Abstract
Aim: This study aimed to compare the local therapeutic efficacy of percutaneous thermal ablation for colorectal liver metastases (CRLM) and hepatocellular carcinoma (HCC).
Methods: One hundred sixty-one CRLM nodules in 101 patients and 122 HCC nodules in 97 patients were treated with thermal ablation. Complications and local efficacy were retrospectively compared.
Results: Major complications were observed in two (2.0%) patients in the CRLM group and one (1.0%) in the HCC group (p = 1.000). The complete ablation (CA) rate of lesions ≤ 3 cm was lower in the CRLM group than in the HCC group (p = 0.018). After a mean follow-up period of 21.1 ± 20.7 months in the CRLM group and 22.1 ± 17.6 months in the HCC group, the local tumour progression (LTP) rate of lesions > 3 cm was higher in the CRLM group than in the HCC group (p = 0.036). The multivariate analysis revealed that only safety margin (≤ 0.5 cm/> 0.5 cm) was a significant predictor of LTP in both CRLM and HCC.
Conclusions: To achieve better local tumour control, thermal ablation should be more aggressive for CRLM than for HCC, especially for large tumours in clinical.
Introduction
Percutaneous thermal ablation has become one of the most widely used minimally invasive therapies to treat liver tumours and is a mature method in clinical practice [Citation1]. Radiofrequency ablation (RFA) and microwave ablation (MWA) are the most commonly used thermal ablation modalities [Citation2]. Thermal ablation is a therapeutic strategy that can be used to treat not only hepatocellular carcinoma (HCC) but also metastatic liver carcinoma (MLC). Approximately 25% of colorectal carcinoma patients also present with liver metastases at diagnosis, and 50 ∼ 75% of patients harbour liver metastases 3 years after the resection of colorectal carcinoma [Citation3]. In the clinic, surgical resection is the first choice and the standard of care for the treatment of resectable colorectal liver metastasis (CRLM) [Citation4]. This approach results in a 5-year survival rate of 20 ∼ 50% [Citation5,Citation6], but only 20 ∼ 30% of patients are deemed suitable for surgery [Citation7,Citation8].
Ablation is recommended as a curative treatment for HCC [Citation9]. For a single lesion with a diameter <3 cm, thermal ablation is considered the first-line therapy [Citation1,Citation10,Citation11]. The efficacy of ablation for the treatment of HCC, particularly small HCC, is equivalent to that of surgical resection [Citation12–14], and the satisfactory safety of ablation was observed in a large-scale trial [Citation15]. Thermal ablation is a promising therapy for MLC and has recently become widely used in clinical practice [Citation16,Citation17]. A tumour size of less than 3 cm is essential for satisfactory local tumour control using RFA for CRLM [Citation4]. However, liver resection is superior to RFA for treating resectable CRLM [Citation18–22]; meanwhile RFA had good oncological outcomes in patients with unresectable CRLM [Citation23]. In contrast to HCC, the local efficacy of ablation for MLC, particularly CRLM, is still under debate.
The purpose of the present study was to retrospectively compare the differences in local efficacy of percutaneous thermal ablation for the treatment of HCC and CRLM.
Materials and methods
Clinical data
This retrospective study was approved by the Institutional Review Board, and informed consent was obtained from all patients. presents a flow diagram of the study population. Of 101 patients with CRLM, 25 (24.8%) patients exhibited liver recurrence after hepatectomy, and 76 (75.2%) patients who were considered to have unresectable disease (n = 23) (22.8%) or who refused hepatectomy (n = 53) (52.5%) underwent thermal ablation instead. Of 97 patients with HCC, 60 (61.9%) patients exhibited liver recurrence after hepatectomy, and 37 (38.1%) patients who were considered to have unresectable disease (n = 7) (7.2%) or who refused hepatectomy (n = 30) (30.9%) underwent thermal ablation instead. In all, 101 patients with 161 CRLM nodules and 97 patients with 122 HCC nodules were treated at our hospital from July 2004 to December 2013. All patients with HCC were selected using the diagnostic algorithm of the American Association for the Study of Liver Diseases (AASLD) practice guidelines for the management of HCC [Citation9]. Patients with CRLM were diagnosed based on one of the following: (a) pathological diagnosis or (b) new lesion after colorectal cancer resection with typical enhanced imaging manifestations of MLC and clinical follow-up. The inclusion criteria for liver thermal ablation in this study were (a) number of treated lesions ≤ 5; (b) maximum diameter of treated lesions ≤ 7 cm; (c) Child-Pugh class A or B; (d) platelet count > 50 × 109/L; (e) prothrombin time ratio > 50%. The exclusion criteria were (a) the presence of vascular invasion in the pre-procedure imaging study; (b) treatment with transcatheter arterial chemoembolization (TACE) before thermal ablation, or (c) ongoing anticoagulant treatment that could not be stopped. presents the demographic and tumour characteristics of the patients.
