Abstract
Introduction: Peritoneal carcinomatosis (PC) is increasingly being treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). We provide a review of a high-volume Asian institute's experience and survival outcomes with this procedure.
Methods: Data were prospectively collected from 201 consecutive CRS and HIPEC procedures performed in a single institution between April 2001 and November 2015. Our primary endpoints were overall survival (OS) and disease-free survival (DFS), and secondary endpoints were morbidity and mortality.
Results: 77% of patients were Chinese, 9% were Malay, 6% were Indian and 8% were other ethnicities. Primary tumours were colorectal (30%), ovarian (32%), appendiceal (20%), primary peritoneal (6.5%), mesothelioma (4.5%) and others (5%). The median peritoneal cancer index (PCI) was 12, and 92% of patients achieved a completeness of cytoreduction score (CC) of 0. High-grade morbidity occurred in 25.8% of cases, and there were no 30-day mortalities. At 5-years, the OS was 55.1% and DFS was 20.3%. Factors associated with improved OS on multivariate analysis were PCI <15 (p < 0.001) and a CC 0 (p = 0.016).
Conclusions: The combined treatment of CRS and HIPEC is beneficial and is associated with reasonable morbidity and mortality in Asian patients with PC from colorectal, ovarian, appendiceal, primary peritoneal and mesothelioma primaries. Complete cytoreduction and extent of disease are the most important prognostic factors for survival.
Introduction
Peritoneal carcinomatosis (PC) from appendiceal, primary peritoneal, ovarian and various gastrointestinal neoplasms are increasingly being treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Traditionally, these patients would have been treated with systemic chemotherapy and/or palliative surgery, and given a dismal prognosis, with a median survival of 6–12 months [Citation1]. CRS and HIPEC have revolutionised the treatment of PC, with significant improvements in overall survival (OS) and disease-free survival (DFS) being reported [Citation2–5]. The principle behind this combined treatment of CRS and HIPEC is the resection of all visible tumours from the peritoneal cavity, followed by the eradication of the remaining microscopic disease by the administration of heated intra-peritoneal chemotherapy. Multiple studies have been published about this combined modality treatment, showing improved survival in selected patients with PC. However, most of these studies are from Western institutions, with the majority of patients being Caucasian. There is growing evidence from Japanese institutions for CRS and HIPEC for PC of gastric origin [Citation5]. The National Cancer Centre Singapore (NCCS) remains the only cancer centre in South East Asia performing CRS and HIPEC for colorectal, ovarian, appendiceal, mesothelioma and primary peritoneal neoplasms, and has the largest known experience in CRS and HIPEC in Asia for all these cancers. We aim to review our institutional experience with the procedure and provide an update on the OS and DFS rates and evaluate our morbidity and mortality, since our initial 100 patient experience [Citation6].
Methods
The study was carried out with the approval of the Centralised Institutional Review Board of the Singapore Health Services. A prospective database of consecutive patients who underwent CRS and HIPEC in NCCS was reviewed. Patients included in the study were treated between April 2001 and November 2015. Our primary end points were OS and DFS and our secondary end points were morbidity and mortality. Clinical characteristics, operative data and 30-day morbidity and mortality were recorded. Post-operative morbidity was evaluated using the common terminology criteria for adverse events version 3.0 of the National Institute of Health criteria [Citation7]. Most of the cases (198 of 201) had surgery performed by either one of the two surgeons (K.C.S. and M.T.)
Patient selection
All patients with PC, who underwent CRS and HIPEC in our institution, were of Eastern Cooperative Group (ECOG) performance status 0 or 1, and had no distant metastases on pre-operative radiological investigation. The patients were evaluated for the presence of extra-abdominal disease either via a thorax CT scan or a positron-emission tomography (PET)–CT scan. The extent of disease was examined on CT scan of the abdomen and pelvis, and the feasibility of complete cytoreduction and tumour clearance was discussed at the multidisciplinary tumour board meetings (MDTM).
