Abstract
Purpose: The aim of this study was to compare the results of microwave ablation (MWA) and hepatic resection (HR) when combined with pericardial devascularisation plus splenectomy (PCDV) for the treatment of patients with cirrhosis complicated by small hepatocellular carcinoma (HCC) and oesophageal variceal bleeding (EVB).
Materials and methods: Between 2001 and 2013, 73 patients (median age 53.2 years, 67% male) with small HCC and concomitant EVB who underwent MWA or HR for HCC and PCDV for cirrhotic portal hypertension were selected retrospectively for inclusion in this study. The overall survival curves and recurrence-free survival curves were calculated using the Kaplan–Meier method and compared using log-rank tests. Multivariate analysis was performed using the Cox regression model.
Results: The 1-, 3- and 5-year overall survival rates were 95.2%, 71.4% and 38.1% and 96.7%, 53.3% and 43.3% for the HR and MWA groups, respectively; these did not differ significantly between the two groups. However, patients in the HR group had more post-operative complications (52.3% vs. 13.7%; p = 0.002). Multivariate analysis identified albumin and bilirubin levels and tumour size to be statistically significant and independent prognostic factors for overall survival, while BCLC stage was associated with poor recurrence-free survival. Furthermore, albumin levels were shown to be an independent predictive factor for post-operative complications.
Conclusions: For patients with small HCC and concomitant EVB, MWA plus PCDV may reduce the incidence of post-operative complications relative to and provide similar therapeutic benefits as HR plus PCDV, especially for patients with low albumin levels.
Introduction
Hepatocellular carcinoma (HCC) is currently the fifth most prevalent cancer and third leading cause of cancer-related mortality worldwide [Citation1]. Despite advances in treatment, such as the introduction of the angiogenesis inhibitor, sorafenib, for advanced stage HCC [Citation2,Citation3], the main curative modalities for HCC are still resection, transplantation and percutaneous ablation. HCC usually arises in the setting of chronic cirrhotic disease [Citation4] and is associated with portal hypertension in most cases. HCC has become the most prevalent complication of cirrhotic disease and is the leading cause of death in patients with initially compensated cirrhosis [Citation5]. The presence of clinically relevant portal hypertension (CSPH) in cirrhotic patients with HCC, assessed by either hepatic venous pressure gradient measurement or clinical parameters, is included in a staging classification model [Citation6]. The presence of oesophageal varices (EV) is used commonly as a marker of CSPH [Citation7,Citation8]. Cirrhotic patients with EV have been found to have a 25% greater risk of mortality due to haemorrhage than those without EV [Citation9]. The presence of oesophageal varices should be taken into account in the therapeutic work-up of HCC patients. However, the Barcelona group identified CSPH as the most powerful predictor of post-operative liver decompensation and poor outcomes in cirrhotic patients with Child-Pugh classification A submitted for hepatic resection [Citation6,Citation10]. As a consequence of these studies, the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) guidelines consider portal hypertension to be a relative contraindication to HR because of the high risk of postoperative liver decompensation [Citation11,Citation12]. However, accumulating evidence suggests that portal hypertension should not be considered an absolute contraindication to hepatectomy in cirrhotic patients [Citation13,Citation14]. Patients with portal hypertension have outcomes similar to patients with normal portal pressure [Citation15–17]. Indeed, as far as prognosis is concerned, the presence of gastroesophageal varices seems to be especially important in identifying HCC patients who are candidates for surgery [Citation10]. Moreover, HCC can lead to worsening portal hypertension, and portal hypertension can lead to worsening HCC [Citation18].
In the past two decades, tumour ablation therapies have been recommended as alternatives to hepatic resection and considered a potentially curative treatment in properly selected candidates [Citation12,Citation19]. Among these therapies, radiofrequency ablation (RFA) and microwave ablation (MWA) has been the most commonly used modalities [Citation20–22]. MWA is common in China and Japan and can provide a similar efficacy to RF ablation [Citation23]. It has been reported that patients who underwent MWA for HCC ≤5 cm had 5-year survival rates of 29–68.6% [Citation24]. MWA appears to be a safe and effective treatment for HCC adjacent to the gallbladder [Citation25,Citation26]. MWA assisted by artificial ascites can also be applied to tumours abutting the gastrointestinal tract [Citation27].
