Abstract
Purpose: Detecting a recurrence after lung radiofrequency ablation (RFA) is based on a group of arguments that include CT, positron emission tomography (PET-CT) at 3 months and clinical patient follow-up. There is no one examination that is absolutely reliable. Recurrences are diagnosed tardily, when the cancers are locally extended, or when the patients are metastatic. The purpose of this article is to investigate the utility of dual-energy computed tomography (DECT) in order to assess therapeutic responses to RFA for lung neoplasia.
Materials and methods: This institutional review board-approved study enroled 70 patients with lung tumours who underwent DECT after RFA. All patients provided a written informed consent for the study.
Results: The study included 70 consecutive patients, and 191 DECT measures were performed. We collected the enhancement values of all scars without establishing a prior threshold of positivity. The optimal threshold value areas appeared to be located between 20 and 35 Hounsfield unit (HU) with sensitivity between 70% and 82%; specificity between 72% and 90%; a negative predictive value (NPV) between 96% and 97% and a diagnostic accuracy index between 73% and 87%.
At the one month follow-up, 53 nodules were analysed with DECT and four nodules had recurred, all of which were detected by DECT. The sensitivity, which was calculated at 100%, was excellent; the NPV was at 100% (CI: 91.62, 100) and the specificity was at 85.71% (CI: 73.33, 92.9). The diagnostic accuracy index was 86.79% (CI: 75.16, 93.45) and the average DECT acquisitions dosimetry was 106 mGy.cm (33mGy.cm 245mGy.cm).
Conclusion: DECT could be a conceivable alternative for detecting early recurrence after lung RFA.
After lung RFA, a PET CT has a high rate of false positives in the initial phase;
The study of enhancement in the follow-up of lung lesions treated with RFA, and especially by DECT, can be relevant;
Dual Energy CT has a good efficiency for a threshold between 20 and 35 HU, especially in the first month after RFA;
DECT could be a conceivable alternative for detecting early recurrence.
Key points
Introduction
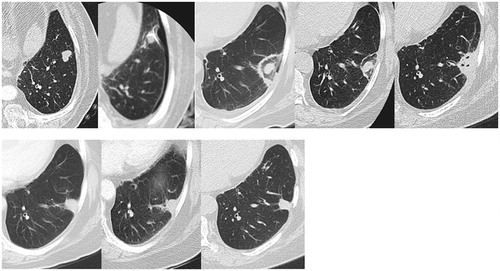
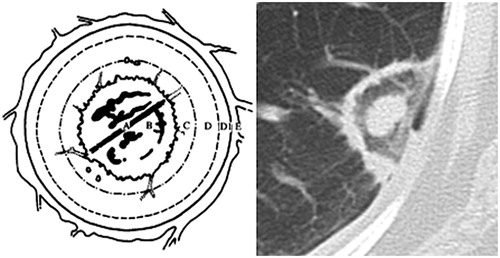
Lung cancer is the first cause of cancer deaths around the world, and the lungs are the second most common metastatic site. Even if surgical excision remains the gold standard for curative treatment, only 15% of patients are operable with more and more of those patients are eligible for the percutaneous radiofrequency ablation (RFA). The size of the destruction area and all induced remodelling far exceeds the size of the initial tumour nodule ().
Figure 1. (A) Central zone: needle electrode; (B) partial items related to vaporisation and coagulation necrosis, the phenomenon of ghost (apparently intact tissue); (C) coagulation necrosis at the boundary of the nodule containing cells, an air trapping and a ghost phenomenon; (D) enzymatic necrosis, partial destruction of the blood and lymphatic capillaries, micro thrombosis and lysosomal enzyme activation; (E) Inflammatory reaction and peripheral hyperaemia.
Detecting a recurrence is based on a group of arguments that include CT, PET-CT at 3 months and clinical patient follow-up. There is no one examination that is absolutely reliable. Recurrences are diagnosed tardily, when the cancers are locally extended, or when the patients are metastatic. The objective of this study is to determine the role of dual-energy computed tomography (DECT) in the follow-up and the detection of post RFA pulmonary recurrences.
