Abstract
Purpose: Transforming growth factor-β-activated kinase1 (TAK1) plays an anti-apoptotic role in response to multiple stresses. TAK1 inhibitor, 5Z-7-oxozeaenol (OZ) has been studied for its apoptotic effects. However, the combined effect of OZ with physical stresses remains to be elusive. Therefore, in this study we focussed to determine the combined effects of OZ with hyperthermia (HT) using Molt-4 cell line.
Materials and methods: Molt-4 cells were pre-treated with OZ for 1 h followed by heat exposure (44 °C, 10 min) and harvested 24 h after incubation at 37 °C, apoptosis was measured by Annexin V-FITC/PI double staining assay using flow cytometry and cell growth was observed by cell counting assay. Further mechanism involved in the combination was investigated by measuring mitochondrial membrane potential (MMP), intracellular ROS generation, expression of apoptosis related protein, intracellular calcium ion level and Fas activity.
Results: Combination of OZ with HT significantly enhances MMP loss and superoxide generation. Furthermore, OZ pre-treatment promotes caspase-8 cleavage, Fas externalisation, caspase 3 activity and intracellular calcium ion levels. OZ pre-treatment decreased the expression of HT-induced Bcl-2 and increased the expression of pro-apoptotic Bax, while markedly suppressed the phosphorylation of JNK and p38. In addition, increased expression of CHOP following combined treatment indicates that ER stress may also involve in the enhancement of HT-induced apoptosis.
Conclusion: Our data showed for the first time that OZ sensitizes Molt-4 cells to HT-induced apoptosis via extrinsic and intrinsic apoptotic pathways. Furthermore, ROS and ER stress may also play role in the enhancement of HT-induced apoptosis by OZ.
Introduction
Local hyperthermia therapy for various histological types of malignant tumours has shown promising progress in anti-tumour effects. A combination of hyperthermia (HT) with radio-or chemotherapy has been used for treatment of various solid tumours [Citation1–3]. However, complete eradication of tumour cells is not always achieved due to biological and technical difficulties. To overcome these difficulties, epoch-making drugs enhancing HT-induced apoptosis, so called thermo-sensitizers should be urgently developed. An ideal heat sensitizer would be non-toxic at normal temperatures but could be cytotoxic at high temperatures.
Transforming growth factor-β-activated kinase 1 (TAK1) was identified as a MAPK kinase kinase (MAP3K), which can be activated by transforming growth factor-β [Citation4]. ΤΑΚ1 is also activated by TNF-α, interleukin-1, and ligand of Toll-like receptors, such as lipopolysaccharides [Citation5]. TAK1 is essential for activating the IκB kinase (IKK/nuclear factor κB) (NF-κB) pathways and the stress kinases (i.e. JNK and p38MAPK) pathways in response to various stimuli [Citation6,Citation7].
It has been reported previously that TAK1 is involved in cell survival against multiple stimuli such as TNF-α, IL-1β, ligands of several TLRs and X-radiation [Citation8–10]. In our previous study it was demonstrated that 44 °C HT for 15 min activate the TAK1 pathway and promote cell survival [Citation11]. Recently, it was reported that TAK1 inhibition mediated by RNAi-silencing or an orally active TAK1 inhibitor significantly suppressed NF-κB activity and sensitized pancreatic cancer cells to gemcitabine-induced cell-death both in vitro and in vivo [Citation12].
5Z-7-oxozeaenol (OZ), a resorcyclic acid lactone, has been reported as a highly potent chemical inhibitor of TAK1 [Citation13]. Exposure of cells to OZ blocked IL-1-induced activation of TAK1, IKK, JNK, p38, and NF-κB. OZ has been known to induce apoptosis in various cell lines [Citation14,Citation15]. Furthermore, blockade of TAK1 kinase activity by OZ sensitised both HeLa and MEF cells to TNF-α, doxorubicin and etoposide-induced cell death [Citation16]. Therefore, in this study, we investigated the effects of OZ on HT-induced cell death in Molt-4 cells.
Materials and methods
Chemicals
OZ was purchased from R&D Systems (Minneapolis, MN). Stock solutions were prepared using dimethyl sulfoxide (DMSO) as a solvent, and further dissolved to make the desired concentrations for experimental use.
Cell line and culture
Human T lymphoblast cell line, Molt-4, was obtained from Human Sciences Research Resource Bank (Japan Human Sciences Foundation, Tokyo, Japan) and maintained in RPMI1640 medium supplemented with 10% heat-inactivated foetal bovine serum (FBS) at 37 °C in humidified air with 5% CO2.