Pericardial devascularisation plus splenectomy (PCDV) remains a viable therapeutic option for treating cirrhotic patients with PHT and oesophageal variceal bleeding [Citation28,Citation29]. Unlike endoscopic and transjugular intrahepatic portosystemic shunt (TIPS) procedures, PCDV has been found to be associated with significant reductions in encephalopathy and diminished rebleeding rates. In cirrhotic patients with HCC, oesophageal variceal bleeding (EVB) as a complication of portal hypertension often requires therapeutic measures to be taken and is an important determinant of patient prognosis [Citation7]. Therefore, simultaneous partial hepatic resection or local ablation and direct interruption procedures, such as PCDV, for gastroesophageal varices are the preferred forms of treatment for these patients [Citation30,Citation31].
To our knowledge, there has been almost no authoritative clinical evidence of MWA plus PCDV for the treatment of HCC complicated by EVB in cirrhotic patients. Therefore, this study compared the effectiveness and clinical outcomes of MWA and HR plus PCDV and aimed to determine the more appropriate mode of treatment for HCC patients with EVB resulting from cirrhotic portal hypertension.
Materials and methods
Patients
Between December 2001 and December 2013, 73 eligible patients (median age 53.2 years, 67% male) diagnosed with HCC complicated by EVB resulting from cirrhotic portal hypertension were enrolled in this retrospective study. Patients underwent combined therapy with either MWA and PCDV (n = 31) or HR and PCDV (n = 42). HR or MWA was performed on patients with HCCs within the Milan criteria: that is, a solitary HCC up to 5 cm in diameter or no more than three tumour nodules each 3 cm or less in diameter. In general, patients were offered both treatment options. The potential advantages and disadvantages of both treatments were fully explained, and the patients made the decision of which treatment option they preferred. However, for those with a centrally located tumour, our team preferred to offer MWA treatment.
For all patients enrolled, HCC diagnosis was based on AASLD radiological criteria [Citation11,Citation12] or histology. All patients were submitted to complete liver function tests (ALT, AST, GGT, bilirubin, and albumin levels and prothrombin time); blood count; chest X-rays; liver ultrasound; esophagogastroduodenoscopy (EGD) and abdominal triple-phase CT or/and contrast-enhanced MR. Oesophageal varices were confirmed to be present in all patients by EGD. The oesophageal variceal grading system used in this study was based on occupancy of the radius of the endoscopic field as described by the North Italian Endoscopy Club (NIEC) for EGD (0 = no varices; 1 = small and with an estimated size <1/3 of the radius; 2 = medium, with an estimated size between 1/3 and 2/3 of the radius and, 3 = large, with an estimated size >2/3 of the radius) [Citation32].
The study was approved by the Qilu Hospital of Shandong University Ethics Committee, and written informed consent for recording and analysis of data was obtained from all patients.
Surgical and microwave ablation procedures
PCDV was performed using the modified Hassab procedure [Citation33]. Briefly, a standard extended left subcostal incision 18–30 cm in length was made, and after the abdomen was opened, the splenic artery was divided and ligated in the conventional manner. The ligaments surrounding the spleen and secondary branches of the splenic pedicle were then dissected and ligated one by one, followed by splenectomy. PCDV then was performed with sequential ligation to devascularise the upper two-thirds of the vessels of both the lesser and greater curvatures of the stomach, including the left gastro-epiploic, short gastric and left gastric veins. The retrogastric venous collaterals running from the upper border of the pancreas to the gastroesophageal junction were meticulously divided and ligated. The lower 5 cm of the oesophagus was devascularised via the transhiatal approach by sequential ligation.
In the hepatic resection group, hepatic resection was performed directly following completion of PCDV. The incision was extended subcostally to the right side and 15–18 cm in length, and partial hepatectomy was performed in a standardised fashion with a resection margin of at least 1 cm around the tumour.
In the MWA group, all patients underwent PCDV first and received MWA with ultrasonographic guidance 7–14 days later. For microwave ablation, MW generators (FORSEA MTC-3; Forsea Microwave & Electronic Research Institute, Nanjing, China) with 14-gauge, flexible coaxial cables and cooled shaft antennae were used. The machine was capable of producing 2450 MHz and 1–120 W. After the patients were given local anaesthesia with 1% lidocaine, the antennae were percutaneously inserted into the tumours under ultrasound (US) guidance. Then, MW emission was initiated, and the region of ablation was monitored by US. During the MWA process, the output of power was 40–60 W for 5–10 min per session. Finally, when the antennae were withdrawn, the applicator track was heated with sufficient microwave energy by stopping the cooling-shaft water dump.