CRS and HIPEC
CRS was performed as described by Sugarbaker [Citation8] and aimed to remove all macroscopic peritoneal disease. Bowel anastomoses were typically performed after HIPEC. HIPEC targeted the microscopic disease, specifically being effective only in lesions less than 3 mm [Citation9,Citation10]. Hence the importance of the CRS to achieve a complete cytoreduction, with no macroscopic residual disease, was pertinent. Besides the stripping of the parietal peritoneum, individual visceral resections were recorded (i.e. gastrectomy, colectomy, splenectomy etc.), and documented as individual CRS procedures. The sub-diaphragmatic peritoneum was removed on either or both sides when macroscopic disease was visible but was left intact if no gross disease was visualised, as was the case for all other parietal peritoneal surfaces. All patients who underwent stripping of the sub-diaphragmatic peritoneum had placement of chest-tubes on the corresponding side intra-operatively [Citation11].
All patients received HIPEC with chemotherapeutic agents prescribed by the medical oncologist. The chemotherapeutic agents used for the HIPEC differed based on the organ of the primary tumour, but within the same tumour histology group, the drug prescribed, the duration of the HIPEC, and the temperature at which it was administered remained similar throughout the years. Mitomycin C was the drug provided for colorectal and appendiceal PC, while cisplatin was the drug provided for the patients with ovarian, primary peritoneal and mesothelioma PC.
At our institution, a closed technique for HIPEC, with the chemotherapy agent diluted in 2–2.5 L of peritoneal dialysis solution at 42 °C, was used to distend the abdomen. The Belmont hyperthermia pump was used to deliver the intra-peritoneal chemotherapy agent via a single inflow catheter, and drainage was via four intra-abdominal drains. HIPEC was administered for 60 min in all cases.
Peritoneal cancer index and completeness of cytoreduction score
The peritoneal cancer index (PCI) score as described by Sugarbaker [Citation8] was used to describe the extent of disease. The completeness of resection was measured prospectively in all patients by the completeness of cytoreduction (CC) score. This score measures the amount of disease left behind and has been shown in most studies to be the strongest prognostic indicator in patients with PC undergoing CRS and HIPEC [Citation12,Citation13]. Patients with a CC score of 0 and 1 are considered to have achieved optimal cytoreduction. In patients with CC scores of 2 or more, surgery does not provide additional survival benefit when compared with conservative management [Citation14].
Post-operative care and course
Post-operatively, the patients were either transferred to the surgical intensive care unit (SICU) or the high-dependence unit at the anesthetist’s discretion. Before November 2012, early post-operative intraperitoneal chemotherapy (EPIC) was delivered through the four intra-abdominal drains that were left in place after CRS and HIPEC. The initiation of EPIC was a combined decision between the surgical and medical oncology teams, and was based on the patients’ post-operative recovery. If the patient remained well, EPIC was initiated by post-operative day 2, and was delivered for 5 days. 5-Fluorouracil (5-FU) was the chemotherapy agent administered for EPIC in our colorectal, appendiceal and primary peritoneal patients, while paclitaxel was the drug administered for the ovarian cancer and mesothelioma patients.
After November 2012, an institutional decision regarding EPIC was made and the delivery of EPIC was stopped. The four intra-abdominal drains used for the delivery of HIPEC were left in place, and gradually removed over the post-operative period when their outputs were minimal.
Follow-up
All patients were followed up at the outpatient unit at the NCCS at approximately 2 weeks after the surgery, and at least every 3 months thereafter for 1 year. After 1 year, follow-up was every 6 months. CT scans of the thorax, abdomen and pelvis, together with tumour markers (as appropriate), were performed at each follow-up visit and when clinically indicated. The patients were also followed up with medical oncologists and received adjuvant systemic chemotherapy at their discretion. Events of recurrent disease were recorded.
Statistical analysis
The Kaplan–Meier method was used to estimate the survival functions for OS and DFS. Median OS and DFS were derived, and 95% confidence intervals (95% CI) were calculated using the log-log method. The log-rank test was used to determine if there was a difference in survival curves between different groups of patients. The Wilcoxon rank-sum test was used to test for differences in continuous variables between two groups of patients. For categorical variables, the Pearson chi-squared test or the Fisher’s exact test was used. Multivariate Cox proportional hazards models were built for OS and DFS, and multivariate logistic regression models were built for occurrence of post-operative complications and occurrence of high-grade complications using a forward stepwise variable selection method. A two-sided p value of less than 0.05 was taken as significant. All analyses were performed in PASW Statistics 18.0.2.