Drug treatment and hyperthermia
A cell suspension containing 1 × 106 cells/ml was pre-treated with OZ for 1 h at 37 °C. For hyperthermia treatment, Molt-4 cells were seeded at a density of 1 × 106/ml in plastic tubes, and exposed to 44 °C by immersing tubes containing 1 ml of cell suspension into a precision-controlled water bath. After the treatment, cells were incubated at 37 °C for indicated time.
Apoptosis assay by flow cytometry
Flow cytometry was performed with propidium iodide (PI) and fluorescein isothiocyanate (FITC)-labeled annexin V (Immunotech, Marseille, France) to detect phosphatidylserine externalisation (on the surface of cell membrane) as an endpoint indicator of early apoptosis [Citation17]. After the treatments, the remaining intact cells were incubated at 37 °C for 6 h, collected, washed with cold PBS at 4 °C and centrifuged at 500 ×g for 5 min. FITC-labeled Annexin V (5 μl) and PI (5 μl) were added to 490 μl of the cell suspension and mixed gently. After incubation at 4 °C for 30 min in the dark, the cells were analysed by flow cytometry (Epics XL, Beckman-Coulter, Miami, FL).
Cell counting assay
Cell counting assay was performed using a Cell Counting Kit-8 (CCK-8) according to the protocol provided by the manufacturers (Dojindo Laboratories Co., Ltd., Kumamoto, Japan). Briefly, cells were pre-treated with OZ for 1 h, and exposed to HT (44 °C, 10 min). After 24 h incubation, cells were incubated in 100 μl RPMI medium (containing 10 μl CCK-8) in 96-well plate and incubated for 2 h at 37 °C in 5% CO2. The absorbance at 450 nm was determined by using Microplate Reader (Bio-Rad Laboratories, Inc., Hercules, CA).
Measurement of intracellular ROS production
Intracellular ROS generation in the Molt-4 cells was evaluated by flow cytometry using the fluorescence generated in the cells loaded with the sensitive fluorescent probes, a hydroxyl radical and peroxynitrite sensitive dye, hydroxyphenyl fluorescein (HPF) (Sekisui Medical Co., Tokyo, Japan). Hydroethidine (HE) (Molecular Probes, Eugene, OR) was used to determine superoxide generation and MitoSOX Red (Invitrogen, Carlsbad, CA) to detect mitochondrial superoxide. Cells were incubated at 37 °C with HPF, HE and MitoSOX Red at a final concentration of 5 μM for 15 min. For all of them, the fluorescence emission was analysed using flow cytometry [Citation18–20].
Measurement of mitochondrial membrane potential (MMP)
To measure changes in MMP, Molt-4 cells were stained with 10 nM tetramethylrhodamine methyl ester (TMRM) (Molecular Probes, Eugene, OR) for 15 min at 37 °C in PBS. The fluorescence of red TMRM was analysed by flow cytometry (excitation at 488 nm; emission at 575 nm). The percentage of low-MMP cells was determined from cell counts falling into the 0.1–12 low window of the TMRM log scale.
Western blot analysis
The cells were collected and lysed in lysis buffer (1 M Tris–HCl, 5 M NaCl, 1% Nonidet P-40 (v/v), 1% sodium deoxycholate, 0.05% SDS, 1 mM phenylmethylsulfonyl fluoride) for 20 min. After brief sonication, the lysates were centrifuged at 12 000 ×g 10 min at 4 °C, and the protein content in the supernatant was measured using a Bio-Rad Protein Assay kit (Bio-Rad, Hercules, CA). The protein lysates were denatured by heating at 96 °C for 5 min after mixing with SDS-loading buffer and were applied on an SDS polyacrylamide gel (Daiichi Pure Chemicals Co., Ltd, Tokyo, Japan) for electrophoresis, and transferred to nitrocellulose membranes (Amersham Biosciences, Buchinghamshire, UK). Western blot analysis was performed using anti-Bax polyclonal antibody (pAb), anti-p38 (Santa Cruz Biotechnology Inc., Dallas, TX), anti-caspase-3 pAb, anti-caspase-8 pAb, anti-JNK, anti-phospho-JNK mAb, anti-phospho-p38 mAb, anti-CHOP mAb, anti-Bip mAb (Cell Signalling Technology) and anti-β-actin mAb (Sigma-Aldrich, St. Louis, MO). Blots were then probed with either secondary horseradish peroxide (HRP)-conjugated anti-rabbit or anti-mouse IgG antibodies obtained from Cell Signalling. Band signals were visualised using a luminescent image analyser (LAS4000, Fujifilm Co., Tokyo, Japan) with chemi-luminescence ECL system (Amersham Biosciences). Where indicated, the relative amounts of proteins associated with specific antibodies were normalised to the intensities of β-actin. Band density was quantified by a BIO-ID image analyser, and the relative amounts of proteins associated with specific antibodies were normalised to the intensities of β-actin.