Post-operative follow-up
Perioperative mortality was defined as death that occurred within a month after treatment. Follow-up was initiated the day of HR or MWA, based on 3-month US and serum AFP assay results, and ended at the patient’s death or last visit. The vast majority of patients underwent annual dynamic CT or MRI scan for follow-up.
Statistical analysis
The primary outcome was overall survival. Liver decompensation was defined by the presence of ascites, acute encephalopathy and/or jaundice (bilirubin level greater than 3 mg/dl on post-operative biochemical examination). The follow-up time was defined as the number of months from surgical or ablation treatment of HCC to death or last contact with the patient. Recurrence-free survival was computed from the day of surgery to the day of evidence of any recurrence (local, regional or distant) or death due to any cause. If recurrence was not diagnosed, patients were censored on the date of death or last follow up. Data following a normal distribution were expressed as the mean and standard deviation; if the data were nonparametric, median and range are reported. Univariate associations between each parameter and survival rate were estimated by comparing actuarial curves (Kaplan–Meier product-limit method and log-rank test) to more accurately characterise the final outcomes. Relative hazards (Cox regression-based test) were used to evaluate the weight of each subgroup in determining significance. A multivariate stepwise logistic regression model was used to assess the risk of post-operative complication development.
Results
Patients’ characteristics
Of the 73 patients diagnosed with HCC complicated by oesophageal varices bleeding, 42 received HR and simultaneous PCDV, whereas the other 31 underwent PCDV followed by MWA 1–2 weeks later. Typical MRI and CT images are shown in . Demographic and clinical characteristics of the patients are summarised in . Patient age, gender, Child-Pugh classification, severity of variceal grading and BCLC staging were statistically similar between the two groups. Of note, seven (16.7%) patients in the HR group were admitted because of bleeding, whereas four (12.9%) patients in MWA group who received conservative therapies first and attained haemostasis were admitted. While 23 (54.8%) patients in the HR group had history of intermittent haematemesis or haematochezia, only 19 (61.3%) patients in MWA group had a similar history. Faecal occult blood was detected in patients in both groups. No emergency surgery was necessary for patients in either group. Four patients lost follow-up in two groups and the status of these patients was considered as censored. Typical MRI and CT images are shown in .
Table 1. Baseline characteristics of patients undergoing MWA or HR for HCC with oesophageal variceal bleeding.
Complications and survival
One patient in the HR group died 7 days after the procedure as a result of perioperative irreversible liver failure resulting in a 30-day mortality rate of 2%; however, this was not significantly different than the mortality rate in the MWA group, which had no treatment-related hospital mortality. Postoperative complications in the HR group included massive ascites (12), pleural effusion (4), portal vein thrombosis (3), inferior diaphragmatic effusion (2) and cardiopulmonary insufficiency (1). Complications in the MWA group included mild ascites (3) and pleural effusion (1). The overall complication rate was 47.6% in the HR group and 12.9% in the MWA group (p = .002) (). Albumin levels were found to be significantly associated with the development of postoperative complications in the logistic regression model with an odds ratio of 0.768 (p < .01) ().
Table 2. Post-operative results in two groups.
Table 3. Logistic analyses of factors associated with post-complications.
The 1-, 3- and 5-year overall survival rates were 95.2%, 71.4%, 38.1% and 96.7%, 53.3%, 43.3% for the HR and MWA groups, respectively. There were no significant differences between the two groups in overall and recurrence-free survival rates (). The most frequently identified causes of death were tumour progression and liver failure. No patient died due to rebleeding of EV. Forty-seven patients developed local or regional recurrence, including twenty-nine cases in HR group and eighteen cases in MWA group. One case with single hepatic nodule underwent surgical resection. Nine cases with single hepatic nodule underwent local ablation including six cases with microwave ablation and three cases with radio-frequency ablation. Twelve cases with multiple hepatic nodules underwent TACE. Other patients had no additional treatment due to tumour recurrence complicated by hepatic insufficiency. The median survival times of BCLC-A and BCLS-B patients were 61 and 36 months, respectively; this difference was statistically significant (). Interestingly, BCLC-B patients in the HR group had better survival than those in the MWA group; however, there was no significant difference between the groups for BCLC-A patients (). Multivariate analysis with Cox’s regression confirmed that levels of plasma albumin and bilirubin and tumour size were associated with survival, while BCLC stage was associated with poor RFS ().
