Abstract
Purpose: To investigate whether cavitation enhances the degree of coagulation during pulsed high-intensity focussed ultrasound (HIFU) in an isolated liver perfusion system.
Methods: Isolated liver was treated by pulsed HIFU or continuous-wave HIFU with different portal vein flow rates. The cavitation emission during exposure was recorded, and real-time ultrasound images were used to observe changes in the grey scale. The coagulation size was measured and calculated.
Results: HIFU treatment led to complete coagulation necrosis and total cell destruction in the target regions. Compared to exposure at a duty cycle (DC) of 100%, the mean volumes of lesions induced by 6 s exposure at DCs of 50% and 10% were significantly larger (P < .01) but were smaller at a DC of 5%. The necrosis volume was negatively related to the perfusion rate in the pulsed HIFU at a DC of 50% for exposure durations of 4 and 6 s, while the perfusion flow rate did not affect the necrosis volume for exposure durations of 1, 2 and 3 s. For increased perfusion flow rates, there was no significant decrease in the cavitation activity for the pulsed-HIFU (P > .05). For continuous-wave HIFU exposure, there was a significant decrease in the necrosis volume and cavitation activity for exposure times of 1, 2, 3, 4, and 6 s with increasing portal perfusion rates.
Conclusion: Perfusion flow rates negatively influence cavitation activity and coagulation volume. Ablation is significantly enhanced during pulsed HIFU exposure compared with continuous-wave HIFU.
Introduction
High-intensity focussed ultrasound (HIFU) is a rapidly developing and widely used therapeutic treatment for a variety of solid tumours in clinical settings. HIFU exposure can rapidly elevate tissue temperature within a limited region and induce coagulation necrosis by nesting lesions side-by-side without damaging the intervening tissues and structures along the acoustic path. It is generally believed that the cooling effect of blood flow and perfusion can be eliminated during short HIFU exposure [Citation1–4]. This concept originated from both the theoretical models and in vivo animal experiments performed in the 1990s [Citation5–11]. However, in clinical applications of HIFU, higher therapeutic energy is always needed to ablate hypervascularized tumours with abundant blood perfusion. If a vascular embolisation forms in the tumours before HIFU treatment, the energy used for ablation would be much lower [Citation12]. The pre-administration of gonadotropin-releasing hormone (GnRH) agonists can significantly reduce the vascularity of large uterine fibroids and thus potentiates the ablative effect of HIFU in these patients who undergo treatment of uterine fibroids [Citation13]. As the fibroid hyperintensity during T2-weighted MR imaging is correlated with vascularisation [Citation14,15], prior studies have demonstrated that HIFU ablation has poor clinical outcomes in patients with hyperintense fibroids, with significant decreases in non-perfusion volume, insignificant decreases in symptom severity scores (SSS), and in the resultant fibroid size [Citation16–20]. Therefore, blood flow and perfusion might be important influencing factors for the HIFU treatment of clinically relevant large volume tumours.
Methods for enlarging the thermal lesion and reducing treatment duration are needed in the clinical applications of HIFU. Increased acoustic intensity or elongated exposure durations would enlarge the lesion but also deliver more heat energy to the surrounding healthy tissues, leading to undesired damage. Inertial cavitation could enhance the HIFU-mediated heating effect by inducing broadband acoustic emission, thus increasing tissue absorption and enhancing HIFU ablation and pulsed HIFU exposure can induce inertial cavitation and increase tissue damage [Citation21–23]. In this study, using an isolated porcine liver perfusion system, the influence of perfusion flow rates on the size of coagulation necrosis size during HIFU was investigated. The inertial cavitation activities of the HIFU treatment with varying perfusion rates were also assessed.
Materials and methods
Organ harvesting
A total of fifty porcine livers weighing 1926 ± 230 g were obtained immediately from a commercial slaughterhouse. The warm ischaemia time was kept within 30 min. Each liver was immersed in 3 L heparinised perfusate and stored in a cold box at 4 °C with autologous blood for 2–4 h. Autologous blood was collected after opening the cervical vessels. The blood was anticoagulated with heparin (10,000 U/L), filtered using a transfusion device and stored in blood bags. Then, the main hepatic artery and portal vein were cannulated and perfused with fresh heparinised perfusate (7 g/L sodium chloride, 0.3 g/L potassium chloride, 0.2 g/L calcium chloride, 3.1 g/L sodium citrate, 30 g/L 6% hydroxyethyl starch, 34 g/L mannitol and 3000 IU heparin). Following perfusion with the solution, reperfusion was initiated with autologous blood. After ligating the cystic duct, the proximal segment of the common bile duct was cannulated to continuously drain out the bile by negative pressure. All the experimental procedures were approved by the Institutional Care and Animal Use Committee of Chongqing Medical University.