Patients who underwent repeat CRS and HIPEC during the study period, had each procedure treated as an independent event. OS was measured from the date of CRS and HIPEC to the date of death from any cause. DFS was measured from the date of CRS and HIPEC to the date of recurrence or death. Patients who did not develop any of these time-to-event endpoints were censored at the date of last follow-up.
Results
Patient and treatment characteristics
Between April 2001 and November 2015, there were a total of 187 patients who underwent 201 CRS and HIPEC procedures at our institution. 12 patients had one repeat CRS and HIPEC, and one patient underwent three CRS and HIPEC procedures. Data regarding these 187 patients are summarised in , while the procedures and operative factors are summarised in . Disease-free interval was calculated as the time (in months) from date of primary surgery to date of CRS and HIPEC. This was only applicable to patients with colorectal and ovarian PC. The majority (62%) of patients had colorectal and ovarian primaries, followed by appendiceal, primary peritoneal and mesothelioma. The remaining 10 patients comprised of four patients with gastric PC, three patients with small bowel PC, two patients with endometrial PC and two patients with sarcomatosis. The 10 patients were grouped together as “others”.
Table 1. Demographic characteristics of 187 patients who underwent CRS and HIPEC.
Table 2. Summary of operative factors and resections performed.
A median of three visceral resections was performed during CRS, with the most common resections being colectomy, small bowel resection, cholecystectomy and splenectomy in decreasing frequency. Diaphragmatic stripping was also considered as a separate resection and was the most common procedure performed. The median PCI score was 12, but data on PCI were missing for 35 procedures. Optimal cytoreduction was achieved in the majority of cases (92%), with only 2% of patients having CC scores of >1; data on CC score were missing in 10 cases.
Postoperative morbidities
There were a total of 102 post-operative complications, of which 50 were low-grade complications (grades 1 and 2), and 52 were high-grade (grades 3, 4 and 5). The high-grade complications are listed in . Of the high-grade complications, 39 occurred during the period while EPIC was being administered routinely (before November 2012), while 13 were during the time when EPIC had been stopped (after November 2012). In our analysis, comparing patients who received EPIC with those who did not receive EPIC, patients who received EPIC had a higher proportion of grade 3 and above complications (58% vs. 25%; p = 0.048) and a longer duration of hospitalisation (16 days vs. 13 days; p = 0.019) than patients without EPIC [Citation15].
Table 3. High-grade post-operative complications.
There were no 30-day mortalities. 29 patients stayed in the hospital beyond 30 days, and there were two deaths on post-operative days 86 and 187, respectively. Both patients suffered from enterocutaneous fistulas and related complications, and eventually died of spontaneous intracranial haemorrhage. Since then, there have been no post-operative mortalities.
Univariate and multivariate analyses were performed to determine factors that could predict for the occurrence of any complication and of high-grade complications. Patients who had a colectomy (HR 1.93 (1.1–3.39) p = 0.021), cholecystectomy (HR 1.9 (1–3.6) p = 0.049) or gastrectomy (HR 2.9 (1–8.6) p = 0.043) as part of their CRS, and patients who received post-operative blood transfusions (HR 1.5 (1.2–1.9) p = 0.001) were more likely to suffer a complication on univariate analysis. None of the factors were significant on multivariate analysis (Supplementary Table 2).
Looking at the high-grade complications, undergoing sub-diaphragmatic stripping, a colectomy or splenectomy was significant on univariate analysis. However, on multivariate analysis, only receiving post-operative blood transfusions were significant, with a 45% (95% CI: 1%–110%) increase in the odds of experiencing a high-grade post-operative complication for every pint of blood received (Supplementary Table 2).