Figure 1. Typical images showing an HCC nodule treated with MWA or HR and CSPH treated with PCDV. (A) Preoperative contrast enhanced venous phase MR image (T1W1) of EV (arrow). (B) Pre-operative T1 weighted image (T1WI) of an HCC nodule (arrow). (C) Post-operative contrast enhanced arterial phase CT image of the same patient who was treated with MWA and PCDV and no enhancement was observed in the ablation zone (arrow). (D) Pre-operative contrast enhanced venous phase CT image of EV (arrow). (E) Pre-operative contrast enhanced venous phase CT image of an HCC nodule (arrow). (F) Post-operative plain CT image of the operative region of the same patient who was treated with HR and PCDV (arrow).
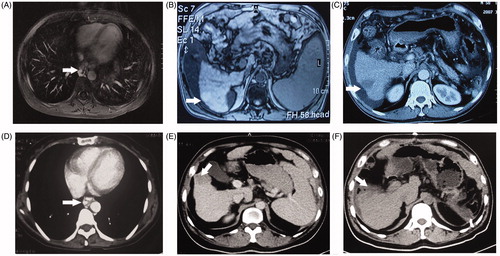
Figure 2. Overall survival and recurrence free survival curves for the entire study population of HCC patients with EVB who underwent MWA or HR for HCC combined with PCDV for EVB. There were no significant differences between the HR and MWA groups in terms of overall and recurrence-free survival rates.
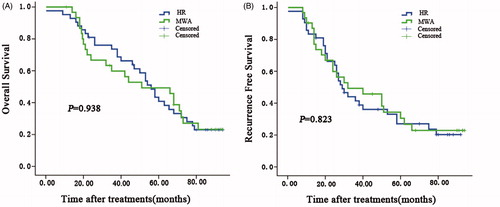
Figure 3. Overall and recurrence-free survival curves for patients undergoing MWA or HR for HCC complicated by EVB by BCLC stage. BCLC-A patients had better overall and recurrence free survival than BCLC-B patients (p < .01).
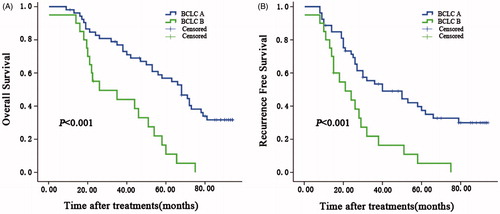
Figure 4. Overall survival curves for subgroup analysis of patients by BCLC stage. The curves show overall survival for patients treated with HR or MWA in the (A) BCLC-A and (B) BCLC-B groups. BCLC-B patients in the HR group had better survival than those in the MWA group, and there was no significant difference between the groups for BCLC-A patients.
Table 4. Univariate and multivariate analyses of factors associated with survival and recurrence.
Discussion
Our results demonstrated that MWA or HR combined with PCDV were similarly effective in the treatment of patients enrolled in this study. Fewer postoperative complications were identified in patients who underwent MWA combined with PCDV than patients who underwent HR therapy; however, both treatments provided similar survival outcomes. To the best of our knowledge, there have been no prior published studies investigating the effectiveness of MWA and HR when combined with PCDV for the treatment of HCC patients with concomitant EVB.