Materials and methods
The institutional review board approved this retrospective study and all patients provided a written informed consent for the study.
Monitoring protocols
Between October 2014 and December 2015, we included all consecutive patients referred for lung RFA and patients followed in our centre for a previous RFA. RFA lung therapy was indicated as the curative treatment of early stage, non-small cell lung carcinomas (NSCLC) and for patients not suitable for surgery and palliative treatment in case of a painful or haemorrhagic clinical manifestation.
For secondary locations, RFA was indicated for the treatment of multiple or bilateral locations when the neoplastic disease was stabilised with the possibility to treat all the secondary sites [Citation1].
Patients were followed by DECT at 1, 3, 6, 9 months and a year, afterwards, they were monitored every year. The patients with lesions that did not fit the 80kVp field of view were excluded. A positron emission tomography (PET) was performed between 6 months and one year.
The criteria for recurrence were the morphological criteria usually recognised in the literature [Citation2]: increase in size of the RFA zone after the 3 month follow-up over 20% even though there are no quantitative criteria for local recurrence well-defined, the occurrence of a nodular enhancement, the transformation from a ground glass opacity to solid nodule, development of nodules along electrode track or tines. All these criteria were confirmed with a second CT one month later.
We did not perform biopsies on all patients, but only when a recurrence was suspected (based on the preceding criteria and the DECT criteria), and when a decision of the multidisciplinary meeting was proposed. The review was performed by a thoracic imaging expert.
The CT acquisition protocol (Somatom Definition Flash, Siemens Healthcare, Forchheim, Germany) was a “classic” chest acquisition from 30–40 s after injection of 80 mL iodine contrast (Xenetix® 350 Guerbet, Roissy, France) at a rate of 3 ml/sec. At 80 s after this injection, a dual energy acquisition was performed on the area of interest (limited by the size of sensor FOV 80 kV).
The two X-ray tubes had 80 and 140 kV with intensities of 235 and 47mAs. Collimating was 14 × 1.2 mm slice thickness between 1.5 and 2 mm with an increment between 1 and 1.5 mm.
The literature’s perfusion studies demonstrated that the optimal moment for measuring the enhancement should be around 80 s.
Two “conventional” acquisitions without injection followed by a routine contrast enhanced CT was not done for two reasons. Firstly, we observed that the dose length product delivered during this protocol would have been similar to that achieved in our protocol and secondly, an acquisition of 80 s after injection on the entire thorax seemed too late to explore the mediastinum in a satisfactory manner.
RF procedure
Procedures were performed under general anaesthesia with antibiotic prophylaxis. The identification of the nodule and the puncture were performed under CT guidance allowing the introduction of a deployable electrode (LeVeen CoAccess™ Electrode System Boston Scientific SCIMED®, Inc., Maple Grove, MN). The size of the electrode was chosen according to the need of a circumferential safety margin greater than 15 mm. The needle was then connected to an RFA generator® 3000 (Boston Scientific SCIMED®, Inc.). Plates were dispersed on the patients’ lower limbs to avoid burns.