Survival outcomes
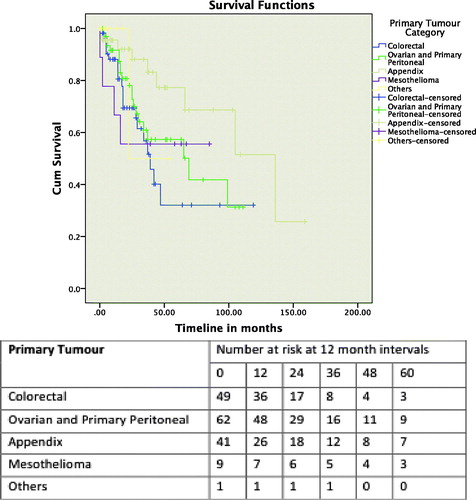
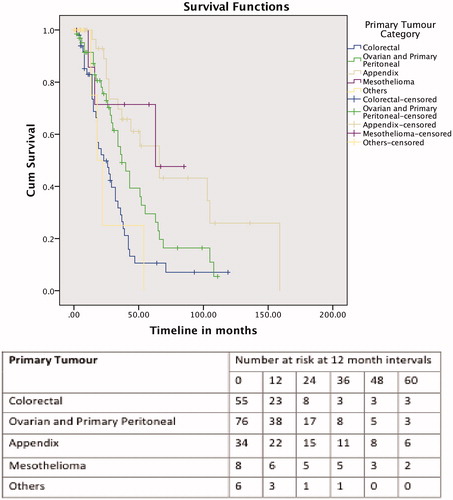
The median follow-up duration was 16 months (range, 0–153 months) for all the patients. The median OS for all patients was 66 months (17.12–114.878), with 1-, 3- and 5-year OS rates of 89.1, 61.4 and 55.1%, respectively. The 201 procedures were further divided into five groups according to the primary tumour types – colorectal cancer, ovarian cancer and primary peritoneal, appendiceal, mesothelioma, and the fifth group consisting of the other cancers (i.e. gastric, small bowel, endometrial and sarcomatosis). The median survivals for the patients who underwent CRS and HIPEC for colorectal primaries was 36 months (25.5–46.4), for patients with ovarian primaries and primary peritoneal malignancies was 65 months (29.1–100.8), and the appendiceal primaries patients was 136 months (65.5–206.4). This was not defined for the mesothelioma and “other tumour” groups. The 1-, 3- and 5-year OS for the groups are shown in . Factors influencing OS were PCI score of less than 15 (p = 0.014), CC score (p < 0.001) and disease-free interval of less than 12 months (p = 0.007) on log-rank tests. PCI score and CC score remained significant on multivariate analysis (Supplementary Table 1).
The median DFS for the 187 patients was 24 months (18.742–29.258), with 1-, 3- and 5-year DFS of 72.8, 31.8 and 20.3%, respectively. The 201 procedures were again divided into five groups, as for the OS analysis. The 1-, 3- and 5-year DFS for the groups are shown in . Primary cancer (p = 0.002) and CC score (p = 0.047) were significant on log-rank test, and remained significant on multivariate analysis (Supplementary Table 1).
Discussion
There is limited data supporting the use of CRS and HIPEC in Asian patients. In our population of patients, most patients who underwent CRS and HIPEC were Chinese, with a smaller percentage of patients from Malay, Indian and other ethnicities. Some evidence of the relevance of this procedure in selected patients has emerged from Japanese institutions, which have recently published their experience with CRS and HIPEC for small bowel [Citation16] and urachal tumours [Citation17]. There have also been Chinese institutions reporting their outcomes with CRS and HIPEC for colorectal PC in recent times [Citation18]. There are no reports of CRS and HIPEC for colorectal, ovarian, appendiceal, primary peritoneal and mesothelioma patients in an Asian centre, apart from our publication on our initial experience with the first 100 patients [Citation6]. In this current study with 201 CRS and HIPECs, we report a median OS of 66 months (17.12–114.878), with 1-, 3- and 5-year OS rates of 89.1, 61.4 and 55.1%, respectively, which is comparable to that reported in Western centres [Citation2–4].
OS was influenced by the origin of the PC, with the primary peritoneal, appendiceal and mesothelioma group of patients performing better compared with the ovarian and colorectal patients. This was similar to what we found with our initial experience [Citation6], and is likely due to tumour biology, and the superficial spreading pattern of growth of these tumours [Citation19], as compared with the deeper penetrating nature of the colorectal and ovarian PC. This group of patients had an OS of 76.9% at 5 years, which is similar to that reported in the literature [Citation20].