HCC patients with EV had significantly shorter survival times compared to those without EV; in another study, 12.3% of patients with large and medium EVs died from bleeding varices [Citation7]. Therefore, the presence of CSPH or EV in cirrhotic patients often requires prophylactic measures to prevent bleeding [Citation9]. A multicentre case study found that secondary prophylaxis could offer survival benefit in patients with HCC and variceal bleeding [Citation34]. The results of our study suggest patients benefitted from combined therapy of both HCC and oesophageal varices bleeding because the main causes of death were tumour recurrence and liver failure within our patients, while death as a result of variceal bleeding was not observed in either the HR group or the MWA group. However, high rebleeding rates caused by recurrent varices and high rates of residual varices and changes in gastric mucosa following PCDV have been observed in other studies [Citation35–37]. In our study, no rebleeding episodes were observed over the 5-year follow-up period, which might be attributed to the short follow-up interval. Nevertheless, portal hypertension is, according to EASL and AASLD guidelines, considered to be a relative contraindication for HR due to the high risk of post-operative liver failure [Citation11,Citation12]. HCC patients with EVB have been found to have similar short- and long-term outcomes as patients without EVB or CSPH when resection of two or fewer segments was performed [Citation13,Citation14,Citation17,Citation38–40]. No mortality was observed in the MWA plus PCDV group, while one perioperative death was observed in the HR plus PCDV group in this study. Therefore, our findings support the benefit of eradication of EV in cirrhotic HCC patients.
Although RFA has been the most widely used local ablative modality for liver tumours, a similar efficacy between RF ablation and MWA for local tumour control, complication rates, and long-term survival in patients with small HCC has been identified, and an apparent superiority of MWA was found for larger neoplasms [Citation23,Citation41]. MWA therapy may allow for larger ablation zones, higher intratumoral temperature [Citation42], and compensation for defects resulting from the heat-sink effect caused by large vessels [Citation43]. Moreover, MWA therapy can be performed in tumours larger than 4 cm with a complete ablation rate of more than 90% [Citation44]. In this study, for patients where nodules were adjacent to large vessels, MWA therapy was associated with better outcomes than those obtained from RFA therapy. We observed that the therapeutic effect of MWA therapy for HCC was similar to that of HR in HCC patients with EVB. Patients with HCC complicated by EVB survived a median of 46 months, and there was no significant difference in the overall or recurrence free survival between the two groups. Meanwhile, MWA therapy dramatically reduced the overall postoperative complications observed in HR therapy.
In the present study, the median survival of BCLC-A and BCLC-B patients was 63 and 39 months, respectively. In the BCLC-A group, the two treatment modalities had a similar influence on patient survival. However, HR therapy was associated with better outcomes than MWA therapy in the BCLC-B group, which may be attributed to the small number of BCLC-B patients in our study.
The most interesting aspect of our study was that MWA with PCDV proved to be a safe and effective therapy even for patients with mildly impaired liver function. Judging from the results of our study, preoperative serum albumin level was a negative prognostic factor for postoperative complications; therefore, preoperative albumin levels should be taken into account during the treatment selection process.
Of note, 18 patients in the MWA group in our study developed local progression or recurrence, which might be attributed, in part, to incomplete ablation or untreated lesions. At present, contrast-enhanced ultrasound (CEUS) is always performed intraprocedurally immediately following ablation, and CEUS-guided targeted retreatment is performed during the same treatment session when incomplete ablation is detected [Citation45,Citation46]. Virtual navigation and fusion imaging between ultrasonography and CT, MRI or PET images have been reported as effective ways to successfully perform percutaneous ablations of lesions with full conspicuity [Citation47,Citation48]. We aspire to apply similar fusion imaging systems for the treatment of lesions undetectable with conventional ultrasound or CEUS.
This study had some limitations. First, the data were obtained from a single centre over a long time interval (almost 15 years), which might lead to variable results in complete ablation rates. A multicentre study with a larger number of patients should be performed. Second, the retrospective nature of this study precludes a uniform approach to follow-up, which might have influenced the evaluation of clinical outcomes. Therefore, we plan to carry out a prospective multi-institutional trial with clinical validation models.
In conclusion, our findings indicate that HCC patients with concomitant EVB could be considered for HR or MWA with simultaneous PCDV, while patients with abnormal levels of albumin should be referred for MWA plus PCDV. According to the results from our study, we conclude that MWA combined with PCDV is a better choice for HCC patients with EVB.
| Abbreviations | ||
| AASLD | = | American Association for the Study of Liver Diseases |
| BCLC | = | Barcelona Clinic Liver Cancer |
| CEUS | = | Contrast-enhanced ultrasound |
| CSPH | = | Clinically significant portal hypertension |
| EASL | = | European Association for the Study of the Liver |
| EGD | = | Oesophagogastroduodenoscopy |
| EVB | = | Oesophageal variceal bleeding |
| HCC | = | Hepatocellular carcinoma |
| HR | = | Hepatic resection |
| HVPG | = | Hepatic venous pressure gradient |
| MELD | = | Model for end-stage liver disease |
| MWA | = | Microwave ablation |
| PCDV | = | Pericardial devascularisation plus splenectomy |
| PHTN | = | Portal hypertension |
| TACE | = | Transcatheter hepatic arterial chemoembolisation |
| TIPS | = | Transjugular intrahepatic portosystemic shunt |
| US | = | Ultrasound |
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Jemal A, Siegel R, Ward E, et al. (2009). Cancer statistics, 2009. CA Cancer J Clin 59:225–49.
