Abstract
Purpose: Non-surgical treatments for benign breast tumours have clinical goals of stopping growth and/or reducing (removing) palpable tumours effect without leaving a surgical scar. The purpose of this non-randomised prospective clinical trial was to assess imaging and clinical outcomes of microwave ablation (MWA) in the treatment of benign breast tumours regardless of the distance from the tumour to the skin and chest wall.
Methods: With approval of the institutional ethics committee and written informed consent, 39 patients with 44 core-biopsy-proved benign breast tumours 3.0 cm or less in diameter assessed by using ultrasound (US) and contrast-enhanced ultrasound (CEUS) were prospectively recruited. US-guided MWA was performed under local anaesthesia. The patients were followed up with physical examination, ultrasound elastography and CEUS.
Results: The MWA procedure with a mean duration of 74.3 s ± 26.5 was well accepted and tolerated in 41 cases except for three cases. Of 41 tumours with follow-up data, 40 (97.5%; 95% confidence interval: 87.1%, 99.9%) showed complete ablation assessed by using CEUS. The mean volume of the ablated tumours decreased significantly (p = .005) during follow-up. The strain ratio 1-3 months after ablation was higher than that before ablation, and became low 6 months after ablation (p = .022). No epidermal burn was observed in all cases with a mean distance of 7.5 mm ±3.3 from the tumour to the skin.
Conclusions: MWA is a safe and effective minimally invasive “patient-friendly” procedure with a very short duration for the treatment of benign breast tumours.
Introduction
Breast masses are the most common breast complaints, and up to 80% biopsies are benign when biopsy is recommended [Citation1,Citation2]. The most common form of benign breast tumour is the fibroadenoma [Citation3]. The common approach of these benign masses is surgical excision. However, the patients may face a surgical procedure, and following complications. Especially for young patients, leaving an unsightly scar and cosmetic defect may be the problem what they most worry about. Therefore, most patients choose serial observation. Another problem will arise, anxiety. Anxiety will develop in some part of the patients with palpable prominence, localised discomfort, interval growth and peace of mind [Citation1,Citation4]. Considering the problems above, an ideal treatment, which is safe and effective minimally invasive “patient-friendly” procedure, is urgently needed for patients with benign breast lesions.
Non-surgical treatments for benign breast tumours have clinical goals of stopping growth and/or reducing (removing) palpable tumours effect without leaving a surgical scar. Vacuum-assisted biopsy [Citation5,Citation6] and minimally invasive thermal therapies [Citation1,Citation7–10] are applied to manage benign breast tumours. Vacuum-assisted biopsy is widely used to resect benign breast tumours. However, complete resection may still be limited by targeting and visualisation difficulties due to local haemorrhage during the procedure [Citation7,Citation11]. Moreover, the complications including haematoma limit the application of this technique in the treatment of benign breast tumours [Citation11]. Minimally invasive thermal therapies, including cryotherapy, radiofrequency ablation, laser therapy and focussed ultrasound surgery, are attempted to be used in treating benign breast tumours. Among these therapies, cryotherapy for benign breast lumps is mostly reported, and seems to be promising [Citation1,Citation2,Citation4,Citation7,Citation12]. However, over-the-counter analgesics is given to 78% of the patients for pain control; ecchymosis is found in 41% of the patients; haematoma is observed in 4% of the patients [Citation12]. The treatment duration is about 10–30 min for cryotherapy [Citation7]. What we always want is a rapid, safe and effective approach. Conspicuously, radiofrequency ablation is effective for the treatment of breast cancers with short treatment durations [Citation9,Citation13]. However, the pain under local anaesthesia and the inability to monitor the changes in the tissues with ultrasonography (US) are the two major limitations for thermal therapies [Citation14] especially for radiofrequency ablation.
Microwave ablation (MWA) is a promising minimally invasive local therapy with many advantages, including large ablation zones, very short ablation durations and improved convection profile compared with other techniques [Citation15,Citation16]. Our previous ablation-resection study [Citation17] shows MWA of small breast cancer is feasible under general anaesthesia with about 4.5 min. The following study [Citation18] shows 2 min MWA can cause an ablation zone with three diameters larger than 2 cm under local anaesthesia. To the best of knowledge, MWA of benign breast lumps has not been reported.
The purpose of this non-randomized prospective clinical trial was to assess imaging and clinical outcomes of MWA in the treatment of benign breast tumours regardless of the distance from the tumour to the skin and chest wall.