Flow cytometric detection of Fas on the cell surface
The cells were washed twice with PBS, suspended in 20 μl of washing buffer containing 2.5 μg/ml FITC-labeled anti-Fas monoclonal antibody (clone: UB3, MBL, Nagoya, Japan), incubated for 30 min at 37 °C, and then analysed by flow cytometry [Citation21].
Measurement of intercellular free calcium ions
To monitor the effects of OZ treatment on intracellular calcium homeostasis, intracellular free Ca2+ was measured using calcium probe Fluo-3/AM (Dojindo Laboratories Co., Ltd., Kumamoto, Japan). Cells were treated with OZ for 1 h, and then were exposed to HT (44 °C, 10 min). Cells were harvested 12 h after incubation, and loaded with 5 μM Fluo-3/AM for 30 min at 37 °C. Excess Fluo-3/AM was removed by washing three times with PBS. The fluorescence intensity of free Ca2+ levels was measured by flow cytometry.
Statistical analysis
Data are expressed as the means ± SD. Statistical analysis was carried out using the Student's t-test. p Values <0.05 were considered to be significant. All the experiments were performed in triplicate.
Results
Enhancement of HT-induced apoptosis by OZ
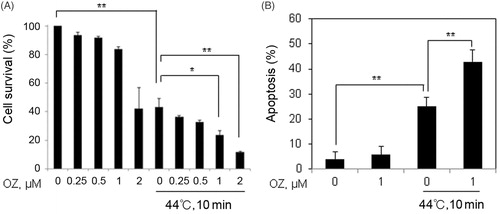
To investigate the effects of OZ on HT-induced apoptosis, cell counting assay was carried out using CCK-8 kit; cells were treated with OZ in a concentration-dependent manner with or without HT. The results showed that 1 μM concentration of OZ is non-toxic while significant decrease in cell survival was observed 24 h after combined treatment of OZ and HT. On the basis of this result 1 μM concentration of OZ is selected for further series of experiments (). Next, we examined the effects of 1 μM OZ with HT on cell death by Annexin V FITC/PI staining using flow cytometry. The results showed significant enhancement of apoptosis 24 h after combined treatment (). These results suggest that OZ may sensitize Molt-4 cells to HT treatment.
Figure 1. OZ increased HT-induced apoptosis in Molt-4 cells. (A) The cells were treated with HT (44 °C, 10 min) combined with or without OZ (0.25, 0.5, 1 and 2 μM) for 1 h and then incubated at 37 °C. Cell survival analysis was carried out by cell counting kit-8 after 24 h. (B) Cells were pre-treated with 1 μM OZ for 1 h and then exposed to HT (44 °C, 10 min). Cells were harvested 24 h after incubation and stained with Annexin V-FITC and PI for flow cytometry. The results are presented as mean ± SD (n = 3). **p < 0.01 vs. control, *p < 0.05, **p < 0.01 compared with HT-treated cells.
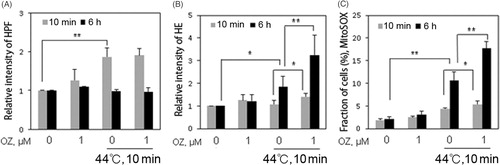
OZ potentiates HT-induced ROS generation
Excess production of intracellular ROS causes oxidative stress, which is an important signal in apoptosis [Citation22,Citation23]. To determine the role of ROS in the combined treatment, we measured generation of hydroxyl radical and superoxide by flow cytometry. HT alone is able to induce ROS generation including hydroxyl radicals and superoxide after 10 min and 6 h, respectively. However, in the combined treatment only superoxide generation was observed at 10 min and 6 h, while no enhancement in the generation of hydroxyl radical was observed (). Mitochondria are potent producers of cellular superoxide, and mitochondrial superoxide production is a major cause of the cellular oxidative damage [Citation24–26]. To examine whether mitochondrial superoxide was involved in the enhancement of apoptosis following combined treatment, MitoSOX red staining was performed and examined by flow cytometry. Mitochondrial superoxide generation was observed after 10 min in HT treated cells and significantly increased after 6 h in the combined treatment (). Taken together, these data suggests that OZ enhanced HT-induced ROS generation especially mitochondrial superoxide generation.