- Cheng AL, Kang YK, Chen Z, et al. (2009). Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10:25–34.
- Llovet JM, Ricci S, Mazzaferro V, et al. (2008). Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–90.
- Stroffolini T, Andreone P, Andriulli A, et al. (1998). Characteristics of hepatocellular carcinoma in Italy. J Hepatol 29:944–52.
- Benvegnu L, Gios M, Boccato S, Alberti A. (2004). Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut 53:744–9.
- Llovet JM, Bru C, Bruix J. (1999). Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 19:329–38.
- Giannini EG, Risso D, Testa R, et al. (2006). Prevalence and prognostic significance of the presence of esophageal varices in patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol 4:1378–84.
- Ueno S, Tanabe G, Nuruki K, et al. (2002). Prognosis of hepatocellular carcinoma associated with child class B and C cirrhosis in relation to treatment: a multivariate analysis of 411 patients at a single center. J Hepatobiliary Pancreat Surg 9:469–77.
- Jensen DM. (2002). Endoscopic screening for varices in cirrhosis: findings, implications, and outcomes. Gastroenterology 122:1620–30.
- Bruix J, Castells A, Bosch J, et al. (1996). Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 111:1018–22.
- Bruix J, Sherman M. (2011). Management of hepatocellular carcinoma: an update. Hepatology 53:1020–2.
- Bruix J, Sherman M. Practice Guidelines Committee AAftSoLD. (2005). Management of hepatocellular carcinoma. Hepatology 42:1208–36.
- Capussotti L, Ferrero A, Vigano L, et al. (2006). Portal hypertension: contraindication to liver surgery? World J Surg 30:992–9.
- Kawano Y, Sasaki A, Kai S, et al. (2008). Short- and long-term outcomes after hepatic resection for hepatocellular carcinoma with concomitant esophageal varices in patients with cirrhosis. Ann Surg Oncol 15:1670–6.
- Santambrogio R, Kluger MD, Costa M, et al. (2013). Hepatic resection for hepatocellular carcinoma in patients with Child-Pugh's A cirrhosis: is clinical evidence of portal hypertension a contraindication? HPB (Oxford) 15:78–84.
- Cucchetti A, Ercolani G, Vivarelli M, et al. (2009). Is portal hypertension a contraindication to hepatic resection? Ann Surg 250:922–8.
- Capussotti L, Ferrero A, Vigano L, et al. (2009). Liver resection for HCC with cirrhosis: surgical perspectives out of EASL/AASLD guidelines. Eur J Surg Oncol 35:11–15.
- Tandon P, Garcia-Tsao G. (2006). Portal hypertension and hepatocellular carcinoma: prognosis and beyond. Clin Gastroenterol Hepatol 4:1318–19.
- Llovet JM, Burroughs A, Bruix J. (2003). Hepatocellular carcinoma. Lancet 362:1907–17.
- EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. (2012). J Hepatol 56:908–43.
- Livraghi T, Solbiati L, Meloni MF, et al. (2003). Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 226:441–51.
- Dong B, Liang P, Yu X, et al. (2003). Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: results in 234 patients. AJR Am J Roentgenol 180:1547–55.
- Facciorusso A, Di Maso M, Muscatiello N. (2016). Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia32:339–44.
- Liang P, Dong B, Yu X, et al. (2003). Prognostic factors for percutaneous microwave coagulation therapy of hepatic metastases. AJR Am J Roentgenol 181:1319–25.
- Huang H, Liang P, Yu XL, et al. (2015). Safety assessment and therapeutic efficacy of percutaneous microwave ablation therapy combined with percutaneous ethanol injection for hepatocellular carcinoma adjacent to the gallbladder. Int J Hyperthermia 31:40–7.