Perfusion system
In total, 4 L perfusate was pumped from the perfusate reservoir using an extracorporeal circulation unit (Polystan, Copenhagen, Denmark). The hepatic artery, portal vein, and retro-hepatic inferior vena cava were used to track the liver inflow and outflow with via three mono-head roller pumps (. The fluid pressure in the main hepatic vessels was monitored using a PT-100 multichannel biological blood pressure sensor (Taimeng, Chengdu, Sichuan, China), with the sensor tips vertically inserted into both the main hepatic artery and portal vein. Perfusion pressure was adjusted to 70–80 mmHg in the hepatic artery and 8–10 mmHg in the portal vein. Flow volume was controlled using an extracorporeal circulation unit, according to the mean pressure in each vessel, and flow velocity was quantified based on the main vessel using colour Doppler flow imaging and pulse waves (7.5–12 MHz probe, Esaote, Florence, Italy).
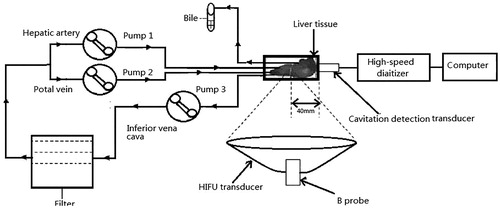
Figure 1. Schematic diagram of the isolated porcine liver perfusion system. Bile: the bile drainage from the proximal portion of the common bile dust; Pump: the mono-head roller pump of the extracorporeal circulation unit. The porcine liver was placed above a 0.9-MHz HIFU transducer with real-time monitoring of ultrasound imaging. A passive cavitation detection (PCD) system was placed around the liver.
High-intensity focussed ultrasound (hifu) system
Experiments were carried out using a clinical CE-approved ultrasound-guided High-Intensity Focussed Ultrasound System (HIFU) therapeutic system (Model JC200, Chongqing Haifu Medical Technology Co., Ltd., Chongqing, China). A 3.5-MHz colour Doppler diagnostic ultrasound device (Esaote, Genoa, Italy) was mounted in the centre of the concave HIFU transducer, which functioned at 0.9 MHz. The integrated transducer could automatically move in six directions. The acoustic power output was measured using a radiation force balance technique. The focal intensity of the HIFU of each acoustic power output was calculated as the acoustic power output divided by the area of the acoustic focal region. The HIFU transducer diameter was 220 mm with a focal length of 140 mm. The focal region was an ellipsoid, with a dimension of 8 mm along the beam’s longitudinal axis and 3 mm in the transverse direction.
After 30 min of circulation, the target region within the isolated porcine liver was treated by continuous-wave HIFU (control) or pulsed HIFU. This study included two parts: (1) At the same exposure dose and acoustic power, the target region within the isolated porcine liver was treated by continuous-wave HIFU exposure (control) or pulsed HIFU at 50%-DC, 10%-DC or 5%-duty cycle (DC) with an input power of 200 W, which was equal to a spatial-peak temporal-average intensity (ISPTA) of 12998 W/cm2. The exposure duration for each DC was set to 6, 12, 60, and 120 s, respectively. (2) At the same irradiation dose and time, the target region was treated by continuous-wave HIFU with 200 W (control) or pulsed HIFU with an input power of 400 W at a duty cycle of 50%, which was equal to ISPTA values of 12 998 W/cm2 and 25 996 W/cm2, respectively. The exposure duration for each input power was set to 1, 2, 3, 4 and 6 s.
The treatment parameters were as follows: (1) the pulse repetition frequency was 100 Hz, (2) the focus depth was 25 mm under the surface, (3) the distance between two irradiation points was at least 20 mm within the same liver lobe, and (4) the irradiation points were at least 5 mm away from the walls of main hepatic vessels. Grey-scale changes in the irradiated region were monitored by ultrasound.
The perfusion flow rates in the portal vein were set at 0 (no flow), 100, 200, 400, and 800 mL/min (approximately equal to flow velocities of 4.3–5.2, 6.7–8.2, 10.6–11.2, and 13.9–16.7 cm/s), respectively. Pulsed HIFU or continuous-wave HIFU exposure was carried out with different portal vein perfusion flow rates.
Experimental setup
A schematic diagram of experimental setup is shown in . The porcine liver was placed above the integrated HIFU transducer and the diagnostic probe for real-time monitoring with US imaging. The isolated liver was placed in a cylindrical tank in a 20 °C water bath with a 25-μm Mylar membrane at the tank bottom. With this system, the major part of the liver could be exposed to the HIFU treatment.