Figure 1. Flow chart of the present study. Random assignment was performed using SPSS version 19.0. MLC: metastatic liver carcinoma; TACE: transcatheter arterial chemoembolization; MWA: microwave ablation; RFA: radiofrequency ablation; CRLM: colorectal liver metastases; HCC: hepatocellular carcinoma.
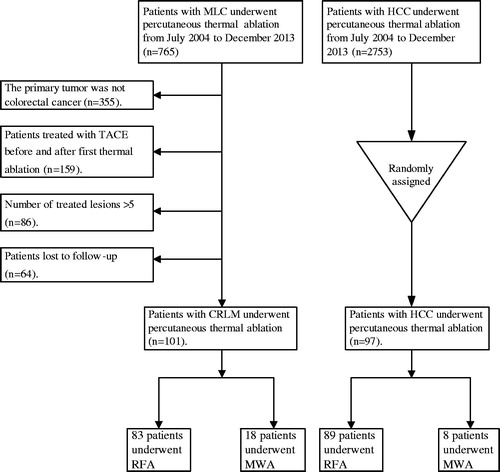
Table 1. Clinical characteristics of CRLM and HCC patients.
Treatment procedures
Each patient underwent one session of thermal ablation. Two of the authors (X. Y. X and M. K with 10 years and 8 years of experience with ablation, respectively) performed the ablation. The treatment was performed according to the standard ablation specification for lesions. After ablation, all the lesions were observed to be completely covered by gas.
The thermal ablations were performed percutaneously under real-time ultrasound guidance using an Acuson Sequoia 512 scanner (Siemens Medical Solutions, Mountain View, CA), with a 1.0–4.0 MHz vector probe and an ALOKA Prosound α10 ultrasound scanner (ALOKA, Tokyo, Japan) with a 1.0–6.0 MHz convex array probe.
Radiofrequency ablation
We used the RITA Medical System (RITA Medical System, Mountain View, CA) and Cool-tip™ Radiofrequency Ablation System (Covidien, Mansfield, MA). The radiofrequency needle electrodes included a StarBurst® XL (nine arrays plus an active trocar tip, five thermocouples and a 5-mm active tip), a StarBurst® Talon (four active arrays plus an active trocar tip and thermocouple at the tip of each active array) and a Cool-tip™ system (a unipolar needle with 15 ∼ 20 cm in length and a tip exposed 2 ∼ 3 cm). In the CRLM group, a total of 83 patients with 134 lesions underwent RFA. In the HCC group, a total of 89 patients with 113 lesions underwent RFA.
Microwave ablation
We chose microwave ablation for the patients with relatively large tumours in safe locations in the liver and not near important organs (e.g. the gallbladder, gastrointestinal tract, heart and diaphragm) [Citation24–26]. We used the FORSEA MWA system (FORSEA, Qinghai, Microwave Electronic Institute, Nanjing, China), which consists of an MTC-3 microwave generator (frequency 2.45 GHz, power output 0–120 W), a flexible low-loss cable, a 14-gauge cooled-shaft antenna and a steady-flow pump (BT01–100 LanGe-Pump, LanGe Steady Flow Pump, Baoding, China). In the CRLM group, a total of 18 patients with 27 lesions underwent MWA. In the HCC group, a total of 8 patients with 9 lesions underwent MWA.
Therapeutic efficacy and follow-up
All complications related to thermal ablation were categorised consistently according to severity (SIR, the Society of Interventional Radiology, classifications C-E) [Citation27].