Figure 2. OZ enhanced HT-induced ROS generation. (A) Cells were pre-treated with 1 μM OZ for 1 h, and then loaded with HPF for 15 min. Fluorescence intensity was detected by flow cytometry at indicated time after HT exposure. (B) Cells were pre-treated with 1 μM OZ for 1 h, and then loaded with HE for 15 min. Fluorescence intensity was detected by flow cytometry time-dependently after HT exposure. (C) Cells were pre-treated with MitoSox red for 10 min. Fluorescence intensity was detected time-dependently by flow cytometry after HT exposure. The results are presented as mean ± SD (n = 3). *p < 0.05, **p < 0.01.
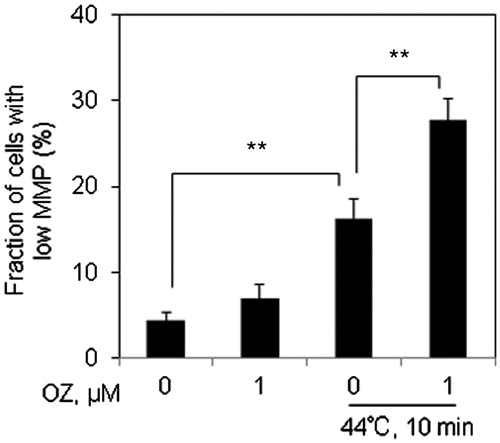
OZ enhances HT-induced MMP loss
As mitochondria constitute the main source of ROS generation, we then attempted to examine the changes in MMP by flow cytometry 12 h after combined treatment of OZ and HT. Significant loss of MMP was observed in the cells following combined treatment as compared to HT alone (.
Effects of combined treatment on cell death related proteins
In order to investigate the molecular mechanism of the enhancement of HT-induced apoptosis with OZ, western blot analysis was performed 12 h after incubation. Caspases are the important executioners of apoptosis induced by various apoptotic stimuli. It was observed that combination of OZ and HT increased the expression of cleaved caspase-3 as compared to HT treatment alone (). Bcl-2 family proteins with anti- or pro-apoptotic functions can control the release of mitochondrial apoptosis factors including cytochrome c and the apoptosis-inducing factor (AIF) [Citation27]. Here, slight increase in the expression of pro-apoptotic Bax and down regulation in the expression of anti-apoptotic Bcl2 was observed after combined treatment ().
Figure 4. Assessment of cell death related proteins. After 12 h incubation, protein was extracted from the cells and western blot analysis was performed to detect the expressions of diverse proteins. (A) Expressions of caspase-3, Bax, Bcl-2, and HSP70 were detected by western blot analysis. The Bcl-2, Bax and HSP70 signals were normalised to the β-actin signals, and the relative ratios are shown below each band. The anti-β-actin antibody was used as an internal control for the western blot analysis. (B) Expressions of MAPK pathway-related proteins such as p-JNK, JNK, p-p38, p38 were detected by western blot analysis 1 h after treatment.
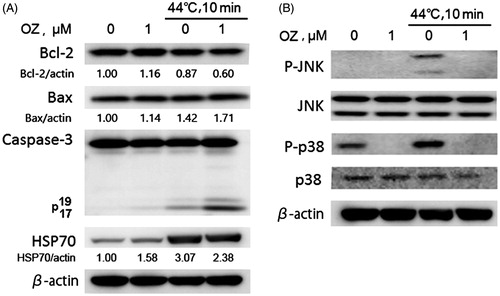
Heat shock is known to induce the upregulation of heat shock proteins (HSPs). Among HSPs, HSP70 has been shown to play a critical role in the cell survival and thermo-tolerance in response to stresses [Citation11]. Western blot data revealed decrease in the expression of HSP 70 after combined treatment ().
Activation of MAPKs plays a critical role in apoptosis induced by a variety of cellular stresses, and oxidative stress is known to activate the MAPK family members by protein phosphorylation [Citation28]. In order to investigate whether MAPKs signalling pathways are involved in OZ-mediated apoptosis enhancement, the activities of JNK and p38 were measured by western blot after 1 h HT or in combination with OZ. It was observed that HT treatment alone increases the phosphorylation of JNK and p38, while OZ treated cells markedly suppressed the phosphorylation of JNK and p38 (). Furthermore, no changes in JNK and p38 expressions were observed in cells treated with HT alone or in combination with OZ ().