Passive cavitation detection (PCD) system was used to detect the acoustic emission at the focus of the HIFU transducer. It consisted of a 5 MHz focussed transducer (V309-SU, Panametrics, Waltham, MA) and a high-speed digitiser (PXIe-5122; National Instrument, Austin, TX). The PCD transducer aperture was 13 mm, and the focal length was 40 mm with a bandwidth of 3.3–7 MHz at the level of–6 dB. To detect the acoustic emission from the focus, the transducer was placed perpendicular to and confocal with the HIFU beams. The acoustic emission signals were obtained by the digitiser at 20 MHz and analysed using a computer. LabVIEW software (National Instruments) graphical programming language was used to create the displacement calculation algorithms used in the spectral analysis. Using fast Fourier transform routines, all the waveforms were first transformed to the frequency domain. The inertial cavitation level was determined by calculating the root mean square (RMS) amplitude of the broadband noise for each spectrum, according to the previously published method by Chen et al. [Citation24]. The RMS amplitude was superimposed on the background noise, which was subtracted as the baseline signal. The inertial cavitation dose (ICD) was quantified as the integrated area under the time-amplitude curve over the entire recording duration.
Real-time ultrasound images before and after HIFU exposure were immediately analysed and compared to determine whether a hyper-echoic region appeared at the HIFU focus, which was defined as a region with a distinct increase in the grey-scale intensity that could be easily recognised by a HIFU operator. It was a clinically useful indicator for the coagulative necrosis. In addition, real-time ultrasound imaging videos were recorded during the pulsed HIFU exposure.
Lesion volume assessment
After HIFU ablation, The livers was sliced into 1–2 mm sections along the beam axis, and the lesion area was measured using a Vernier calliper. The lesion volume was calculated according to the following formula: Volume = π × maximum length × maximum width2/6, in which the maximum length of the lesion was obtained along the beam axis, and the maximal width was obtained along the perpendicular axis.
Histological examination
The tissue samples were obtained and subjected to histological examination. The sample was fixed in 4% formalin, dehydrated by a series of ethanol solutions and xylene, and then embedded in paraffin. The sample was cut into 5-μm sections, stained with haematoxylin and eosin, and then observed under a light microscope.
Statistical analysis
Data were expressed as the mean ± SD. SPSS 17.0 software (SPSS, Chicago, IL) was used for statistical analysis. One-way analysis of variance (ANOVA) and the least significant difference (LSD) t-test were performed for multiple comparisons. P < .05 was considered statistically significant.
Results
Effects of perfusion flow on isolated porcine livers
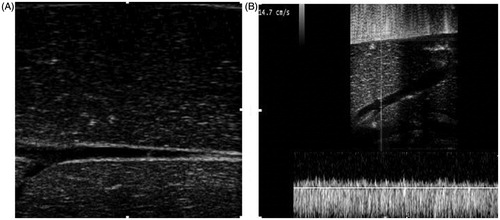
Ultrasound imaging indicated that after the 30-min perfusion, the liver parenchyma echo was more even compared with before perfusion, and the liver colour became beige (. The flow pressure and flow velocity in the main hepatic artery and portal vein were monitored using a flow pressure sensor and colour Doppler flow imaging, respectively, for 3 h. Our results showed that the intra-hepatic vessels were clear and regular, with smooth vascular walls. The flow pressure and flow velocity were relatively stable and within the normal range throughout the entire perfusion period (. Moreover, HE staining indicated limited local monocyte infiltration and hydropic degeneration. The parenchymal morphology and architecture of the livers remained intact throughout the 3-h perfusion. Based on this isolated porcine liver perfusion system, the effects of the perfusion flow rate in the HIFU treatment were investigated, as described in the following sections.
Figure 2. Grey-scale and colour Doppler ultrasound images of the perfused liver. (A) Grey-scale image after perfusion. The perfused liver parenchyma was homogeneously hypoechoic, the intrahepatic vessels were clear and regular, and the vascular wall was smooth. (B) Normal spectrum of the portal vein after perfusion with colour Doppler ultrasound imaging with a mean velocity of 14.7 cm/s.
Pulsed-HIFU ablation with varied duty cycle
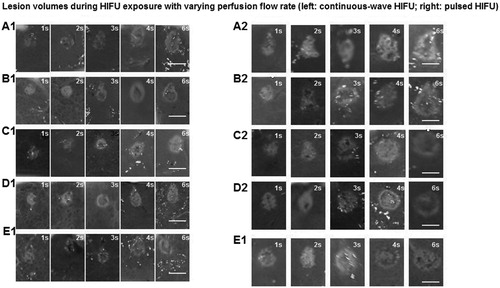
To investigate the effects of HIFU treatment with varying duty cycles, the livers were treated by continuous-wave HIFU or pulsed HIFU with no perfusion or at perfusion rates of 800 mL/min for 6 s. Gross observation showed that after the HIFU treatment, the target tissues became greyish-white, and the lesion region was regularly shaped with clear boundaries. Representative images of the macroscopic lesions induced by HIFU exposure are shown in , which included cross- and longitude-sections of the HIFU lesions for the pulsed and continuous-wave HIFU exposures.