Image post-processing
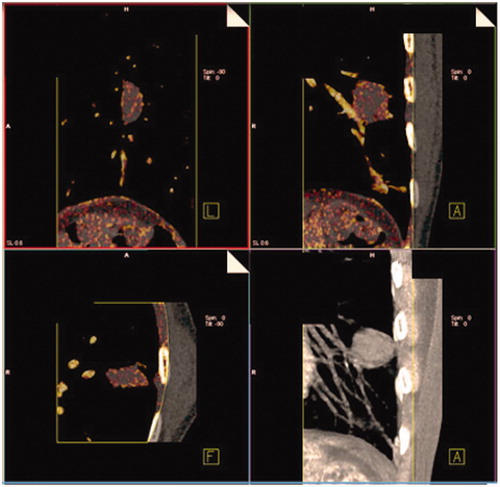
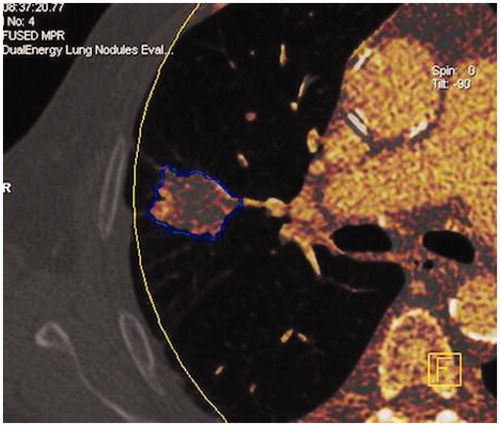
We retrospectively analysed images of these 70 consecutive patients. The images were post-processed using Syngo MMWP software (DE lung nodules Siemens Healthcare). Once loaded on the workstation, the software displayed the images which included standard linearly blended images, virtual non-contrast images, and iodine overlay images. The iodine overlay could be superimposed with the virtual non-contrast images, which were indicated by the reddish colour. The intensity of the colour correlated with the relative amount of iodine content detected ().
The assessment of nodule enhancement is very dependent on the region of interest (ROI) placement, which is why a segmentation software “CAD lung care” was used in order to ensure a better reproducibility. The software automatically isolates the entire nodule from the pulmonary parenchyma and determines, by a volumetric ROI (), the maximum size of the nodule, its volume, the mean density inside the nodule in the virtual non-contrast (VNC) mapping and its average enhancement. For each CT, the enhancement values and the dose-length product (DLP) were recorded. The reader of the test was blinded to the results of other imaging tests. If the coefficient of variance was greater than 30%, a more appropriate ROI measurement was systematically done for the second time manually. A limited number of missing data (CT acquisitions outside the lesion) have led some patients to be excluded from the study.
Figure 3. DECT image. Software automatically isolating the entire nodule from the pulmonary parenchyma to determine a volumetric ROI. (a) Diagnostic efficacy of DECT. (b) Example of the evolution of the enhancement in a patient with an early recurrence and in a patient with no recurrence.
Statistical analysis
Statistical analysis was performed using SPSS Version 17 Statistic (Inc., Chicago, IL) software. Qualitative values were presented as effective and percentages, while quantitative values were presented as mean with their standard deviation. Testing was presented with 95% confidence intervals. We used statistical tests that considered clustering effects when a patient had more than one lesion.
Results
Population
There were 70 consecutive patients (44 men and 26 women, mean age 65.56 years SD 14.2) included and 191 DECT measures were performed. A total of 144 measures were realised in the first year after the treatment. We excluded 18 measures because the lesions were outside the 80kVp field of view. There were 47 measures performed after the first year, including 8 measures after 3 years with one at 5 years. Median follow-up was 31 months (6 months, 68 months). The average lesion size was 14.5 mm (SD 17 mm). Patients had primary tumours (31% of patients) or secondary locations (33% colorectal, 23.7% sarcoma, 8.4% Thymus, 7% kidney, 7% breast, 5.7% uterus, 2.8% stomach/oesophagus, 1.4% neuroendocrine tumours, 1.4% pancreas and 1.4% melanoma).
Overall reliability of dual-energy
We collected the enhancement values of all scars without establishing a prior threshold of positivity. and summarises the diagnostic efficacy of DECT based on different thresholds. Among all the RFA lesions, 10 nodules showed local recurrence (12.8%) demonstrated by “classic” criteria. This number is comparable to the recurrence rates in the literature [Citation3].