The initial complete ablation (CA) performed 1 month after thermal therapy in the CRLM and HCC groups was assessed using contrast-enhanced computed tomography (CECT). All patients were scanned by an Aquilion™ 64-slice helical CT machine (Toshiba, Tokyo, Japan) with the following parameters: 0.5 mm × 64 mm collimation, 120 kV and 150–200 mA. CA was defined as non-enhancement in the ablated zone 1 month after ablation. Follow-up was conducted at regular intervals post-ablation (at 3-month intervals for the first year and biannually thereafter). The evaluation included common blood chemistry, serum carcinoembryonic antigen (CEA) or alpha-fetoprotein (AFP) and abdomen CECT. Local tumour progression (LTP) was defined as the appearance of tumour foci at the edge of the ablation zone after at least one contrast-enhanced follow-up study documented adequate ablation and the absence of viable tissue in the target tumour and surrounding ablation margin according to imaging criteria [Citation27]. LTP was assessed on a tumour-by-tumour basis.
Based on the size of tumours and the ablated area measured on CT images before and after thermal ablation, the safety margin was measured as the smallest distance between each side of the tumour and the minimal margin of coagulation using a PACS workstation [Citation28]. We evaluated the ablation margin based on anatomic landmarks and reviewed the pre- and post-ablation portal venous phase CT images side by side to calculate the size of the ablation zone [Citation29]. The safety margins were evaluated in consensus by two experienced radiologists (K. G. Z and G. L. H with 15 years and 5 years of experience with abdominal CT, respectively), who were blinded to the clinical data.
Additional treatment after thermal ablation
In the CRLM group, 35 patients with multifocal liver recurrences and/or extrahepatic progression underwent systemic chemotherapy after the ablation. In the HCC group, 20 patients with multifocal liver recurrences underwent TACE after the ablation. Considering that the current study was focussed on local efficacy, the patients who underwent re-ablation for other lesions and those who underwent liver resection or liver transplantation after ablation were excluded from this analysis.
Statistical analysis
All statistical analyses were performed using commercially available software (SPSS version 19.0; SPSS Inc., Chicago, IL). For all statistical tests, significance was assumed at p values <0.05. The tumour size, the size of the ablated area, the time to LTP and the time of follow-up are presented as the mean ± the SD (x ± s). Categorical variables were analysed using the chi-squared test, correction for continuity or Fisher’s exact probability test. The LTP rate was compared using the Kaplan–Meier method. The relationship between each of the variables and LTP was estimated with a log-rank test. The variables included the following: gender, age, presence of hepatitis B or C virus infection, Child-Pugh class for liver function, alanine aminotransferase level, total bilirubin, serum albumin level, prothrombin time, tumour size (≤ 3 cm/> 3cm), prior liver resection (yes/no), prior systemic chemotherapy (yes/no), systemic chemotherapy after ablation (yes/no), TACE after ablation (yes/no), type of thermal ablation (MWA/RFA) and safety margin (≤ 0.5 cm/> 0.5 cm).
Results
Patients and tumour profile
The demographic and clinical data of the two groups are compared in . Age, the diameter of the lesion and the follow-up time did not differ significantly between groups, whereas the sex ratio and the ratio of the number of lesions ≤ 3 cm/> 3 cm differed significantly between groups.
Complications
No deaths occurred in the CRLM and HCC groups. In the CRLM group, two patients (2.0%, 2/101) experienced major complications. Specifically, these patients developed liver abscesses, which presented with abdominal pain and high fever and occurred 30 days and 7 days, respectively, in the two patients after thermal ablation. These two cases were treated with ultrasound-guided abscess drainage. One case was confirmed as a Klebsiella pneumoniae infection, and the other case was an Escherichia coli infection. Both patients were successfully treated with antibiotics and catheter drainage. In the HCC group, one patient (1.0%, 1/97) experienced a major complication (pleural effusion) that required aspiration. The incidence rate of major complications did not differ significantly between the two groups (p = 1.000).
In the CRLM group, ablation-related minor complications occurred in two patients (2.0%, 2/101), including one case of arterioportal shunting and one case of small subcapsular haematoma. In the HCC group, ablation-related minor complications occurred in two patients (2.1%, 2/97), including one case of minor intra-abdominal haemorrhage and one case of asymptomatic biloma. The incidence rate of minor complications did not differ significantly between the two groups (p = 1.000).