Figure 3. Gross observation of HIFU ablation effects with different portal vein perfusion rates. (a) (A1-E1) Continuous-wave HIFU with no perfusion (A1) and with perfusion rates of 100 mL/min (B1), 200 mL/min (C1), 400 mL/min (D1) and 800 mL/min (E). (b) (A2-E2) Pulsed-HIFU with no perfusion (A2) and with perfusion rates of 100 mL/min (B2), 200 mL/min (C2), 400 mL/min (D2) and 800 mL/min (F2). Scale bar, 10 mm.
The lesion volume after HIFU exposure was measured. As shown in , the mean volume of the lesions was directly related to the DC of the HIFU. Compared to continuous-wave HIFU exposure, the mean volumes of lesion by exposure at DCs of 10 and 50% were significantly larger (P < .005) but were smaller at a DC of 5% (P < .005) (). The mean necrosis volume was significantly reduced with increasing perfusion rates for continuous-wave HIFU, but there was no significant change for exposure at a DC of 50%. These results suggest that pulsed HIFU treatment could significantly enhance the coagulation necrosis in the target regions.
Table 1. Changes in the necrosis volume in the target region for HIFU exposure with varying duty cycles between the reperfusion and non-reperfusion groups.
Table 2. Mean necrosis volume for HIFU exposure with varying perfusion flow rates.
Pulsed-HIFU ablation with varying perfusion flow rates
To investigate the effects of HIFU treatment with varying perfusion rates on isolated porcine livers, the livers were treated by continuous-wave HIFU or pulsed HIFU with no perfusion or with perfusion rates of 100 , 200 , 400 and 800 mL/min, respectively, for 1, 2, 3, 4 and 6 s. Gross observation revealed that after HIFU treatment, the target tissues became greyish-white, and the lesion region was regularly shaped with clear boundaries. Compared with continuous-wave HIFU treatment, pulsed HIFU induced coagulation necrosis with significant features. The necrotic region was characterised with bubble texture in the centre induced by cavitation/or boiling effects. The inertial cavitation effect may cause mechanical damage in the target region. Representative images of the macroscopic lesions induced by HIFU exposure are shown in , which included cross- and longitude-sections of the HIFU lesions for the pulsed and continuous-wave HIFU exposures.
Subsequently, the lesion volume was measured after HIFU exposure. Compared to continuous-wave HIFU treatment, there was a significant increase in the necrosis volume for pulsed HIFU (P < .05) (, . The mean necrosis volume was significantly reduced with increasing perfusion rates for each value of exposure duration (P < .05). Moreover, HE staining revealed that the HIFU treatment led to complete coagulation necrosis and/or cell cytolysis in the target regions (.
Figure 4. Changes in lesion volumes during HIFU exposure with varying perfusion flow rates. The isolated liver was subjected to HIFU exposure with an input power of 200 W (A) or an input power of 400 W at a duty cycle of 50% (B) for 1, 2, 3, 4, and 6 s. Necrosis volume decreased significantly with increased flow rates for 4 and 6 s after pulsed-HIFU exposure at 400 W (P < .05), while there was not a significant decrease for 1, 2, or 3 s after HIFU exposure (P > .05). Necrosis volume decreased significantly with increased flow rates after continuous-wave HIFU exposure at 200 W (P < .001) in comparison with different perfusion flows for 1, 2, 3, 4 and 6 s (P < .05). The errors bars in Figure 4(A) represent the standard deviation of 586 experiments on 25 different livers. The errors bars in Figure 4(B) represent the standard deviation of 603 experiments on 25 different livers. Compared with the no perfusion flow group at 200 W, *P < .05, **P < .01. Compared with the no perfusion flow group at 400 W, *P < .05
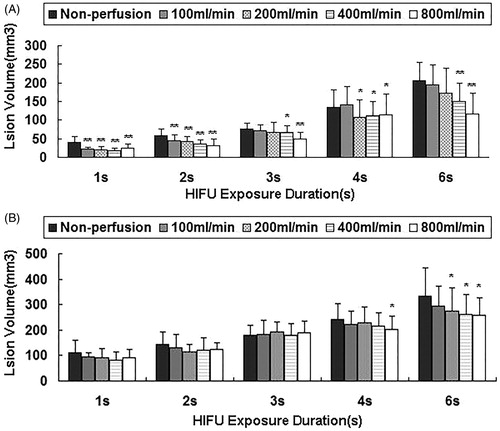
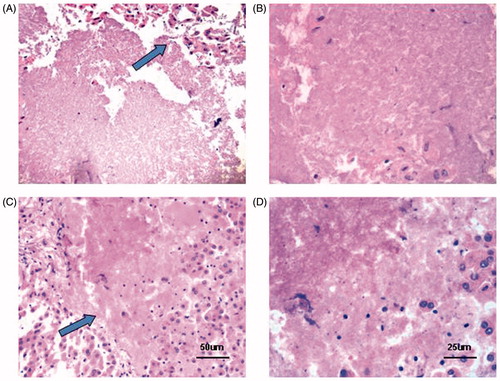
Figure 5. Histological changes after HIFU exposure. (A & B) Continuous-wave HIFU exposure (A, ×200; B, ×400). (C & D) Pulsed HIFU exposure (C, ×200; D, ×400). An abrupt boundary was observed between the treated region and the normal tissue (×200) (Arrowhead). Treated tissues showed pyknotic nuclei, karyolytic nuclei, or complete cell debris.
