Abstract
Purpose: There is no standard treatment for peritoneal metastases (PM) from gastric cancer (GC). The aim of this review is to evaluate the clinical trials on cytoreductive surgery (CRS) plus hyperthermic intraperitoneal chemotherapy (HIPEC) for GC PM.
Materials and methods: The published clinical trials on CRS + HIPEC for GC PM are critically evaluated, and survival and safety are the primary endpoints. In addition, the registered ongoing clinical trials are summarised.
Results: The natural course of GC PM is <5 months. CRS + HIPEC could improve the overall survival (OS). In prospective studies, the median OS was 11.0 months in the CRS + HIPEC group vs. 5.4 months in the CRS alone group. In case-control studies, the median OS was 13.3 months in the CRS + HIPEC group vs. 7.9 months in the CRS alone group. In cohort studies, the median OS after CRS + HIPEC was 13.3. The median 1-, 2- and 5-year survival rates after CRS + HIPEC were 50.0%, 35.8% and 13.0%, respectively. There is no statistically significant increase in serious adverse events that are directly attributed to CRS + HIPEC.
Conclusions: The combination of CRS and HIPEC is a promising integrated treatment strategy for GC PM that has encouraging initial results, calling for urgent further evaluation of this strategy in randomised control trials (RCTs).
Introduction
Gastric cancer (GC) accounts for 9% of all cancer-related deaths [Citation1], and China accounts for over 40% of all new cases worldwide [Citation1,Citation2]. Approximately 30% of GC patients have regional spread at diagnosis, and the locoregional progression of GC generally results in peritoneal metastases (PM), which are characterised by the presence of tumour nodules of various sizes, numbers, and distributions on the peritoneal surface [Citation3,Citation4]. PM have a significant negative impact on the overall survival (OS) and quality of life (QoL) as a result of refractory ascites, progressive intestinal obstruction and uncontrollable abdominal pain. The traditional therapies for such patients are systemic chemotherapy, palliative surgery and the best supportive care. The prognosis for GC PM is poor because of its recurrent patterns, and it has a median survival of approximately half a year [Citation5].
However, over the past three decades, significant improvements in the diagnosis and treatment of PM have been achieved as a result of the in-depth understanding of the fundamental mechanisms of PM and treatment technology improvements. It is now widely accepted in the oncology community that at least some PM are due to regional and not terminal disease, and proactive local-regional therapy that combines cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) has the advantages of surgical removal of a visible bulky tumour burden and regional hyperthermic chemotherapy to eradicate micrometastases and invisible free tumour cells [Citation6].
Focussing on GC PM, this work aims to review the milestone developments from perspectives of published and ongoing clinical trials. The former depict the real-life natural course of GC PM, and the latter is critically evaluating the efficacy and safety issues of CRS + HIPEC. Finally, the registered clinical trials involving GC PM are also summarised to inspire future developments.
Materials and methods
Literature search strategy
A comprehensive search of electronic databases was performed using Medline, PubMed, Web of Science and Cochrane library with the search terms “gastric cancer”, “hyperthermic intraperitoneal chemotherapy” and “HIPEC”, from January 1, 1980 to August 1, 2016. The literature search also included all Mesh Terms, as spontaneously matched by PubMed. Review articles were also obtained to determine other relevant studies. Only the latest version was considered for duplicate published trials with accumulating numbers of patients or increased lengths of follow-up. The reference lists of all retrieved articles were examined for further identification of potentially relevant studies. A comprehensive search of registered clinical trials was performed in clinicaltrials.gov with the search terms “gastric” and “peritoneal”.
Selection criteria
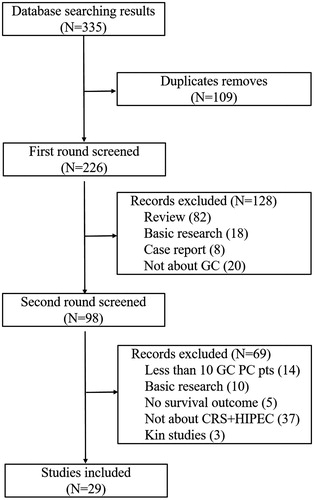
All articles were assessed in two rounds by two independent experienced reviewers (Y. Li and Z.H. Ji). The first round of the literature search focussed on reading the titles, abstracts and keywords in published review articles, basic research articles, case reports and clinical trials, and any paper that did not include GC was excluded. Papers on prospective or retrospective clinical trials about CRS + HIPEC in treating GC PM were selected for the second round of assessment. In the second assessment, the full texts were reviewed, and the articles included in this review had over 10 cases reporting on the median and/or mean OS or 1-, 2-, 3- and 5-year survival rates, with or without morbidity and mortality information ().
Figure 1. Flow chart of the search process and results. A total of 335 relevant papers were selected from all the published literature; 109 duplicated papers were removed, 226 papers underwent the first round of assessment and 98 papers underwent the second round of assessment. Only 29 qualified papers were identified for inclusion in the final evaluation.