Tumour response
One hundred forty-three of 161 lesions (88.8%) exhibited CA after the first ablation in the CRLM group vs. 115 of 122 lesions (94.3%) in the HCC group; this difference was not significant (p = 0.110). For lesions ≤ 3 cm, the CA rate of the CRLM group was lower than that of the HCC group (p = 0.018). For lesions > 3 cm, the CA rate did not differ significantly between groups (p = 1.000) (). The mean diameter of the ablation zone was 3.5 ± 1.0 cm (range: 1.2–6.4 cm) in the CRLM group, compared with 4.0 ± 1.0 cm (range: 0.8–7.0 cm) in the HCC group (p = 0.492). The mean size of the safety margin was 0.5 ± 0.4 (range: 0–1.2 cm) in the CRLM group, compared with 0.5 ± 0.3 cm (range from 0–1.3 cm) in the HCC group (p = 0.106).
Table 2. Comparison of local therapeutic efficacy in the CRLM and HCC groups.
Local tumour progression
In the CRLM group, 36 (25.2%) of 143 lesions exhibited LTP during a mean follow-up period of 21.1 ± 20.7 months (range, 1.0–89.6 months), and the mean time of LTP was 11.8 ± 9.0 months (range, 2.1–29.6 months). In the HCC group, 20 (17.4%) of 115 lesions exhibited LTP during a mean follow-up period of 22.1 ± 17.6 months (range, 1–71.6 months), and the mean time of LTP was 13.8 ± 10.6 months (range, 3.2–41.1 months). The LTP rate (p = 0.132) and LTP time (p = 0.347) did not differ significantly between the two groups for lesions ≤ 3 cm. For lesions > 3 cm, the LTP rate was significantly higher in the CRLM group than the HCC group (p = 0.036) ().
The risk factors for LTP were analysed using the log-rank test. Among the 15 variables examined, hepatitis B/C infection, tumour size (≤ 3 cm/> 3 cm), type of thermal ablation (MWA/RFA) and safety margin (≤ 0.5 cm/> 0.5 cm) were identified as independent predictors of LTP in CRLM. Prothrombin time (> 14 s) and safety margin (≤ 0.5 cm/> 0.5 cm) were identified as independent predictors of LTP in HCC (). reveals that tumour size (≤ 3 cm/> 3 cm) was an independent predictor of LTP in CRLM but not in HCC. shows that the safety margin (≤ 0.5 cm/> 0.5 cm) was an independent predictor of LTP in both CRLM and HCC. The multivariate analysis revealed that only the safety margin (≤ 0.5 cm/> 0.5 cm) was a significant predictor of LTP in both CRLM and HCC ().
Figure 2. Curves of local tumour progression (LTP), calculated by the Kaplan–Meier method, according to tumour size (≤ 3 cm/> 3 cm). (a) The log-rank test demonstrates that over time, the presence of tumour size >3 cm was associated with a significantly higher rate of LTP in the CRLM group (p = 0.002). (b) The log-rank test demonstrates that over time, the presence of tumour size >3 cm was not associated with LTP in the HCC group (p = 0.979).
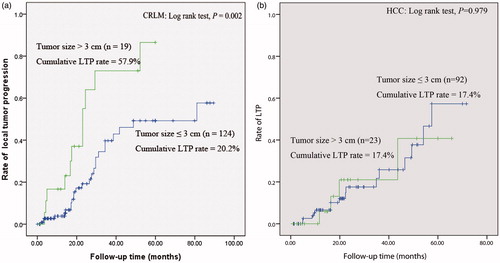
Figure 3. Curves of local tumour progression (LTP), calculated by the Kaplan–Meier method, according to safety margin (≤ 0.5 cm/> 0.5 cm). (a) and (b), The log-rank test demonstrates that over time, the presence of a safety margin ≤0.5 cm was associated with a significantly higher rate of LTP in the CRLM and HCC group (p < 0.001, p = 0.013).
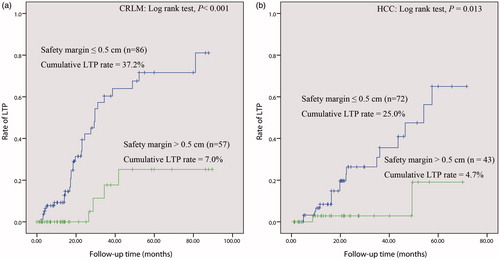
Figure 4. HCC in a 57-year-old patient. (a) The image obtained by CECT before thermal ablation indicates a lesion diameter of 4.1 cm in segment VIII, with hyper-enhancement in the arterial period (arrow). (b) One month after thermal ablation, the ablated area did not exhibit enhancement in CECT (arrow), which confirms complete ablation of the tumour. The diameter of the ablated area was 4.2 cm. We calculated a safety margin of 0 cm. (c) and (d) Approximately 7.1 months after ablation, the arterial phase in CECT revealed nodular hyper-enhancement at the lateral margin of the ablated area, which indicates LTP (arrow); washout of this nodule was observed in the portal venous phase of CECT.