Materials and methods
Patient enrollment
Between 1 April 2015 and 15 January 2016, patients clinically diagnosed with breast fibroadenoma in our hospital were enrolled in this prospective non-randomized clinical trial, with the approval (No. 2013-NT-138) of the institutional ethics committee of The First Affiliated Hospital with Nanjing Medical University. And written informed consent was received from each patient.
The inclusion criterion included the following: (a) less than 3 tumours in unilateral breast; (b) US BI-RADS (the Breast Imaging Recording and Data System) score 3 and mammogram BI-RADS 3 if it was given to patients before biopsy; (c) the tumour smaller than 3 cm in greatest diameter confirmed by using US and contrast-enhanced US (CEUS); (d) benign breast disease proved by using core biopsy; (e) Karnofsky performance status greater than 70%. Patient exclusion criteria were as following: (a) patients with medical contraindications to CEUS; (b) patients who were pregnant or breast-feeding; (c) patients with evidence of coagulopathy, chronic liver diseases or renal failure; (d) US BI-RADS score ≥4; (e) patients with breast implants.
The procedure of US-guided MWA
Pre-operative US, ultrasound elastography (UE) and CEUS evaluations were performed by one radiologist with 15 years of experience in breast US. Similar to the previous study, US was applied to identify the lesion and measure it in three dimensions. Then, core biopsy was performed by two surgeons with 20 and 7 years of experience in breast surgery, respectively. One to three days after core biopsy, MWA was performed by one surgeon with 5 years of experience in breast intervention (especially in MWA) under local anaesthesia. Cooled-shaft microwave antenna (14 G) was used in this study. The radiating segment embedded in the front, 11 mm from the antenna tip. Each tumour was ablated with one antenna. Patients were monitored by the surgeons during the biopsy, the MWA procedure, and one week after MWA for any complications that might be induced by the interventions.
Local anaesthesia was induced by using 1% lidocaine. Under local anaesthesia, the antenna was placed into the lump along the longest axis with US guidance through a small skin incision (). The protocols of antenna placement and treatment were the same as the previous study [Citation17]. Hydrodissection () was applied for skin protection and avoiding pains in all patients. When the antenna was accurately placed, 1% lidocaine was injected into subcutaneous space and retromammary space to form isolation belts (). The microwave irradiation frequency of the system (Nanjing Yigao Microwave Electric Institute, Nanjing, China) was 2450 MHz, and an output power of 40 W was selected in this study. The antenna was connected to the microwave generator through a flexible coaxial cable, and the two cooling-water tubes were connected. After testing the water cycling system, MWA was started immediately to cover the entire tumour. When the patient could not tolerate the pain, the procedure was stopped and lidocaine was injected again. Pain was evaluated with a (0–10) numerical rating scale. Score 1–3 was recognised as slight pain; 4–6 was recognised as moderate pain; and 7–10 was recognised as severe pain. After ablation, patients were monitored in our hospital for about half an hour for immediate complications. No antibiotics were given to any patient in this study.
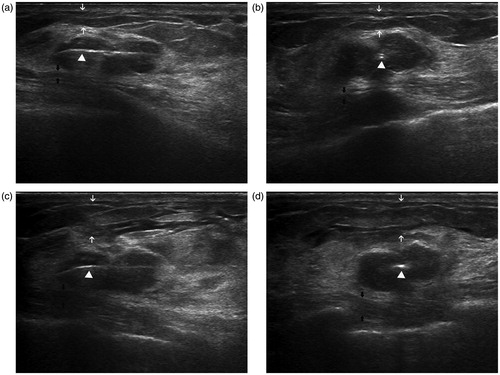
Figure 1. Intraoperative US in a 34-year-old woman demonstrates the accurate placement of the antenna and successful local anaesthesia. Longitudinal (a) and coronal (b) sonograms show the central placement of the antenna (arrow-head) within the tumour, indicating that the antenna was located in the centre of the tumour. After accurate placement of the antenna, 1% lidocaine was injected into subcutaneous space (white arrow) and retromammary space (black arrow). Longitudinal (c) and coronal (d) sonograms show the isolation belt was formed in subcutaneous space (white arrow) and retromammary space (black arrow).