Our ovarian patients fared better than the colorectal patients, with a 5-year OS of 50%, and a median OS of 65 months. This was not unexpected, given the chemosensitive nature of these tumours. As optimal debulking with systemic chemotherapy have been reported to achieve an OS of 20–57 months [Citation21,Citation22], HIPEC is possibly a significant contributor to the improved survival. A randomised controlled trial looking at HIPEC for recurrent epithelial ovarian cancer reported a superior survival of 75% at 3 years for the patients who received HIPEC, compared with 18% at 3 years for patients who did not receive HIPEC. In that study, complete cytoreduction and a PCI <15 were also associated with better survival [Citation23].
There has been improvement in the OS for our patients with colorectal peritoneal carcinomatosis, with an OS of 35.8% at 5 years. In our initial experience, there were 28 patients with colorectal PC, and their OS at 5 years was 18.2%. This improvement is likely due to a refinement of our selection of colorectal patients with PC, and the experience we have attained with the additional 101 procedures. As with all the cases for consideration of CRS ad HIPEC, the patients are discussed at the MDTM, where the surgical oncologists, medical oncologists, oncology radiologist and pathologists are present. We now often choose to administer “pseudo-neoadjuvant” chemotherapy for selected colorectal patients with PC, in an attempt to sieve out the patients who are likely to fail distally. These patients typically have nodal disease at their original surgery, with a short disease-free interval [Citation24]. The use of PET scans, and increasingly so, magnetic resonance imaging (MRI) scans of the abdomen and pelvis may also contribute to the more precise patient selection for the procedure.
The PCI and CC scores continue to be important determinants of prognosis for patients with PC of various origins. In our cohort, a PCI of less than 15 significantly influenced OS on univariate and multivariate analyses. CC score was also proven to affect OS and DFS, with the majority (92%) of our patients having optimal cytoreduction with a CC score of 0 and 1, and only 2% of patients having CC scores of 2 and 3. Consequently, achieving optimal cytoreduction is crucial, and if a CC score of 2 or 3 is likely, the patient is unlikely to benefit from CRS and HIPEC and should not be considered for the procedure. In these patients, consideration for a diagnostic laparoscopy to assess the extent of the disease may be helpful [Citation25].
As with every complex procedure, CRS and HIPEC have widely reported morbidity and mortality rates. These ranged from 40–80% and 3–20%, respectively in the earlier years but have since improved to 20–40% and 3%, respectively. However, this does not take away the fact that many surgeons and oncologists who are unfamiliar with this treatment modality continue to quote the earlier figures, often in a bid to dissuade patients who have read about CRS and HIPEC as a potentially viable treatment option for their disease. In our experience, the risks of high-grade complications were 25%, but decreased to 6% without the administration of EPIC. This reduction of our morbidity rates is likely a reflection that as an institution, we have overcome the initial learning curve that is associated with CRS/HIPEC [Citation26], and have likely improved in our patient selection over time. Our analyses also found that receiving post-operative blood transfusions were associated with high-grade morbidity, however, this finding is likely confounded by other time-associated factors and may possibly be a surrogate of morbidity rather than a true association.
As an institution, we have learnt that the ideal situation would be to welcome the discussion of all cases with peritoneal disease at a multidisciplinary tumour board meeting, and decide if there is enough supporting infrastructure, fuelled by committed multidisciplinary parties to embark on this. And as with every complex procedure, there will necessarily be a learning curve. It is possible to eventually reduce the morbidity and mortality rates to those similar to that of a whipple’s resection, in experienced centres, which is likely to be accompanied by improved survival outcomes. Kusamura et al.’s [Citation26] paper describes a need to have completed 140 cases before a surgical team or institution becomes proficient at CRS and HIPEC. An in-depth look at our learning curve, comparing the first 100 cases with the subsequent 100 cases of CRS and HIPEC is the focus of a subsequent paper.
Conclusions
PC is a common end-point for many gastrointestinal and ovarian cancers, often with debilitating symptoms when it occurs. Our updated data indicate that CRS and HIPEC remain beneficial and are associated with reasonable morbidity and mortality in Asian patients with PC from colorectal, ovarian, appendiceal, primary peritoneal and mesothelioma primaries. Complete cytoreduction is the most important prognostic factor for OS and DFS, and primary tumour biology must continue to influence patient selection for this aggressive procedure.