Table 3. Univariate analysis of risk factors for LTP in CRLM and HCC after ablation.
Table 4. Multivariate analysis of risk factors for LTP in CRLM and HCC after ablation.
Discussion
The reported ablation-related major complication rates are 0 ∼ 33% for CRLM and 0 ∼ 20% for HCC [Citation7,Citation30]. The incidence rates of major and minor complications were similar and relatively low in both groups in the current study, which indicates that thermal ablation is a safe therapy for both types of liver tumours.
The CA rate was 88.8% in the CRLM group, which is consistent with the 81 ∼ 94% CA rates reported in the literature [Citation31–33]. The CA rate of the CRLM group was lower than that of the HCC group for all lesions, but this difference was not significant. For lesions ≤ 3 cm, the CA rate of the CRLM group was significantly lower than that of the HCC group, which may be attributable to the infiltrative growth of CRLM without a capsule and the fact that the actual sizes of CRLM lesions cannot be accurately assessed before ablation by imaging modalities with limited resolution [Citation34]. Furthermore, CA is the final desirable outcome resulting from many elements, including the employed technology, skill of the operator and anatomical location of the target. The high rates of CA are representative of the skill of the operators and the accuracy of the planification of the ablation treatment, and could predict the reliability of the treatment. Thus, a lower CA rate in the CRLM group indicates the technical difficulty of thermal ablation for CRLM, and suggests that for achieving a higher CA rate, the ablation of a small CRLM lesion is more challenging than that of a small HCC lesion; in addition, a small CRLM lesion should be very carefully ablated to guarantee complete clinical necrosis; such care includes precise tumour ablation planning prior to the ablation.
According to the literature, the LTP rate of CRLM varies significantly from 2% to 60% [Citation7,Citation17,Citation32,Citation35–39]. In the present study, the LTP rate was 25.2%, and the mean time to LTP was 11.8 months. The LTP rate was higher in the CRLM group than in the HCC group with no statistically significant difference. For lesions > 3 cm, the LTP rate of the CRLM group was significantly higher than that of the HCC group, which indicates that thermal ablation of larger tumours is more effective for local tumour control for HCC than CRLM. LTP is generally caused by the growth of microscopic viable tumours at the site of ablation of liver tumours [Citation40,Citation41]. Several reasons exist for the higher LTP rate for CRLM. First, LTP may be caused by peri-metastasis and infiltration of cancer cells in CRLM lesions, which does not occur in HCC [Citation34]. Second, the de novo growth of viable cells has been described in the lesions after hyperthermia treatments, especially if the tumour is in close contact with a vessel; moreover, rather than the proliferation of residual viable cancer cells that remaining in the ablation zone, the growth of viable cells from another lesion can also occur [Citation29]. Meanwhile, the presence of a microscopic nest of tumour cells is beyond the resolution limits of imaging before ablation [Citation42]. In addition, the reason that LTP occurs in patients with HCC is that HCC tumours may have many micrometastases that can appear up to 1.0 cm away from the main tumour, even if the tumour is encapsulated and is 3.0 cm or smaller. Our study suggests that as the size of CRLM lesions increases, the local control of the tumour becomes more difficult than in HCC.
Tumour size [Citation17,Citation28,Citation38,Citation39,Citation43,Citation44] and safety margin size [Citation4,Citation29,Citation38,Citation43] have been reported as predictors of LTP in liver tumours. The safety margin of ablation therapy is defined as the minimal margin of coagulation at each side of the tumour [Citation28]. The safety margin is considered an independent risk factor for survival after percutaneous radiofrequency ablation of HCC, and 0.5 cm has been recommended as an oncologic safety margin for ablation therapy [Citation45]. In an evaluation of the relationship between the safety margin and LTP in HCC and CRLM after RFA, Wang et al. concluded that a minimal margin > 5 mm in all directions around the target CRLM at 4–8 weeks post-RFA CT is associated with improved local tumour control [Citation29]. In contrast, Liu et al. observed that the safety margin was correlated with LTP in the HCC group, but not in the CRLM group, and concluded that for CRLM, the size of the post-ablation margin does not influence the rate of LTP [Citation43]. In the present study, the safety margin (≤ 0.5 cm/> 0.5 cm) correlated with the LTP rate in both the HCC and CRLM groups. Therefore, the safety margin during thermal ablation for CRLM and HCC should be > 0.5 cm to encompass the tissue surrounding the tumour so that the LTP rate can be reduced and the local therapeutic efficacy can be improved .