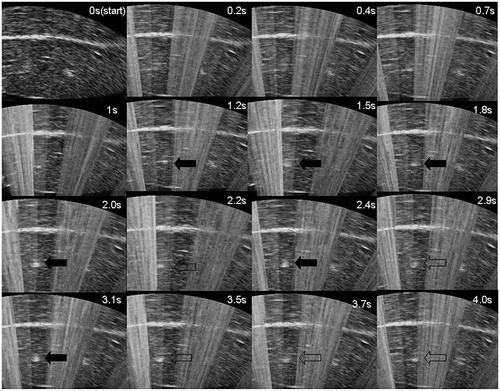
Changes in ultrasound imaging during pulsed HIFU exposure with different perfusion flow rates
To reduce the interference of HIFU on imaging, pulsed HIFU exposure was performed to aid the real-time ultrasound imaging recording. The grey scale first gradually increased, followed by a decreasing trend with a final increasing segment during the same exposure (. In addition, the perfusion flow rate influenced the time at which changes in the grey scale emerged. The time required for the emergence of the grey-scale change was delayed with increasing perfusion flow rate.
Figure 6. Representative real-time ultrasound images of the time evolution of a hyperechoic region (arrowhead) with 4 s of HIFU exposure during pulsed HIFU exposure at the perfusion flow of 800 mL/min. A bright hyper-echoic region can be observed on the US imaging at 1.2 s after HIFU exposure. During pulsed-HIFU exposure, there was a dynamic process by which the grey scale increased gradually (solid arrowhead), then decreased (empty arrowhead), and finally increased again.
Changes in inertial cavitation activities with varied perfusion flow rates
The PCD system was set up to monitor the inertial cavitation activities at the focus during HIFU exposure. In the Fourier spectrum of the radiofrequency signals, broadband noise was interpreted as inertial cavitation, and subharmonic signals were read as stable cavitation. Broadband noise and harmonics were deleted for both groups.
As shown in , significantly increased broadband and sub-harmonic signals were observed during HIFU exposure. Erratic changes referring to the rise and fall in the inertial cavitation level were also noted, with an overall increased cavitation level during HIFU exposure (). The ICD data for these two groups are given in . As illustrated in , compared with no flow, the ICD was significantly reduced with increasing perfusion rates for the continuous-wave HIFU exposure (P < .05). No significant decrease in the ICD was observed for the pulsed HIFU exposure with increasing perfusion flow rates (P > .05). Moreover, the ICD was stronger for the pulsed HIFU exposure (50%-DC) than for the continuous-wave HIFU exposure (100%-DC).
Figure 7. Representative images of the Fourier spectra of the radiofrequency signals (A) and typical time evolutions of inertial cavitation activity as a function of time for PCD signals (B) at the HIFU focus during exposures with varying perfusion flow rates.
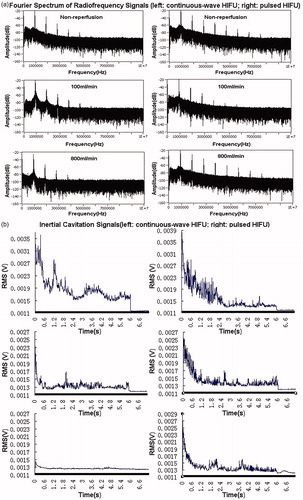
Figure 8. ICD during HIFU exposure with different perfusion flow rates. The isolated liver was subjected to HIFU exposure with an input power of 200 W (A) or with an input power of 400 W at a duty cycle of 50% (B) for 1, 2, 3, 4 and 6 s. Compared with the no perfusion flow group at 200 W, *P < .05.