Data extraction and management
Two reviewers independently extracted data, including the publication time, type of design (randomised control, prospective, retrospective case-control or retrospective cohort), country, number of patients, treatment arms, HIPEC characteristics, survival outcomes (median OS; 1-, 2-, 3- and 5-year survival rates; and other survival-related parameters), mortality and morbidity and multivariate analysis results.
Statistical analysis
Descriptive statistics (simple counts, means and medians) were used to summarise the extracted data. The management of data and construction of tables was performed with Excel 2010 (Microsoft Corp., Redmond, WA, USA), and the drawing of figures was performed with Graph Pad Prime 6 (GraphPad Software, Inc. La Jolla, CA, USA).
Results
Importance of PM in GC patients
Several large-sample clinical studies across major regions of the world demonstrated that GC PM is a long-standing but ignored clinical problem () [Citation7–11]. These studies at different time periods and through different geographic regions have provided convincing evidence that PM is the most frequent and aggressive type of GC metastasis, and it remains the most important challenge in GC treatment.
Table 1. Information from large-sample clinical trials on postoperative recurrence of gastric cancer.
The natural course of GC PM
Two large-sample studies, one institution-based [Citation12] and one population-based [Citation5], have provided convincing data on the natural history of GC PM, from two different time periods and geographic areas. In the institution-based study, performed in France in 2000 [Citation12], the natural course of 125 patients with GC PM was first systematically described. The mean and median OS values were 6.5 months and 3.1 months. In the population-based study, conducted in the Netherlands in 2014 [Citation5], the median OS values were 4.6 months for patients with PM as the only metastatic site and 3.3 months for those with PM combined with other metastases. Taken together, these studies firmly established that the natural course of GC PM is less than five months.
CRS + HIPEC trials for GC PM
In terms of clinical studies on CRS + HIPEC to treat GC PM, retrospective, cohort and prospective studies have been conducted throughout the world, demonstrating improved clinical efficacy and acceptable safety profiles. At the time of this review, a total of 1863 patients with GC PM were included in CRS + HIPEC studies, including 1659 patients in retrospective studies and 204 patients in prospective studies. The major features of these studies are summarised in [Citation13–41].
Table 2. Major characteristics of CRS + HIPEC studies for peritoneal carcinomatosis from gastric cancer.
Technical analyses of these studies revealed several eminent features. In terms of the geographic distribution, 12 studies were from Europe (France 6, Germany 3, Sweden 1, Greece 1 and Ukraine 1), 13 from Asia (Japan 7, Mainland China 5 and Taiwan China 1), and 4 from America (US 4). In terms of the publication time, 15 studies were published during 2010–2016, 8 during 2000–2010, and 6 during 1990–2000. In terms of major CRS + HIPEC protocols, 14 studies adopted open HIPEC, 10 studies used closed HIPEC, 1 study used both open and closed techniques, and 4 studies did not specify the technical modality.
The most promising result is the survival outcome. Five prospective studies and 24 retrospective studies were available for survival analysis, including the median OS; 1-, 2-, 3- and 5-year survival rates, and other survival-related parameters () [Citation5,Citation12–41]. There are slight differences in the median OS among different types of studies. For the five prospective studies, the median OS was 11.0 months (range 10.0–11.3 months) in the CRS + HIPEC group vs. 5.4 months (range 4.3–6.5 months) in the CRS alone group. For the eight retrospective case-control studies, the median OS was 13.3 months (range 8.0–24.5 months) in the CRS + HIPEC group vs. 7.9 months (range 5.0–11.0 months) in the CRS alone group. For the 16 retrospective cohort studies, the median OS after CRS + HIPEC was 13.3 (range 6.6–37.0 months). The median 1-, 2- and 5-year survival rates after CRS + HIPEC were 50.0% (range 41.2%–83.4%), 35.8% (range 19.9%–69.0%) and 13.0% (range 6.4%–31.0%), respectively.
Table 3. Survival outcomes of CRS + HIPEC treating peritoneal carcinomatosis from gastric cancer.
Prognostic factors have also been explored in these studies. Multivariate analyses were conducted in 16 studies to identify independent prognostic factors (). In terms of the frequency, the following parameters were identified as independent prognostic factors: the completeness of cytoreduction (CCR) in 12 studies; peritoneal cancer index (PCI) in four studies; HIPEC, systemic chemotherapy and TNM stage each in three studies; and ascites, tumour histopathology, performance status, peritonectomy and postoperative complications each in two studies.
There is adequate evidence to judge the safety profile of CRS + HIPEC for treating GC PM () [Citation13,Citation15–20,Citation25,Citation27–29,Citation31,Citation35–39]. In terms of adverse events, the most frequent adverse events include pleural effusion, ileus, sepsis, wound infection, occlusion, hypoalbuminemia and anastomosis leakage.
Table 4. Mortality, morbidity, and serious adverse events (SAEs) of CRS + HIPEC.