Our study is subject to several limitations, including its retrospective nature. Another limitation is that only tumours that were completely ablated were included in the evaluation of the safety margin, which may have led to selection bias. Furthermore, unlike the resected specimen, the measurement of the ablation zone and the corresponding safety margin was very challenging as it depended solely on CT imaging; CT imaging is associated with variables such as different patient positions and variations in the respiratory phases during pre- and post-ablation imaging. In addition, the safety margin data calculated by CT imaging were not confirmed by any pathological proof.
In conclusion, due to the biological characteristics of CRLM, thermal ablation is more effective in patients with HCC than in those with CRLM. To achieve better local tumour control, thermal ablation for CRLM should be more aggressive than it is for HCC, especially for large tumours encountered in clinical practice. A safety margin of 0.5 cm is recommended as appropriate for thermal ablation therapy.
| Abbreviations | ||
| CRLM | = | colorectal liver metastases |
| HCC | = | hepatocellular carcinoma |
| CA | = | complete ablation |
| RFA | = | radiofrequency ablation |
| MWA | = | microwave ablation |
| MLC | = | metastatic liver carcinoma |
| TACE | = | transcatheter arterial chemoembolization |
| CECT | = | contrast-enhanced computed tomography |
| LTP | = | local tumour progression |
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Additional information
Funding
References
- Nishikawa H, Kimura T, Kita R, et al. (2013). Radiofrequency ablation for hepatocellular carcinoma. Int J Hyperthermia 29:558–68.
- Chen MH, Yang W, Yan K, et al. (2004). Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients-mathematic model, overlapping mode, and electrode placement process. Radiology 232:260–71.
- Fong Y, Kemeny N, Paty P, et al. (1996). Treatment of colorectal cancer: hepatic metastasis. Semin Surg Oncol 12:219–52.
- Shady W, Petre EN, Gonen M, et al. (2016). Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes–a 10-year experience at a single center. Radiology 278:601–11.
- Solbiati L, Ahmed M, Cova L, et al. (2012). Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 265:958–68.
- Sorensen SM, Mortensen FV, Nielsen DT. (2007). Radiofrequency ablation of colorectal liver metastases: long-term survival. Acta Radiol 48:253–8.
- Pathak S, Jones R, Tang JM, et al. (2011). Ablative therapies for colorectal liver metastases: a systematic review. Colorectal Dis 13:e252–65.
- Alberts SR. (2012). Update on the optimal management of patients with colorectal liver metastases. Crit Rev Oncol Hematol 84:59–70.
- Bruix J, Sherman M. (2011). Management of hepatocellular carcinoma: an update. Hepatology 53:1020–2.
- N'Kontchou G, Mahamoudi A, Aout M, et al. (2009). Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 50:1475–83.
- Lee DH, Lee JM, Lee JY, et al. (2014). Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 270:900–9.
- Naugler WE, Sonnenberg A. (2010). Survival and cost-effectiveness analysis of competing strategies in the management of small hepatocellular carcinoma. Liver Transpl 16:1186–94.
- Kim KH, Yoon YS, Yu CS, et al. (2011). Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J Korean Surg SOC 81:25–34.
- Lu MD, Yin XY, Xie XY, et al. (2005). Percutaneous thermal ablation for recurrent hepatocellular carcinoma after hepatectomy. Br J Surg 92:1393–8.
- Tateishi R, Shiina S, Teratani T, et al. (2005). Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 103:1201–9.
- Flanders VL, Gervais DA. (2010). Ablation of liver metastases: current status. J Vasc Interv Radiol 21:S214–22.
- Solbiati L, Livraghi T, Goldberg SN, et al. (2001). Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology 221:159–66.
- He N, Jin QN, Wang D, et al. (2016). Radiofrequency ablation vs. hepatic resection for resectable colorectal liver metastases. J Huazhong Univ Sci Technol Med Sci 36:514–18.