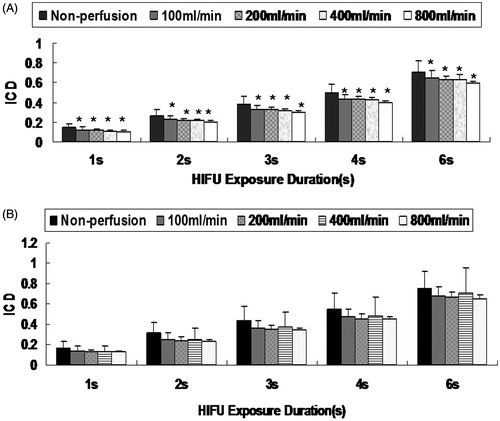
Table 3. ICD during HIFU exposure with varying perfusion flow rates.
Discussion
Compared with cavitation, the effects of heating are more repeatable and predictable, making it was the preferred mode in the early clinical application of HIFU [Citation25]. The preference of heating is now fading as clinical applications increase.
Studies have shown that inertial cavitation can enhance heating at the focus, and pulsed HIFU exposure can nucleate the cavitation activities and has been shown to produce obvious cavitation, which precedes the thermal effects and accelerates heating during coagulation necrosis [Citation26–28]. Inertial cavitation can induce mechanical tissue destruction and have some advantages over heat alone for ablation. Therefore, the combination of cavitation and thermal effects may be a solution to coagulate hypervascularized tissues [Citation29].
Compared with no flow perfusion, the blood flow and perfusion could simulate the main effects in the perfusion system, such as the eliminating effects of micro-bubbles induced by cavitation. These effects could also be confirmed because when compared with before perfusion, the liver parenchyma subjected to perfusion was associated with a homogeneous low echo. However, it remains unknown whether blood and perfusion flow can significantly influence cavitation activity during HIFU exposure. In the present study, the effects of the perfusion flow rate on cavitation activity and coagulated size during HIFU exposure were investigated in an isolated porcine liver perfusion system. Hepatic vessels have been haemodynamically characterised by the low pressure and high flow of the portal vein as well as the high pressure and low flow of the hepatic artery. In this study, the dual-vessel route of perfusion was selected to preserve the integrated microcirculation within the liver. Liver organs were kept functional even after 1 h of warm ischaemia and 4 h of normothermic extracorporeal perfusion [Citation30]. Freshly harvested livers were transported under hypothermic conditions for 30 min before the experiments were carried out under normothermic conditions. Although oxygenation was not taken into consideration in this study, normal organ function could be indirectly confirmed by various parameters, such as constant perfusion pressure, a histologically intact tissue structure, and bile production [Citation31–36].
The organ perfusion system consists of the harvested blood and saline solution, which is sufficient to meet the subsequent research requirements concerning thermal conduction and the elimination of micro-bubbles produced by cavitation due to perfusion flow, which was induced by the fluid in the major hepatic vessels; Therefore, this perfused liver model is clinically relevant in terms of possible thermal and cavitation features. Biological parameters remained steady during the experiments (1–1.5 h). And, no major circulatory changes could have influenced the shape and size of lesions.
We found that DC could directly influence the volume of the HIFU-induced lesion. For both no perfusion and reperfusion, there were significant differences in the lesion volumes between continuous-wave (100%-DC) exposure and 50%-DC, 10%-DC exposures, with the largest lesion observed for 50%-DC HIFU exposure. These results demonstrated that appropriate pulsed HIFU exposure can significantly increase the volume of lesion with the same total US energy.
Our results showed that for continuous-wave HIFU, significant differences in the necrosis volume were observed for exposure times of 1, 2, 3, 4 and 6 s, with increasing portal perfusion rates. In contrast, for pulsed HIFU, the necrosis volume was independent on the portal flow rates for exposure times of 1, 2 and 3 s.
The influence of varying perfusion rates on the inertial cavitation was further investigated for the pulsed HIFU and continuous-wave HIFU treatments with exposure times of 1, 2, 3, 4 and 6 s. Our results revealed significant influencing effects of the varying perfusion rates on inertial cavitation for continuous-wave HIFU. This may be caused by the eliminating effect of cavitation bubbles induced by perfusion flow, which weakened the cavitation activity during continuous-wave HIFU exposure and negatively influenced HIFU ablation. Therefore, the eliminating effect of cavitation bubbles might be involved in weakening the inertial cavitation. However, the perfusion rates did not have a significant effect on the inertial cavitation for pulsed HIFU. For the pulsed HIFU exposure, high-power pulses generated micro-bubbles that caused energy reflection, resulting in greater energy absorption and heating at the target, thereby enhancing the ablation. With increasing perfusion flow rates, there was no significant decrease in the inertial cavitation dose. Therefore, even with the same total energy, the ablated tissue volume could be enlarged in shorter treatment durations.