Table 1. Diagnostic efficacy of DECT: sensibility, specificity, PPV and NPV variation with the threshold
For a threshold of 15HU, determined by Swensen et al., the sensibility was 88.2%, the specificity was 59.6% and the NPV was 97.9%. The negative predictive value always seemed to be excellent, whatever the threshold, and was always over 90%. The optimal threshold value areas appeared to be located between 20 and 35 HU where we found the sensitivity between 70% and 82%, specificity between 72% and 90%, NPV between 96% and 97% and diagnostic accuracy index between 73% and 87%.
Above the threshold of 35 HU, the sensitivity becomes very poor and goes below 50%. Below the threshold of 20 HU, the specificity drops under 60%. As an example, when considering a threshold at 25 HU, the sensitivity is around 80% (CI: 49.02, 94.33).
Among the RFA sites without recurrence (68 nodules), 73.6% had an enhancement under 25 HU. For a threshold of 25 HU, the NPV was excellent at 96.15% (CI: 87.02; 98.94); the positive predictive value (PPV) was low, 30.7% (CI: 16.5; 49.99). The diagnostic accuracy index was 74.36% (CI: 63.69, 82.74) ().
Regarding the two cases of recurrence where the PET was positive at 3–6 months and the DECT negative for a threshold between 20 and 35 HU, one of the two “false negatives” showed a recurrence which was demonstrated by PET at 18 months after the RFA (no earlier PET). Due to the lack of monitoring, only one DECT was performed at 12 months, showing an enhancement below 20 HU. We were not able to determine if this recurrence occurred before 18 months. The second false negative presented an enhancement below the threshold of 20HU a few days before performing a PET, which attested to the recurrence.
Reliability of dual energy at one month
In the one-month follow-up group, 53 nodules were analysed by DECT and the four nodules that recurred were all detected by DECT with a threshold between 20 and 35 HU. The sensitivity was excellent and calculated at 100% (CI: 51, 100), and the VPN was at 100% (CI: 91.62, 100). The specificity was at 85.71% (CI: 73.33, 92.9). The diagnostic accuracy index was 86.79% (CI: 75.16, 93.45) with a threshold between 20 and 35 HU.
Dose study
With regards to dosimetry, the mean overall DLP acquisitions were 213 mGy.cm (73–500 mGy.cm). The average dosimetry of DECT acquisitions was 106 mGy.cm (33–245 mGy.cm).
Discussion
Role of FDG PET/CT and Chest CT in the follow-up of lung lesions treated with RFA
Early detection of post RFA lung recurrences is a major challenge for an initiation of specific treatment. Monitoring RFA sites, especially during the early phase, is delicate due to its mode of evolution [Citation2]. The normal evolution of the RFA scar is an increase in size reported to the area of tissue destructed around the initial lesion, the safety margins and the local inflammatory phenomenon.
The decrease in size occurs only after 6 months and then the scar evolves into an atelectasis, fibrosis, nodule or cavity (). During the early phase, response evaluation criteria in solid tumours (RECIST) 1.1 cannot be used. Studies have shown that PET had a high rate of false positives in the initial phase until the sixth month. Many have looked at PET CT [Citation4–6] and there is a general agreement that after 3–6 months post treatment changes have generally been assumed to be not present.
Role of enhancement in the follow-up of lung lesions treated with RFA
One of the research tracks is to study the enhancement of RFA lesions. The most important study was carried out by Swensen [Citation7] who studied the enhancement of 550 indeterminate and untreated solid nodules and concluded that the absence of enhancement above 15UH was strongly predictive of benignity with a sensitivity of 98%. In 2001, Lee et al. [Citation8] and Park et al. [Citation9] demonstrated the reliability of the DECT in finding an early recurrence after RFA, respectively in tumours of the liver and kidney. No study had yet demonstrated the value of DECT in monitoring recurrences after lung RFA.
In this study, we demonstrated that DECT could be a conceivable alternative for detecting early recurrences, using a threshold between 20 and 35 HU.