Follow up
Physical examination, UE and CEUS were performed to the patients at 1 week, 1 month, 3 months and 6 months after MWA. Tumour palpability and cosmetic outcomes were recorded at follow-up by two surgeons. UE was applied to assess the stiffness of targeted tumour before and after MWA. Moreover, the strain ratio was calculated to reflect the property of stiffness of the lesion. The ablated zone and the effect of MWA were assessed by CEUS before and after ablation.
Conventional US and CEUS were performed using a MylabTwice ultrasound unit (The Esaote Group, Genova, Italy). US was performed with a LA523 transducer (4–13 MHz), and CEUS was performed with a LA522 transducer (3–9 MHz). SonoVue®, a contrast agent, was injected as an intravenous bolus of 2.4 ml, followed by a 5-ml saline flush. The tumours were scanned for about 4 min after bolus injection. The video clip was digitally recorded.
Data and statistical analysis
Median, mean, percentiles, range and standard deviation (SD) were analysed for continuous variables. Fisher’s exact test was used to identify the differences of the grade of pain in different subgroups. The volume of the tumour was calculated by using the three-dimensional axis (a, b, c) with the equation V = 4π(a/2)(b/2)(c/2)/3. One-way ANOVA was used to identify differences of longest diameter, volume, elasticity score and strain ratio of the tumour among different time points. The rate and 95% confidence interval (CI) were applied to assess the effectiveness of MWA. The 95% CI was calculated by using the binomial exact test. All statistical analyses were performed by using statistics software (State version 11.0, StataCorp, College Station, TX), and all p values were two-tailed with 5% significance levels.
Results
Basic characteristics
Thirty-nine patients with 44 tumours were enrolled in this study. Of these 39 patients, three patients had a single tumour in bilateral breasts, and two patients had two tumours in unilateral breast. The mean age of these 39 patients was 34.9 years ±9.8, with a range of 15–50 years. The median longest diameter of these 44 tumours was 13 mm assessed by using US, with a range of 7–24 mm. Of these 44 tumours, the distance from the tumour to the skin or chest wall of three tumours cannot be available. Of the remaining 41 tumours, the mean distance from the tumour to the skin was 7.5 mm ±3.3, with a range of 2–16 mm; the mean distance from the tumour to the chest wall was 3.1 mm ±2.4, with a range of 0–12.3 mm. The three diameters assessed by using CEUS were almost the same as assessed by using US before MWA. Of these 44 tumours by using core biopsy, 30 were diagnosed with fibroadenomas and 14 were diagnosed with other benign breast disease, including adenosis tumour, mastoplasia and collagen fibre hyperplasia.
Therapeutic response
After accurate placement of the antenna and second anaesthesia (), MWA was performed. A gradual and diffuse increase in the echogenicity from the irradiating segment to the whole tumour was observed. The mean duration of MWA was 74.3 s ± 26.5, with a range 40–150 s. Patients suffered severe pain in three cases, and the MWA procedure was stopped. Additional local anaesthesia was given, and prescheduled MWA was completed. No sedation was given in any patient. Slight to moderate pain was experienced without additional anaesthesia in 14 cases when MWA was performed. In the remaining 27 cases, patients did not experience any pain at all. There was no significant relationship between the grade of pain and the distance from the tumour to neither the skin nor the chest wall (, both p > .05). No analgesis was needed to all patients after MWA.
Table 1. The grade of pain experienced in the procedure of MWA according to the distance from the tumour to the skin and chest wall.
Follow-up imaging and clinical outcomes
Before ablation, all tumours showed enhancement (homogeneous or heterogeneous; strong or slight) in CEUS (). Of 39 patients, two were lost during follow-up, and the remaining 37 patients received CEUS at least once after ablation. Of these 37 patients with 41 tumours, US and CEUS were performed to 33 patients with 36 tumours (87.8%) 1 week after ablation, and US and CEUS were performed to the other four patients with five tumours in the following days. Of 37 patients with 41 tumours ablated, 40 (97.5%; 95% CI: 87.1%, 99.9%) showed complete ablation assessed by using CEUS (). In the only one case without complete ablation (), failure may attribute to poor placement of the antenna. The diameters and volumes of the ablation zone 1 week after MWA are shown in . CEUS showed that the ablation zone became smaller than before during follow-up (data not shown).