Supplemental File
Download Zip (97.8 KB)Acknowledgements
Ms. Thakshayeni Skanthakumar, Database manager.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Sadeghi B, Arvieux C, Glehen O, et al. (2000). Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 88:358–63.
- Glehen O, Kwiatkowski F, Sugarbaker PH, et al. (2004). Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 22:3284–92.
- Verwaal VJ, Bruin S, Boot H, et al. (2008). 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 15:2426–32.
- Di Giorgio A, Naticchioni E, Biacchi D, et al. (2008). Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer 113:315–25.
- Yonemura Y, Kawamura T, Bandou E, et al. (2005). Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg 92:370–75.
- Teo M, Tan G, Lim C, et al. (2013). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in Asian patients: 100 consecutive patients in a single institution. Ann Surg Oncol 20:2968–74.
- Younan R, Kusamura S, Baratti D, et al. (2008). Morbidity, toxicity, and mortality classification systems in the local regional treatment of peritoneal surface malignancy. J Surg Oncol 98:253–57.
- Sugarbaker PH. (1995). Peritonectomy procedures. Ann Surg 221:29–42.
- Witkamp AJ, de Bree E, Van Goethem R, Zoetmulder FA. (2001). Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev 27:365–74.
- Jacquet P, Sugarbaker PH. (1996). Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 82:359–74.
- Teo M, Foo KF, Koo WH, et al. (2006). Lessons learnt from initial experience with peritonectomy and intra-peritoneal chemotherapy infusion. World J Surg 30:2132–36.
- Sugarbaker PH, Ronnett BM, Archer A, et al. (1997). Pseudomyxoma peritonei syndrome. Adv Surg 30:233–80.
- Sugarbaker PH, Jablonsky KA. (1995). Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg 221:124–32.
- Sugarbaker PH. (1999). Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol 43:S15–S25.
- Tan G, Ong WS, Chia CS, et al. (2016). Does early post-operative intraperitoneal chemotherapy (EPIC) for patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) make a difference? Int J Hyperthermia 32:281–88.
- Liu Y, Ishibashi H, Takeshita K, et al. (2016). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal dissemination from small bowel malignancy: results from a single specialized center. Ann Surg Oncol 23:1625–31.
- Liu Y, Ishibashi H, Hirano M, et al. (2015). Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei arising from urachus. Ann Surg Oncol 22:2799–805.
- Huang CQ, Yang XJ, Yu Y, et al. (2014). Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for patients with peritoneal carcinomatosis from colorectal cancer: a phase II study from a Chinese center. PLoS One 9:e108509.
- Mohamed F, Cecil TD, Moran BJ, Sugarbaker PH. (2011). A new standard of care for the management of peritoneal surface malignancy. Curr Oncol 18:e84–96.
- Lord AC, Shihab O, Chandrakumaran K, et al. (2015). Recurrence and outcome after complete tumour removal and hyperthermic intraperitoneal chemotherapy in 512 patients with pseudomyxoma peritonei from perforated appendiceal mucinous tumours. Eur J Surg Oncol 41:396–99.
- Bae JH, Lee JM, Ryu KS, et al. (2007). Treatment of ovarian cancer with paclitaxel- or carboplatin-based intraperitoneal hyperthermic chemotherapy during secondary surgery. Gynecol Oncol 106:193–200.
- Mulier S, Claes JP, Dierieck V, et al. (2012). Survival benefit of adding hyperthermic intraperitoneal chemotherapy (HIPEC) at the different time-points of treatment of ovarian cancer: review of evidence. Curr Pharm Des 18:3793–803. Review.
- Spiliotis J, Halkia E, Lianos E, et al. (2015). Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol 22:1570–75.
- Tan GHC, Teo MC, Chen W, et al. (2013). Surgical management of colorectal peritoneal metastases: treatment and outcomes compared with hepatic metastases. J Gastrointest Cancer 44:170–76.
- Iversen LH, Rasmussen PC, Laurberg S. (2013). Value of laparoscopy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Br J Surg 100:285–92.
- Kusamura S, Baratti D, Hutanu I, et al. (2012). The importance of the learning curve and surveillance of surgical performance in peritoneal surface malignancy programs. Surg Oncol Clin N Am 21:559–76.