The use of DECT chest imaging is best known in studying the pulmonary perfusion to find a pulmonary embolism. The advantages of dual energy are reduced dosages, a simultaneous acquisition that eliminates the respiratory movements’ artefacts and partial volume effects, which have a significant impact in the study of small nodules.
Schmid-Bindert et al. [Citation10] demonstrated a significant correlation between the SUV in 18 FDG PET CT and enhancement measured in DECT, thus making the DECT a useful functional imaging modality for patients with NSCLC.
In our study, the total dosimetry of our protocol was lower than that which would have represented two acquisitions across the thorax, both without and after injection of contrast, and being much less important than with a dynamic perfusion. Many studies [Citation11] have shown that dual-energy CT mode can be performed routinely without compromising the dose or the quality of the images.
Limitations of study
In our study, not all nodules could be investigated due to an acquisition limit of fields, especially in patients with a surgical history. Indeed, in order to limit radiation, we did not make acquisitions on the entire chest but only focussed on the lesions. Another limitation to our study was the small number of recurrences after lung RFA; however this number remains higher than in similar studies [Citation5]. Because of the retrospective nature of this study, the DECT follow-up imaging is variable between patients. The establishment of a reference standard of recurrence is critical but there are no clear criteria of recurrence applied in clinical practice. Even if these criteria are not specific, it seems they are conventionally used in clinical practice without resorting systematically to histological data.
Conclusions
The study of enhancement in the follow-up of lung lesion treated with RFA and especially by DECT can be relevant. DECT is easy to perform in common practice and can avoid respiratory motion artefacts and partial volumes. For a threshold between 20 and 35 HU, the diagnostic efficiency in terms of NVP, sensibility and specificity is effective, especially in the first month after RFA.
| Abbreviations | ||
| DECT | = | Dual Energy computed tomography |
| RFA | = | Radiofrequency ablation |
Supplementary File
Download PDF (7.7 KB)Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Chhajed PN, famm M. (2003). Radiofrequency heat ablation for lung tumors: potential applications. Med Sci Monit 9:ED5–7.
- Fereidoun G. (2012). Radiofrequency ablation of lung tumors: imaging features of the postablation zone. RadioGraphics 32:947–69.
- Simon CJ, Dupuy DE, DiPetrillo TA, et al. (2007). Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology 243:268–75.
- Marine S, Bacharach SL, Buvat I. (2007). Partial-volume effect in PET tumor imaging UMR 678 INSERM-UPMC, CHU Pitie-Salpetrière, Paris, France. J Nucl Med 48:932–45.
- Okuma T, Okamura T, Matsuoka T, et al. (2006). Fluorine-18-fluorodeoxyglucose positron emission tomography for assessment of patients with unresectable recurrent or metastatic lung cancers after CT-guided radiofrequency ablation: preliminary results. Ann Nucl Med 20:115–21.
- Higaki F, Okumura Y, Sato S, et al. (2008). Preliminary retrospective investigation of FDG-PET/CT timing in follow-up of ablated lung tumor. Ann Nucl Med 22:157–63.
- Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. (1997). The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 157:849–55.
- Lee SH, Lee JM, Kim KW, et al. (2011). Dual-energy computed tomography to assess tumor response to hepatic radiofrequency ablation: potential diagnostic value of virtual noncontrast images and iodine maps. Invest Radiol 46:77–84.
- Park SY, Kim CK, Park BK. (2014). Dual-energy CT in assessing therapeutic response to radiofrequency ablation of renal cell carcinomas. Eur J Radiol 83:e73–9.
- Schmid-Bindert G, Henzler T, et al. (2012). Functional imaging of lung cancer using dual energy CT: how does iodine related attenuation correlate with standardized uptake value of 18FDG-PET-CT? Eur Radiol 22:93–103.
- Schenzle JC1, Sommer WH, Neumaier K, et al. (2010). Dual energy CT of the chest: how about the dose? Invest Radiol 45:347–53.