Figure 2. Conventional US and CEUS images in a 28-year-old woman before ablation and during follow-up. (a) US shows a clear tumour before MWA. (b) CEUS shows enhancement before MWA. (c) The ablation zone (arrow) seems to be discernible but not very clear in conventional US 1 week after ablation. (d) No enhancement was observed in the ablation zone (arrow) in CEUS 1 week after ablation. (e) In conventional US 2 months after MWA, the margin (white arrow) of the ablated zone is clear. Moreover, three typical zones are observed, including the hypoechoic tumour (black arrow), the surrounding hyperechogenicity and hypoechogenicity (arrow-head) at the margin of the ablation zone. (f) In CEUS 2 months after MWA, enhancement is observed at the margin of the hypoechogenicity (arrow-head) in US. (g) 6 months after ablation, characteristics are similar to those 2 months after MWA; however, the margin of the ablation zone seems to be vague. (h) The area (arrow) without enhancement is smaller than before about 6 months after MWA in CEUS.
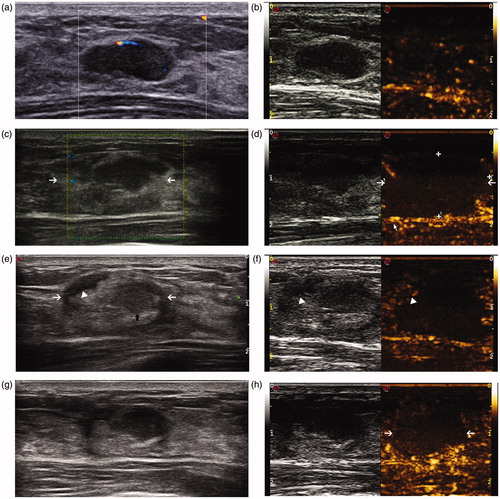
Figure 3. US and CEUS images before and 1 week after MWA in a 15-year-old girl. (a) US shows a hypoechoic tumour with a clear margin before ablation. (b) The tumour shows heterogeneous enhancement in CEUS before ablation. (c) Colour Doppler flow imaging shows blood flow signals at the margin the tumour 1 week after MWA. (d) Part of the tumour (arrow) shows enhancement in CEUS 1 week after MWA, suggesting incomplete ablation.
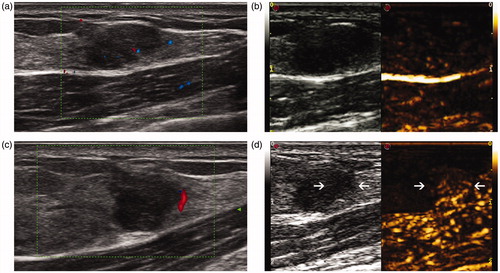
Table 2. Diameters and volumes of ablation zone 1 week after MWA.
All tumours were hypoechoic mass with well-defined margins relative to surrounding normal breast tissue in conventional US images before MWA. About 1 week after ablation, the margins of the tumour became vague, and the tumour showed hypoechogenicity surrounding by hyperechogenicity similar to normal breast tissues (). Moreover, the ablation zone, containing hypoechoic tumour and the hyperechogenicity, seemed to be discernible but not very clear in conventional US, and no enhancement was observed in the ablation zone in CEUS. Interestingly, the margin of the ablated zone was clear 2–3 months after MWA in conventional US images (). Moreover, three typical zones () were observed in several cases, including the hypoechoic tumour, the surrounding hyperechogenicity and hypoechogenicity at the margin of the ablation zone. At the margin of the hypoechogenicity, enhancement was observed in CEUS (). Half a year after MWA, characteristics in US and CEUS were similar to those 2–3 months after MWA. The area without enhancement was smaller than before about 6 months after MWA in CEUS.
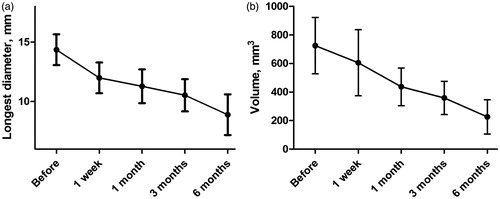
Conventional US demonstrated that the margin of the targeted lesion became less discernible during follow-up. Importantly, the mean longest diameter of the ablated tumours decreased significantly (p < .001, ), from 14.4 mm ±4.3 before MWA, 12.0 mm ±3.7 about 1 week after ablation, 11.3 mm ±3.1 about 1 month after ablation, 10.5 mm ±3.4 about 3 months after ablation, to 8.9 mm ±3.1 about 6 months after ablation. Similarly, the mean volume of the ablated tumours also decreased significantly (p = .005, ), from 725.5 mm3 ± 649.3 before MWA to 226.0 mm3 ± 217.3 half a year after MWA, about 31% of primary volume.