Taken together, these results indicate that OZ might sensitise cells to HT through mitochondrial pathway but independent of p38/JNK pathway.
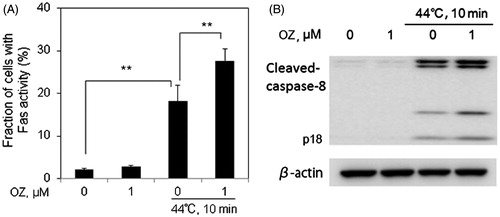
Effects of OZ on HT-induced Fas externalisation and caspase-8 expression
The Fas receptor is a death receptor on the surface of cells that leads to one of the apoptotic pathways, extrinsic pathway, through DISC assembly and subsequent caspase-8 activation. To determine whether OZ could increase HT-induced apoptosis via extrinsic pathway, Fas and caspase-8 activities were detected in Molt-4 cells 12 h after treatment. It was observed that OZ significantly enhanced HT-induced Fas externalisation and caspase-8 expression 12 h after HT exposure ().
Figure 5. OZ enhances HT-induced Fas externalisation and caspase-8 activation in Molt-4 cells. Cells were pre-treated with OZ (1 μM), then treated with HT (44 °C, 10 min). (A) The externalisation of Fas was determined by flow cytometry using anti-Fas FITC-conjugated antibody 12 h after treatment. The results are presented as mean ± SD (n = 3). **p < 0.01. (B) Caspase-8 expression was evaluated by western blot analysis 12 h after treatment.
Effects of OZ on intracellular Ca2+ levels and ER stress-mediated pathway
Calcium homeostasis is essential for various cellular functions, such as protein folding, processing, transport and signal transduction [Citation29]. Also, increased levels of Ca2+ overexcite cells and causes the generation of harmful chemicals like free radicals [Citation30]. HT is known to enhance calcium dependent apoptosis. Hence we assessed intracellular Ca2+ levels by flow cytometry. HT alone treatment showed increased intracellular Ca2+ compared to control; however, intracellular Ca2+ levels were enhanced to approximately 10-folds of basal levels after the combined treatment of OZ and HT ().
Figure 6. OZ pre-treatment enhances HT-induced intracellular Ca2+ levels via ER stress-related pathway in Molt-4 cells. Cells were pre-treated with OZ (1 μM), and then treated with HT (44 °C, 10 min). (A) Cells were loaded with 5 μM calcium probe Fluo-3/AM for 30 min, and intracellular Ca2+ level was measured by flow cytometry 12 after HT exposure. The results are presented as mean ± SD (n = 3). **p < 0.01. (B) Changes in the expressions of CHOP and Bip were detected by western blot analysis 12 h after HT exposure.
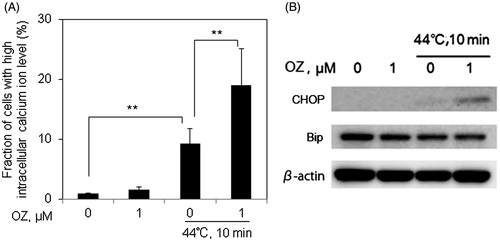
The pro-apoptotic effects of Ca2+ are regulated by a diverse range of Ca2+ sensitive factors that are compartmentalised in many intracellular organelles such as ER [Citation29]. The downstream signalling of ER stress is mainly transduced through CHOP (also known as DDIT3 or GADD153) and Bip (also known as GRP78), which are reported to elevate in ER stress-induced apoptosis [Citation31,Citation32]. It was observed that 12 h after incubation expression of CHOP was slightly induced by HT treatment alone, and this induction was markedly increased by OZ pre-treatment (). However, the expression of Bip was remain unchanged (), indicating that ER stress might involve in OZ-mediated enhancement of HT-induced apoptosis.
