
- Horsman MR, Overgaard J. (2007). Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol) 19:418–26.
- Winslow TB, Eranki A, Ullas S, et al. (2015). A pilot study of the effects of mild systemic heating on human head and neck tumour xenografts: analysis of tumour perfusion, interstitial fluid pressure, hypoxia and efficacy of radiation therapy. Int J Hyperthermia 31:693–701.
- Sun X, Xing L, Ling CC, Li GC. (2010). The effect of mild temperature hyperthermia on tumour hypoxia and blood perfusion: relevance for radiotherapy, vascular targeting and imaging. Int J Hyperthermia 26:224–31.
- Vujaskovic Z, Song CW. (2004). Physiological mechanisms underlying heat-induced radiosensitization. Int J Hyperthermia 20:163–74.
- Brizel DM, Scully SP, Harrelson JM, et al. (1996). Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res 56:5347–50.
- Overgaard J, Nielsen OS. (1983). The importance of thermotolerance for the clinical treatment with hyperthermia. Radiother Oncol 1:167–78.
- Rosner GL, Clegg ST, Prescott DM, Dewhirst MW. (1996). Estimation of cell survival in tumours heated to nonuniform temperature distributions. Int J Hyperthermia 12:223–39.
- Franken NA, Van Bree C, Veltmaat MA, et al. (2001). Radiosensitization by bromodeoxyuridine and hyperthermia: analysis of linear and quadratic parameters of radiation survival curves of two human tumor cell lines. J Radiat Res 42:179–90.