- Li M, Yu X, Liang P, et al. (2015). Ultrasound-guided percutaneous microwave ablation for hepatic malignancy adjacent to the gallbladder. Int J Hyperthermia 31:579–587.
- Zhang D, Xie D, Wei X, et al. (2014). Microwave ablation of the liver abutting the stomach: insulating effect of a chitosan-based thermosensitive hydrogel. Int J Hyperthermia 30:126–33.
- Yin L, Liu H, Zhang Y, Rong W. (2013). The surgical treatment for portal hypertension: a systematic review and meta-analysis. ISRN Gastroenterol 2013:464053. doi: 10.1155/2013/464053.
- Yang L, Yuan LJ, Dong R, et al. (2013). Two surgical procedures for esophagogastric variceal bleeding in patients with portal hypertension. World J Gastroenterol 19:9418–24.
- Higashi H, Matsumata T, Utsunomiya T, et al. (1993). Successful treatment of early hepatocellular carcinoma and concomitant esophageal varices. World J Surg 17:398–402.
- Harada A, Nonami T, Nakao A, et al. (1996). Surgical treatment for hepatocellular carcinoma and concomitant esophagogastric varices. Semin Surg Oncol 12:193–6.
- North Italian Endoscopic Club for the S, Treatment of Esophageal V. (1988). Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med 319:983–9.
- Wu YK, Wang YH, Tsai CH, et al. (2002). Modified Hassab procedure in the management of bleeding esophageal varices–a two-year experience. Hepatogastroenterology 49:205–7.
- Ripoll C, Genesca J, Araujo IK, et al. (2013). Rebleeding prophylaxis improves outcomes in patients with hepatocellular carcinoma. A multicenter case-control study. Hepatology 58:2079–88.
- Chen WC, Lo GH, Lai KH, et al. (2004). Development of hepatocellular carcinoma after successful management of esophageal variceal bleeding. J Chin Med Assoc 67:557–64.
- Thuluvath PJ, Yoo HY. (2002). Portal hypertensive gastropathy. Am J Gastroenterol 97:2973–8.
- Ohta M, Yamaguchi S, Gotoh N, Tomikawa M. (2002). Pathogenesis of portal hypertensive gastropathy: a clinical and experimental review. Surgery 131:S165–S70.
- Ruzzenente A, Valdegamberi A, Campagnaro T, et al. (2011). Hepatocellular carcinoma in cirrhotic patients with portal hypertension: is liver resection always contraindicated? World J Gastroenterol 17:5083–8.
- Choi GH, Park JY, Hwang HK, et al. (2011). Predictive factors for long-term survival in patients with clinically significant portal hypertension following resection of hepatocellular carcinoma. Liver Int 31:485–93.
- Ishizawa T, Hasegawa K, Aoki T, et al. (2008). Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology 134:1908–16.
- Huang Q, Yang H, Lin QN, Qin X. (2016). 'Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis': two issues should be noted. Int J Hyperthermia 32:345. doi: 10.1080/02656736.2016.1257824.
- Qian GJ, Wang N, Shen Q, et al. (2012). Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol 22:1983–90.
- Wright AS, Sampson LA, Warner TF Jr, et al. (2005). Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 236:132–9.
- Kuang M, Lu MD, Xie XY, et al. (2007). Liver cancer: increased microwave delivery to ablation zone with cooled-shaft antenna-experimental and clinical studies. Radiology 242:914–24.
- Pescatori LC, Sconfienza LM, Mauri G. (2016). The role of contrast-enhanced ultrasonography in image-guided liver ablations. Ultrasonography 35:87–8.
- Mauri G, Porazzi E, Cova L, et al. (2014). Intraprocedural contrast-enhanced ultrasound (CEUS) in liver percutaneous radiofrequency ablation: clinical impact and health technology assessment. Insights Imaging 5:209–16.
- Mauri G, Cova L, De Beni S, et al. (2015). Real-time US-CT/MRI image fusion for guidance of thermal ablation of liver tumors undetectable with US: results in 295 cases. Cardiovasc Intervent Radiol 38:143–51.
- Mauri G. (2015). Expanding role of virtual navigation and fusion imaging in percutaneous biopsies and ablation. Abdomin Imaging 40:3238–9.