Discussion
TAK1 has a potent pro-survival role in activating the IκB kinase (IKK)-NF-κB pathway, which has numerous target genes, many of which block apoptosis, promotes cell proliferation and stimulate inflammatory responses [Citation33,Citation34]. HT treatment at 44 °C has been reported to activate TAK1 pathway and promote cell survival in Hela cells [Citation11]. Thus, inhibition of TAK1 may sensitize cells to apoptosis via different pathways. OZ was identified as the potent TAK1 inhibitor and sensitizes cells to apoptosis [Citation16]. In this study, we examined the effects of OZ on HT-induced apoptosis in Molt-4 cells. OZ alone does not induce any marked cytotoxic effects, however treatment of OZ in combination with HT caused significant enhancement of apoptosis in Molt-4 cells ().
To investigate the possible molecular mechanism by which OZ enhances HT-induced apoptosis we examined ROS production, MMP change and activation of caspases all of which are known to be important signal mediators of cell death. The intracellular ROS plays a key role in the induction of apoptosis [Citation26,Citation35,Citation36]. Heat stress to cells induced ROS such as superoxide, H2O2, nitric oxide in various cell lines and tumour tissues [Citation25,Citation37,Citation38]. Previously, it was demonstrated that HT-induced apoptosis was enhanced in U937 cells by agents which were associated with increase in the intracellular superoxide formation [Citation17,Citation21,Citation39]. In consistent, the present study also demonstrates that superoxide generation increased rapidly at a very early stage in Molt-4 cells treated with the combination of OZ and HT (), while enhancement of hydroxyl radical following combined treatment was not observed (). It has been reported that generation of mitochondrial superoxide is one of the major cause of MMP loss [Citation40,Citation41]. It was observed that the combined treatment causes mitochondrial superoxide formation and MMP loss in Molt-4 cells ( and .
Caspases play a critical role in the initiation and execution of apoptosis. They transduce the apoptotic cell death signals in a cascade manner, where the initiator caspases cleave and activate the effector caspases, which then degrade other cellular targets leading to cell death [Citation42]. It is known that death ligands bind to their death receptors (Fas or TRAIL) located in the cytoplasmic membrane, leading to the formation of ligand-receptor complex. This complex activates initiator caspase-8 which then cleaves and activates caspase-3 [Citation43]. Here, the activation of caspase-8 and caspase-3 following combined treatment was observed ( and ). Furthermore, Fas externalisation is also associated with caspase 8 activation after combined treatment (). ROS has been known as an initiator for MMP loss, capase-8 activity and Fas externalisation in HT-induced apoptosis in U937 cells [Citation21,Citation44]. Therefore, it might be possible that immediate ROS generation can enhance HT-induced apoptosis after combined treatment in Molt-4 cells through extrinsic apoptotic pathway.
The Bcl-2 family members play a key role in the regulation of mitochondrial apoptotic pathway. The pro-apoptotic Bcl-2 protein Bax translocate to mitochondria following death signals and triggers mitochondrial outer membrane permeabilization. In the present study slight increase in pro-apoptotic Bax and decrease in anti-apoptotic Bcl-2 protein was observed after combined treatment. This indicates that mitochondrial pathway may also involve in the enhancement of HT-induced apoptosis by OZ. Furthermore, suppression of HSP70 was also observed in the combined treatment, which plays an important role in cell survival and thermo-tolerance.
In previous studies it has been reported that an elevation of [Ca2+]i is involved in the enhancement of HT-induced apoptosis by agents which are associated with increase in the intracellular superoxide formation [Citation17]. Present study also shows the significant increase in [Ca2+]i concentration after combined treatment of OZ and HT ().
Oxidative stress causes rise in [Ca2+]i [Citation45]. In addition, intracellular calcium level also increases during ER stress-mediated apoptosis [Citation46]. Therefore two major ER proteins Bip and CHOP were examined 12 h after treatment. Activation of Bip is demonstrated to regulate toxicants or stimulus-induced apoptotic pathway [Citation47,Citation48]. CHOP play a key role in ER stress-induced apoptosis [Citation49]. Down regulation of pro-survival protein Bcl-2 is involved in the mechanism of CHOP-induced apoptosis [Citation50]. Here, it was demonstrated that Bip was not affected while the expression of CHOP was markedly increased (), and Bcl-2 was decreased () 12 h after combined treatment. This indicates that ER stress may be responsible for the enhancement of HT-induced apoptosis by OZ.
Activation of MAPKs plays an essential role in apoptosis induced by many cellular stresses. TAK1 activation has been known to phosphorylate and activate MAPKs such as ERK, p38 and JNK [Citation7,Citation50], and OZ induced TAK1 inhibition suppress MAPK phosphorylation such as p-JNK and p-p38 [Citation16]. In the present study it was observed that HT significantly enhanced the expression of p-p38 and p-JNK, while OZ pre-treatment inhibits HT-induced phosphorylation of p38 and JNK (). Hence, it is suggested that OZ enhances HT-induced apoptosis independent of p38/JNK pathway.