Figure 4. The changes of the longest diameter (a) and volume (b) of the ablated tumour before and after MWA. The mean longest diameter of the ablated tumours significantly decreased from 14.4 mm ±4.3 before MWA to 8.9 mm ±3.1 about 6 months after ablation. Similarly, the mean volume of the ablated tumours also significantly decreased, from 725.5 mm3 ± 649.3 before MWA to 226.0 mm3 ± 217.3 half a year after MWA.
Although slight skin depression overlying the ablated tumour was observed in two cases, all patients were satisfied with cosmetic outcomes. The data of palpability of the tumours were available in 40 tumours before ablation. A total of 19 (47.5%) were palpable by physical examinations. A palpable discreet treatment site was reported in 100% of cases at 1 week (31/31) and 1 month (21/21), respectively. A palpable treatment site was found in 70.8% (17/24) and 66.7% (8/12) of cases at 3 and 6 months, respectively.
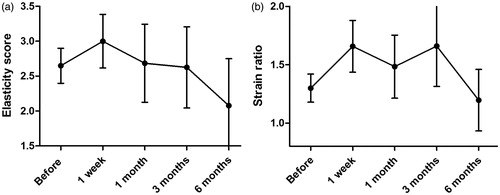
Elasticity scores and strain ratio of the targeted tumour before and after MWA were recorded. According to the results of elasticity score (), the tumours one week after MWA were stiffer than that before ablation and then became soft gradually, although there was no significant difference among different time points (p = .139). However, significant difference was observed about the strain ratios among different time points (p = .022). The strain ratio 1–3 months after ablation was higher than that before ablation, and became low about 6 months after ablation ().
Figure 5. The mean elasticity score (a) and strain ratio (b) of the ablated tumour during follow-up. (a) According to elasticity score, the tumours one week after MWA seemed to be stiffer than that before ablation and then became soft gradually, although there was no significant difference among different time points (p = .139). (b) The strain ratio 1–3 months after ablation was higher than that before ablation, and became low about 6 months after ablation (p = .022).
Adverse events
No epidermal burn was observed in these 39 patients due to isolation belt with 1% lidocaine (). Oedema and swelling at the treatment site were reported about two days after ablation, and disappeared in one week after treatment. No ecchymosis, haematoma, infection, or other adverse events induced by MWA were noted in 37 patients during and after the procedure.
Discussion
We report successful experience of MWA in the treatment of benign breast tumours under local anaesthesia. Of 39 patients with 44 tumours, the MWA procedure was well accepted and tolerated in 41 cases except for three cases with severe pain. Of 37 patients with 41 tumours, 40 showed complete ablation assessed by using CEUS. The mean volume of the ablated tumours decreased significantly. The tumours after MWA were stiffer than before and then became soft gradually. Our results suggest that MWA is a safe and effective minimally invasive “patient-friendly” procedure with a very short duration for the treatment of benign breast tumours.
For previous studies [Citation17,Citation19–23], the distance larger than 1 cm from the tumour to the skin and chest wall was the inclusion criterion. However, it is not the inclusion criterion in this study. Any distance from the tumour to the skin or chest wall was acceptable. To avoid skin burns and chest wall injuries, 1% lidocaine was injected into subcutaneous space and retromammary space after placement of the antenna. No epidermal burn was observed in all 39 patients. We think the distance may be not a limitation for thermal therapies in the treatment of breast tumours.
The pain under local anaesthesia, a major limitation for thermal therapies, is not a limitation for cryotherapy [Citation14]. However, over-the-counter analgesics is given to 78% of the patients for pain control after cryotherapy in a previous study [Citation12]. In this study, the MWA procedure was well accepted and tolerated in 41 cases except for three cases with severe pain. There may be two reasons for severe pain. First, the procedure of making isolation belt between tumour and skin or chest wall was not done well enough, patients may suffer severe pain before skin burns and chest wall injuries happened. Second, local anaesthesia might be not used properly. Importantly, prescheduled MWA was completed after additional local anaesthesia for these three cases. Additional local anaesthesia was given, and prescheduled MWA was completed. No analgesis was needed to all patients after MWA. Our local anaesthesia may contribute to well pain control. Besides, previous studies [Citation1,Citation7–9] have reported that more than 10 min are needed for ablating benign breast tumours by applying radiofrequency, laser and cryotherapy. Compared with these techniques, very short duration was needed for complete ablation of benign breast tumours 3.0 cm or less in diameter by applying MWA. MWA may be convenient for ablation benign breast tumours.