Unlike continuous-wave HIFU exposure in which the ultrasound waves were applied continuously over the exposure course, the pulsed HIFU exposure was characterised by ultrasound waves pulsed on and off during one exposure according to the working duty. Real-time ultrasound imaging can indicate the grey-scale changes in the target region, which first gradually increased, then decreased, and finally again increased. At the beginning of exposure, there were no significant grey-scale changes because the cavitation bubbles easily dissipated during the pulse-off duration. Then, the pulse exposure cycle gradually increased the cavitation bubbles and enhanced the grey-scale changes. Bubbles kept growing to the end of the pulse on time and did not completely dissolve during the pulse off time. They made up a cloud of pre-existing bubbles, which could serve as the cavitation nuclei for the next pulse-on cycle. These results suggest that there would be an optimum duty cycle in which the cavitation effect can be preferably enhanced to achieve an optimal synergistic effect. The time required for the emergence of the grey-scale change was longer with increasing portal vein flow rate. So an increased perfusion flow rate may decrease the cavitation activity and delay the emergence of grey-scale changes during HIFU.
According to our results, the role of cavitation in the formation of coagulation necrosis during HIFU exposure is very important, and enhancing treatment via the cavitation effect is promising. In some necrotic regions, a bubble texture in the centre of the lesion due to the cavitation or boiling effects induced by the rapid temperature elevation was observed in gross examination and HE staining. Our results may provide a reference for the clinical treatment of hyper-vascularized tumours. These findings are important for enhancing the efficacy of HIFU therapy in clinical treatments.
Conclusion
The effects of the perfusion flow on the volume of ablation lesions induced by HIFU exposure were investigated in an isolated liver perfusion system. Our results showed that the necrosis volume was significantly reduced, and the cavitation was dramatically weakened with increasing perfusion flow rates during continuous-wave HIFU exposure. On the other hand, the inertial cavitation activity during pulsed HIFU exposure was more intense, and the perfusion rate did not have a significant effect on the cavitation activity and the necrosis volume. Our results suggest that the inertial cavitation induced by pulsed HIFU exposure would enhance the ablation effect.
Disclosure statement
No potential conflicts of interest are disclosed. The authors alone are responsible for the content and writing of this paper.
Additional information
Funding
References
- Kennedy JE. (2005). High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer 5:321–7.
- Tempany CM, McDannold NJ, Hynynen K, Jolesz FA. (2011). Focused ultrasound surgery in oncology: overview and principles. Radiology 259:39–56.
- Wu F. (2006). Extracorporeal high intensity focused ultrasound in the treatment of patients with solid malignancy. Minim Invasive Ther Allied Technol 15:26–35.
- Thiburce AC, Frulio N, Hocquelet A, et al. (2015). Magnetic resonance-guided high-intensity focused ultrasound for uterine fibroids: mid-term outcomes of 36 patients treated with the Sonalleve system. Int J Hyperthermia 31:764–70.
- Chen L, ter Haar GR, Hill CR, et al. (1993). Effect of blood perfusion on the ablation of liver parenchyma with high-intensity focused ultrasound. Phys Med Biol 38:1661–73.
- Dorr LN, Hynynen K. (1992). The effects of tissue heterogeneities and large blood vessels on the thermal exposure induced by short high-power ultrasound pulses. Int J Hyperthermia 8:45–59.
- Kolios MC, Sherar MD, Hunt JW. (1995). Large blood vessel cooling in heated tissues: a numerical study. Phys Med Biol 40:477–94.
- Kolios MC, Sherar MD, Hunt JW. (1996). Blood flow cooling and ultrasonic lesion formation. Med Phys 23:1287–98.
- Yang R, Sanghvi NT, Rescorla FJ, Kopecky KK, Grosfeld JL. (1993). Liver cancer ablation with extracorporeal high-intensity focused ultrasound. Eur Urol 23:17–22.
- Couret LN, Miller NR, Rivers IH, et al. (2003). Experimental methodologies for studying the effects of perfusion on high intensity focused ultrasound (HIFU). Ultrasonics, IEEE Symposium 1:142–5.
- Huang HW, Shih TC, Liauh CT. (2010). Predicting effects of blood flow rate and size of vessels in a vasculature on hyperthermia treatments using computer simulation. Biomed Eng Online 26:18–38.
- Wu F, Wang ZB, Chen WZ, et al. (2005). Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology 235:659–67.
- Smart OC, Hindley JT, Regan L, Gedroyc WG. (2006). Gonadotrophin-releasing hormone and magnetic-resonance-guided ultrasound surgery for uterine leiomyomata. Obstet Gynecol 108:49–54.
- Murase E, Siegelman ES, Outwater EK, et al. (1999). Uterine leiomyomas: histopathplogic features, MR imaging findings, differential diagnosis and treatment. Radiographics 19:1179–97.