In conclusion, the current study showed that OZ sensitizes Molt-4 cells to HT-induced apoptosis via extrinsic and intrinsic apoptotic pathways. Furthermore, ROS and ER stress may also play role in the enhancement of HT-induced apoptosis by OZ. Based on these results we proposed that OZ might be an effective heat sensitiser. Further studies are needed to examine the effects of OZ on mild HT treated cells.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Urano M, Kuroda M, Nishimura Y. (1999). For the clinical application of thermochemotherapy given at mild temperatures. Int J Hyperthermia 15:79–107.
- Dahl O, Mella O. (2002). Referee: hyperthermia alone or combined with cisplatin in addition to radiotherapy for advanced uterine cervical cancer. Int J Hyperthermia 18:25–30.
- Prosnitz L, Jones E. (2002). Counterpoint: test the value of hyperthermia in patients with carcinoma of the cervix being treated with concurrent chemotherapy and radiation. Int J Hyperthermia 18:13–18.
- Yamaguchi K, Shirakabe K, Shibuya H, et al. (1995). Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science 270:2008–11.
- Singhirunnusorn P, Suzuki S, Kawasaki N, et al. (2005). Critical roles of threonine 187 phosphorylation in cellular stress-induced rapid and transient activation of transforming growth factor-beta-activated kinase 1 (TAK1) in a signaling complex containing TAK1-binding protein TAB1 and TAB2. J Biol Chem 280:7359–68.
- Sato S, Sanjo H, Takeda K, et al. (2005). Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol 6:1087–95.
- Shim JH, Xiao C, Paschal AE, et al. (2005). TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev 19:2668–81.
- Furusawa Y, Wei ZL, Sakurai H, et al. (2012). TGF-beta-activated kinase 1 promotes cell cycle arrest and cell survival of X-ray irradiated HeLa cells dependent on p21 induction but independent of NF-kappaB, p38 MAPK and ERK phosphorylations. Radiat Res 177:766–74.
- Hirata Y, Sugie A, Matsuda A, et al. (2013). TAK1-JNK axis mediates survival signal through Mcl1 stabilization in activated T cells. J Immunol 190:4621–6.
- Roh YS, Song J, Seki E. (2014). TAK1 regulates hepatic cell survival and carcinogenesis. J Gastroenterol 49:185–94.
- Li P, Furusawa Y, Wei ZL, et al. (2013). TAK1 promotes cell survival by TNFAIP3 and IL-8 dependent and NF-κB independent pathway in HeLa cells exposed to heat stress. Int J Hyperthermia 29:688–95.
- Melisi D, Xia Q, Paradiso G, et al. (2011). Modulation of pancreatic cancer chemoresistance by inhibition of TAK1. J Natl Cancer Inst 103:1190–204.
- Ninomiya-Tsuji J, Kajino T, et al. (2003). A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem 278:18485–90.
- Acuna UM, Wittwer J, Ayers S, et al. (2012). Effects of (5Z)-7-oxozeaenol on MDA-MB-231 breast cancer cells. Anticancer Res 32:2415–21.
- Acuna UM, Wittwer J, Ayers S, et al. (2012). Effects of (5Z)-7-oxozeaenol on the oxidative pathway of cancer cells. Anticancer Res 32:2665–71.
- Fan Y, Cheng J, Vasudevan SA, et al. (2013). TAK1 inhibitor 5Z-7-oxozeaenol sensitizes neuroblastoma to chemotherapy. Apoptosis 18:1224–34.
- Zhao QL, Fujiwara Y, Kondo T. (2006). Mechanism of cell death induction by nitroxide and hyperthermia. Free Radic Biol Med 40:1131–43.
- Gorman A, McGowan A, Cotter TG. (1997). Role of peroxide and superoxide anion during tumour cell apoptosis. FEBS Lett 404:27–33.
- Setsukinai K, Urano Y, Kakinuma K, et al. (2003). Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J Biol Chem 278:3170–5.
- Kudin AP, Bimpong-Buta NY, Vielhaber S, et al. (2004). Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem 279:4127–35.
- Yu DY, Zhao QL, Wei ZL, et al. (2009). Enhancement of hyperthermia-induced apoptosis by sanazole in human lymphoma U937 cells. Int J Hyperthermia 25:364–73.