Previous studies suggest that the inability to monitor the changes in the tissues with US is another limitation for thermal therapies [Citation14]. Similar to our previous study [Citation17], MWA was stopped when the tumour disappeared completely with US. However, US monitoring is not accurate [Citation24], and accurate approaches are needed for ablation. In the learning phase, the complete ablation rate may be lower than that under steady conditions [Citation25]. MWA was performed by one surgeon with 5 years of experience in breast intervention, and 40 out of 41 cases showed complete ablation in this study. In the only one case without complete ablation, failure may attribute to poor placement of the antenna. The patient did not accept re-ablation or resection of the residual tumour. Therefore, there is still space to improve the placement of the antenna.
The changes of imaging in US and CEUS after ablation have seldom been reported. It is supposed that CEUS could be used to judge the success of ablation and it shows potential as a modality of radiological follow-up. The ablation zone was clear in US until 2–3 months after MWA, and three typical zones were firstly observed in this study. CEUS showed that no enhancement was observed in the ablation zone about 1 week after MWA, but enhancement was found at the margin of the zone about 2–3 months after MWA, and increased 6 months after MWA. Otherwise, the mean volume of the ablated tumours decreased significantly in US, and the tumour volume 6 months after MWA was about 31% of primary volume. However, long-term follow-up are still needed.
Palpable mass in the breast after thermal therapy may cause discomfort and anxiety to patients [Citation4]. In this study, a palpable discreet treatment site was reported in 100% of cases 1–4 weeks after ablation, and then the tumour became non-palpable gradually. This phenomenon may be demonstrated by elasticity score and strain ratio of the targeted tumour during follow-up. Factors related to the resorption of ablated tumours to non-palpability were not analysed because of limited data. Except for the previously reported factors [Citation4,Citation26], the distance from the tumour to the skin may be very important. Future studies are needed to systemically determine the factors.
Limitations still existed in this study. First, three cases experienced severe pain, although prescheduled MWA was completed after additional local anaesthesia. Several measures should be given to improve the tolerance of MWA in the treatment benign breast tumours. Second, a palpable discreet treatment site was reported in 8 of 12 cases 6 months after ablation. Factors related to the resorption of ablated tumours to non-palpability should be determined for patient inclusion in the future. Third, a long follow-up period is warranted to assess the long-term results in the future. Fourth, we have not compared MWA with other minimally invasive technique. Further clinical studies need to be taken into consideration for the comparisons. Besides, although a previous study [Citation27] reported that CEUS could be used to judge the success of ablation for breast lesions, further clinical studies are needed to demonstrate the valuable use. Maybe more useful tool would be developed to judge the success of ablation.
Conclusions
Taken together, our study demonstrates the promising use of MWA in breast benign tumours. The short-term results suggest that MWA is a safe and effective minimally invasive “patient-friendly” procedure with a very short duration for the treatment of benign breast tumours. Large clinical studies with long-term follow-up are still needed in the future.
Disclosure statement
The authors report no declarations of interest.
Funding
This work was supported in part by the National Natural Science Foundation of China [81572607, 81502299 and 81502286], the Natural Science Foundation of Jiangsu Province [BK20141023] and a project Funded by the Priority Academic Programme Development of Jiangsu Higher Education Institutions (PAPD).
References
- Kaufman CS, Bachman B, Littrup PJ, et al. (2002). Office-based ultrasound-guided cryoablation of breast fibroadenomas. Am J Surg 184:394–400.
- Caleffi M, Filho DD, Borghetti K, et al. (2004). Cryoablation of benign breast tumors: evolution of technique and technology. Breast 13:397–407.
- Dent DM, Cant PJ. (1989). Fibroadenoma. World J Surg 13:706–10.
- Kaufman CS, Bachman B, Littrup PJ, et al. (2004). Cryoablation treatment of benign breast lesions with 12-month follow-up. Am J Surg 188:340–8.