- Yamashita Y, Torashima M, Takahashi M, et al. (1993). Hyperintense uterine leiomyoma at T2-weighted MR imaging: differentiation with dynamic enhanced MR imaging and clinical implications. Radiology 189:721–5.
- Funaki K, Fukunishi H, Funaki T, et al. (2007). Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol 196:184.e1–.e6.
- Lenard ZM, McDannold NJ, Fennessy FM, et al. (2008). Uterine leiomyomas: MR imaging-guided focused ultrasound surgery-imaging predictors of success. Radiology 249:187–94.
- Machtinger R, Inbar Y, Cohen-Eylon S, et al. (2012). MR-guided focus ultrasound (MRgFUS) for symptomatic uterine fibroids: predictors of treatment success. Hum Reprod 27:3425–31.
- Trumm CG, Stahl R, Clevert DA, et al. (2013). Magnetic resonance imaging-guided focused ultrasound treatment of symptomatic uterine fibroids: impact of technology advancement on ablation volumes in 115 patients. Invest Radiol 48:359–65.
- Zhao WP, Chen JY, Zhang L, et al. (2013). Feasibility of ultrasound-guided high intensity focused ultrasound ablating uterine fibroids with hyperintense on T2-weighted MR imaging. Eur J Radiol 82:e43–9.
- McLaughlan J, Rivens I, Leighton T, ter Haar G. (2010). A study of bubble activity generated in ex vivo tissue by high intensity focused ultrasound. Ultrasound Med Biol 36:1327–44.
- Zhou YF, Gao XW. (2013). Variations of bubble cavitation and temperature elevation during lesion formation by high-intensity focused ultrasound. J Acoust Soc Am 134:1683–94.
- Coussios CC, Farny CH, Haar GT, Roy RA. (2007). Role of acoustic cavitation in the delivery and monitoring of cancer treatment by high-intensity focused ultrasound (HIFU). Int J Hyperthermia 23:105–20.
- Chen WS, Brayman AA, Matula TJ, Crum LA, Miller MW. (2003). The pulse length-dependence of inertial cavitation dose and hemolysis. Ultrasound Med Biol 29:739–48.
- Kennedy JE, ter Haar GR, Cranston D. (2003). High intensity focused ultrasound: surgery of the future? Br J Radiol 76:590–9.
- Kieran L, Hall TL, Parsons JE, et al. (2006). Exploring the acoustic parameter space in ultrasound therapy: defining the threshold for cavitational effects. 6th International Symposium on Therapeutic Ultrasound; 2006 Aug 30-Sep 2; Oxford, UK, 185–90.
- Robert WW, Hall TL, Ives K, et al. (2006). Pulsed cavitational ultrasound: a noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J Urol 175:734–8.
- Hall TL, Kieran K, Fowlkes JB, et al. (2006). Temporal trends in the histology of the rabbit kidkey after cavitational tissue ablation. 6th International Symposium on Therapeutic Ultraosund; 2006. 30 August – 2 September 2; Oxford, UK, 191–7.
- Melodelima D, Chapelon JY, Theillère Y, et al. (2004). Combination of thermal and cavitation effects to generate deep lesions with an endocavitary applicator using a plane transducer: ex vivo studies. Ultrasound Med Biol 30:103–11.
- Bell R, Ross Shiel AG, Dolan P, et al. (1993). The evaluation of the isolated perfused liver as a model for the assessment of liver preservation. Aust N Z J Surg 63:44–52.
- Areflev A, Prat F, Chapelon JY, et al. (1998). Ultrasound-induced tissue ablation: studies on isolated, perfused porcine liver. Ultrasound Med Biol 24:1033–43.
- Yagi S, Iida T, Hori T, et al. (2012). Effect of portal haemodynamica on liver graft and intestinal mucosa after small-for-size liver transplantation in swine. Eur Surg Res 48:163–70.
- Gringeri E, Polacco M, D’amico FE, et al. (2011). A new liver autotransplantation technique using subnormothermic machine perfusion for organ preservation in a porcine model. Transplant Proc 43:997–1000.
- Carrano A, Gringeri EF, Violi P, et al. (2007). A new experimental model of isolated perfused pig liver to support acute hepatic failure. Transplant Proc 39:2028–30.
- Monbaliu D, Brassil J. (2010). Machine perfusion of the liver: past, present and future. Curr Opin Organ Transplant 15:160–6.
- Vairetti M, Ferrigno A, Carlucci F, et al. (2009). Subnormothermic machine perfusion protects steatotic livers against preservation injury: a potential for donor pool increase. Liver Transpl 15:20–9.
- Nagel S, Hegemann O, Groneberg DA, Christian-Siestrup C. (2005). An improved model of isolated hemoperfused porcine livers using pneumatically driven pulsating blood pumps. Toxicol Pathol 33:434–40.