- Tripathi P, Hildeman D. (2004). Sensitization of T cells to apoptosis-a role for ROS? Apoptosis 9:515–23.
- Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. (2007). Mitochondria, oxidative stress and cell death. Apoptosis 12:913–22.
- Brand MD, Affourtit C, Esteves TC, et al. (2004). Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med 37:755–67.
- Roti Roti JL. (2008). Cellular responses to hyperthermia (40-46 degrees C): cell killing and molecular events. Int J Hyperthermia 24:3–15.
- Slimen IB, Najar T, Ghram A, et al. (2014). Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int J Hyperthermia 30:513–23.
- Tsujimoto Y, Shimizu S. (2000). Bcl-2 family: life-or-death switch. FEBS Lett 466:6–10.
- Lu TH, Hsieh SY, Yen CC, et al. (2011). Involvement of oxidative stress-mediated ERK1/2 and p38 activation regulated mitochondria-dependent apoptotic signals in methylmercury-induced neuronal cell injury. Toxicol Lett 204:71–80.
- Zhao S, Xiong Z, Mao X, et al. (2013). Atmospheric pressure room temperature plasma jets facilitate oxidative and nitrative stress and lead to endoplasmic reticulum stress dependent apoptosis in HepG2 cells. PLoS One 8:e73665.
- Emam H, Zhao QL, Furusawa Y, et al. (2012). Apoptotic cell death by the novel natural compound, cinobufotalin. Chem Biol Interact 199:154–60.
- Li J, Lee AS. (2006). Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med 6:45–54.
- Han J, Back SH, Hur J, et al. (2013). ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 15:481–90.
- Hayden MS, Ghosh S. (2008). Shared principles in NF-kappaB signaling. Cell 132:344–62.
- Sakurai H. (2012). Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol Sci 33:522–30.
- Shackelford RE, Kaufmann WK, Paules RS. (2000). Oxidative stress and cell cycle checkpoint function. Free Radic Biol Med 28:1387–404.
- Salganik RI. (2001). The benefits and hazards of antioxidants: controlling apoptosis and other protective mechanisms in cancer patients and the human population. J Am Coll Nutr 20(5 Suppl):464S–72S.
- Davidson JF, Whyte B, Bissinger PH, Schiestl RH. (1996). Oxidative stress is involved in heat-induced cell death in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 93:5116–21.
- Flanagan SW, Moseley PL, Buettner GR. (1998). Increased flux of free radicals in cells subjected to hyperthermia: detection by electron paramagnetic resonance spin trapping. FEBS Lett 431:285–6.
- Li FJ, Kondo T, Zhao QL, et al. (2003). A lipophilic free radical initiator, 2,2'-azobis (2,4-dimethylvaleronitrile) (AMVN) enhances caspase-dependent apoptosis induced by hyperthermia. Int J Hyperthermia 19:165–77.
- St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. (2002). Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277:44784–90.
- Malik F, Kumar A, Bhushan S, et al. (2007). Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis 12:2115–33.
- Wolf BB, Schuler M, Echeverri F, Green DR. (1999). Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. J Biol Chem 274:30651–6.
- Kiechle FL, Zhang X. (2002). Apoptosis: biochemical aspects and clinical implications. Clin Chim Acta 326:27–45.
- Yu DY, Matsuya Y, Zhao QL, et al. (2008). Enhancement of hyperthermia-induced apoptosis by a new synthesized class of benzocycloalkene compounds. Apoptosis 13:448–61.
- Asada S, Fukuda K, Nishisaka F, et al. (2001). Hydrogen peroxide induces apoptosis of chondrocytes; involvement of calcium ion and extracellular signal-regulated protein kinase. Inflamm Res 50:19–23.
- Choi AY, Choi JH, Lee JY, et al. (2010). Apigenin protects HT22 murine hippocampal neuronal cells against endoplasmic reticulum stress-induced apoptosis. Neurochem Int 57:143–52.
- Oyadomari S, Mori M. (2004). Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11:381–9.
- Huang SM, Cheung CW, Chang CS, et al. (2011). Phloroglucinol derivative MCPP induces cell apoptosis in human colon cancer. J Cell Biochem 112:643–52.
- Li Y, Guo Y, Tang J, Jiang J, Chen Z. (2014). New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai) 46:629–40.
- Mihaly SR, Ninomiya-Tsuji J, Morioka S. (2014). TAK1 control of cell death. Cell Death Differ 21:1667–76.