- Weng M, Zhang Y, Zhou D, et al. (2012). Radiofrequency ablation versus resection for colorectal cancer liver metastases: a meta-analysis. PLoS One 7:e45493.
- Lee KH, Kim HO, Yoo CH, et al. (2012). Comparison of radiofrequency ablation and resection for hepatic metastasis from colorectal cancer. Korean J Gastroenterol 59:218–23.
- Khajanchee YS, Hammill CW, Cassera MA, et al. (2011). Hepatic resection vs minimally invasive radiofrequency ablation for the treatment of colorectal liver metastases: a Markov analysis. Arch Surg 146:1416–23.
- Wu YZ, Li B, Wang T, et al. (2011). Radiofrequency ablation vs hepatic resection for solitary colorectal liver metastasis: a meta-analysis. World J Gastroenterol 17:4143–8.
- Hof J, Wertenbroek MW, Peeters PM, et al. (2016). Outcomes after resection and/or radiofrequency ablation for recurrence after treatment of colorectal liver metastases. Br J Surg 103:1055–62.
- Facciorusso A, Di Maso M, Muscatiello N. (2016). Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia 32:339–44.
- Li M, Yu X, Liang P, et al. (2015). Ultrasound-guided percutaneous microwave ablation for hepatic malignancy adjacent to the gallbladder. Int J Hyperthermia 31:579–87.
- Li M, Yu XL, Liang P, et al. (2012). Percutaneous microwave ablation for liver cancer adjacent to the diaphragm. Int J Hyperthermia 28:218–26.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. J Vasc Interv Radiol 25:1691–705.
- Mulier S, Ni Y, Jamart J, Ruers T, et al. (2005). Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg 242:158–71.
- Wang X, Sofocleous CT, Erinjeri JP, et al. (2013). Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol 36:166–75.
- Thomasset SC, Dennison AR, Garcea G. (2015). Ablation for recurrent hepatocellular carcinoma: a systematic review of clinical efficacy and prognostic factors. World J Surg 39:1150–60.
- Sofocleous CT, Petre EN, Gonen M, et al. (2011). CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. J Vasc Interv Radiol 22:755–61.
- Veltri A, Sacchetto P, Tosetti I, et al. (2008). Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Intervent Radiol 31:948–56.
- Gillams AR, Lees WR. (2009). Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol 19:1206–13.
- Sofocleous CT, Nascimento RG, Petrovic LM, et al. (2008). Histopathologic and immunohistochemical features of tissue adherent to multitined electrodes after RF ablation of liver malignancies can help predict local tumor progression: initial results. Radiology 249:364–74.
- Bale R, Widmann G, Schullian P, et al. (2012). Percutaneous stereotactic radiofrequency ablation of colorectal liver metastases. Eur Radiol 22:930–37.
- Pawlik TM, Izzo F, Cohen DS, et al. (2003). Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol 10:1059–69.
- Vogl TJ, Straub R, Eichler K, et al. (2004). Colorectal carcinoma metastases in liver: laser-induced interstitial thermotherapy-local tumor control rate and survival data. Radiology 230:450–8.
- Kim YS, Rhim H, Cho OK, et al. (2006). Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol 59:432–41.
- Nakazawa T, Kokubu S, Shibuya A, et al. (2007). Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol 188:480–8.
- Kim YS, Lee WJ, Rhim H, et al. (2010). The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (>2 and <5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol 195:758–65.
- Sofocleous CT, Klein KM, Hubbi B, et al. (2004). Histopathologic evaluation of tissue extracted on the radiofrequency probe after ablation of liver tumors: preliminary findings. AJR Am J Roentgenol 183:209–13.
- Hoffman AL, Wu SS, Obaid AK, et al. (2002). Histologic evaluation and treatment outcome after sequential radiofrequency ablation and hepatic resection for primary and metastatic tumors. Am Surg 68:1038–43.
- Liu CH, Arellano RS, Uppot RN, et al. (2010). Radiofrequency ablation of hepatic tumours: effect of post-ablation margin on local tumour progression. Eur Radiol 20:877–85.
- Ayav A, Germain A, Marchal F, et al. (2010). Radiofrequency ablation of unresectable liver tumors: factors associated with incomplete ablation or local recurrence. Am J Surg 200:435–9.
- Peng ZW, Zhang YJ, Chen MS, et al. (2008). Risk factors of survival after percutaneous radiofrequency ablation of hepatocellular carcinoma. Surg Oncol 17:23–31.