- Parker SH, Klaus AJ, McWey PJ, et al. (2001). Sonographically guided directional vacuum-assisted breast biopsy using a handheld device. AJR Am J Roentgenol 177:405–8.
- March DE, Coughlin BF, Barham RB, et al. (2003). Breast masses: removal of all US evidence during biopsy by using a handheld vacuum-assisted device-initial experience. Radiology 227:549–55.
- Littrup PJ, Freeman-Gibb L, Andea A, et al. (2005). Cryotherapy for breast fibroadenomas. Radiology 234:63–72.
- Dowlatshahi K, Wadhwani S, Alvarado R, et al. (2010). Interstitial laser therapy of breast fibroadenomas with 6 and 8 year follow-up. Breast J 16:73–6.
- Teh HS, Tan SM. (2010). Radiofrequency ablation – a new approach to percutaneous eradication of benign breast lumps. Breast J 16:334–6.
- Peek MC, Ahmed M, Scudder J, et al. (2016). High intensity focused ultrasound in the treatment of breast fibroadenomata: results of the HIFU-F trial. Int J Hyperthermia 32:881–8.
- Luo HJ, Chen X, Tu G, et al. (2011). Therapeutic application of ultrasound-guided 8-gauge Mammotome system in presumed benign breast lesions. Breast J 17:490–7.
- Edwards MJ, Broadwater R, Tafra L, et al. (2004). Progressive adoption of cryoablative therapy for breast fibroadenoma in community practice. Am J Surg 188:221–4.
- Peek MC, Ahmed M, Napoli A, et al. (2016). Minimal invasive ablative techniques in the treatment of breast cancer: a systematic review. Int J Hyperthermia. [Epub ahead of print]. doi: http://dx.doi.org/10.1080/02656736.2016.1230232.
- Fornage BD, Hunt KK. (2015). Image-guided percutaneous ablation of small breast cancer: which technique is leading the pack? Technol Cancer Res Treat 14:209–11.
- Simon CJ, Dupuy DE, Mayo-Smith WW. (2005). Microwave ablation: principles and applications. Radiographics 25:S69–S83.
- Zhou W, Liang M, Pan H, et al. (2013). Comparison of ablation zones among different tissues using 2450-MHz cooled-shaft microwave antenna: results in ex vivo porcine models. PLoS One 8:e71873.
- Zhou W, Zha X, Liu X, et al. (2012). US-guided percutaneous microwave coagulation of small breast cancers: a clinical study. Radiology 263:364–73.
- Zhou W, Jiang Y, Chen L, et al. (2014). Image and pathological changes after microwave ablation of breast cancer: a pilot study. Eur J Radiol 83:1771–7.
- Izzo F, Thomas R, Delrio P, et al. (2001). Radiofrequency ablation in patients with primary breast carcinoma: a pilot study in 26 patients. Cancer 92:2036–44.
- Fornage BD, Sneige N, Ross MI, et al. (2004). Small (≤2-cm) breast cancer treated with US-guided radiofrequency ablation: feasibility study. Radiology 231:215–24.
- Furusawa H, Namba K, Thomsen S, et al. (2006). Magnetic resonance-guided focused ultrasound surgery of breast cancer: reliability and effectiveness. J Am Coll Surg 203:54–63.
- Zippel DB, Papa MZ. (2005). The use of MR imaging guided focused ultrasound in breast cancer patients; a preliminary phase one study and review. Breast Cancer 12:32–8.
- Morin J, Traore A, Dionne G, et al. (2004). Magnetic resonance-guided percutaneous cryosurgery of breast carcinoma: technique and early clinical results. Can J Surg 47:347–51.
- Sag AA, Maybody M, Comstock C, Solomon SB. (2014). Percutaneous image-guided ablation of breast tumors: an overview. Semin Intervent Radiol 31:193–202.
- Dowlatshahi K, Francescatti DS, Bloom KJ. (2002). Laser therapy for small breast cancers. Am J Surg 184:359–63.
- Zhou W, Liu X, Ding Q, et al. (2013). Long-term outcomes of breast cancer ablation. Radiology 269:309–10.
- Schässburger KU, Löfgren L, Lagerstedt U, et al. (2014). Minimally-invasive treatment of early stage breast cancer: a feasibility study using radiofrequency ablation under local anesthesia. Breast 23:152–8.