Although the published survival data are promising, the results should be interpreted with caution because of the low quality of these studies. Among five prospective studies included in this review, only one randomised control trial (RCT) with sample size of 68 patients compared CRS + HIPEC vs. CRS alone, one RCT with sample size of 17 patients compared CRS + HIPEC + postoperative systemic chemotherapy vs. systemic chemotherapy alone, and the other three studies were prospective cohort studies with a total sample size of 119 patients. Of 24 retrospective studies, 16 studies were retrospective cohort studies or cases reports with a sample size ranging from 14 to 231 patients. Small sample size, lack of a control group and retrospective design are the major causes of a low quality of evidence. Therefore, the results from these studies are promising and should be the basis for further high quality research.
Critical appraisal of the ongoing clinical trials in GC PM
We have identified 101 registered clinical trials in clinicaltrials.gov; 28 are ongoing clinical trials focussing on GC PM. Of the 28 ongoing clinical trials, 5 are on the diagnosis or evaluation of GC PM, 15 are on the treatment for GC PM, and 8 are on prophylaxis for GC PM (Supplemental Table 1).
Among five diagnostic trials, three used peritoneal lavage as the study object, and the major objective was to try to identify tumour cells or effective tumour makers to diagnose and evaluate PM.
The 15 therapeutic trials focussed on intraperitoneal (IP) administration of cytotoxic or immunologic drugs, including nine using HIPEC with/without IP and intravenous (IV) chemotherapy, five using IP with/without IV chemotherapy, and one using pressurised IP air-flow chemotherapy. Hyperthermic or normothermic IP administration of anti-tumour drugs combined with conventional systemic chemotherapy is the mainstream model of adjuvant chemotherapy for GC PM. However, only six trials combined CRS and HIPEC with/without IP or IV adjuvant chemotherapy for a curative intent, which means most oncologists still regard GC PM as a terminal incurable condition rather than a locoregional curable metastasis such as liver metastasis from colorectal cancer.
In eight prophylactic trials, three different strategies were used to prevent PM in GC patients, including extensive peritoneal lavage in three trials, HIPEC in three trials and postoperative IP chemotherapy in two trials.
In terms of the study design, 9 of 27 trials had a randomised parallel assignment, and the remaining 18 had non-randomised single group assignment. There were only two multi-centre RCTs, the GASTRIPEC and GASTRICHIP studies.
The GASTRIPEC study is a multi-centre German trial on GC PM patients with the objective of comparing neoadjuvant chemotherapy + CRS + HIPEC + postoperative chemotherapy vs. neoadjuvant chemotherapy + CRS + postoperative chemotherapy. This trial started on May 4, 2014, with established enrolment of 180, and it is still recruiting participants.
The GASTRICHIP study is a multi-centre French trial on advanced GC patients with a high risk for PM, and the objectives are to compare the five-year OS rates and recurrence-free or locoregional-free survival rates in GC patients treated with either curative gastrectomy + adjuvant HIPEC or with curative gastrectomy alone. This study started at May 2013 with an established enrolment of 322, and it is still recruiting participants and opening new centres at present. In 2016, Oliver Glehen reported their partial results at the PSOGI meeting in Washington DC. The first safety evaluation in November 2015 of 50 inclusions demonstrated that there was no difference in the mortality and morbidity between the Gastrectomy + HIPEC and Gastrectomy alone groups. The second safety evaluation in October 2016 of 120 inclusions found that one patient died within 60 days after surgery in the Gastrectomy + HIPEC group and no patients died in the Gastrectomy alone group. The severe morbidity was 28.4% in the Gastrectomy + HIPEC group and 26.2% in the Gastrectomy alone group.
Discussion
In the field of understanding and treating GC PM, what successes have we gained and what questions remain unanswered? From the comprehensive review of the current literature, several facts are evident.
First, there has been considerably increasing awareness of the importance of GC PM. The fundamental data on natural course of the disease obtained from France [Citation12] and the Netherlands [Citation5] demonstrated that the prognosis of GC PM is even worse than conventionally expected; it has an OS of less than five months. Moreover, PM are the most frequent and aggressive sites of metastasis in GC. It has been repeatedly demonstrated that the currently adopted conventional treatments could not improve the OS for such patients. These studies repeatedly imply that, at least for GC treatment, there are fundamental flaws in the concept, design and techniques in the currently universally adopted standardised curative gastrectomy with D2 lymphadenectomy. Under the current treatment paradigm, due attention has been paid to local control by superb resection techniques, and adequate attention has also been paid to systematic controls by improving systemic chemotherapeutic regimens, but far less attention has been paid to regional controls.
Therefore, there should be concept changes in the theory of GC metastasis. After curative resection, GC metastasis seems to follow a pattern of stepwise progression in that peritoneal metastasis is the first, key dissemination site and that other forms of GC spread occur later. Therefore, GC should not be regarded as a disease with early systemic metastasis. Instead, GC should be considered a disease with early intra-abdominal spread that is mostly in the form of PM, which is associated over time with a steadily increasing incidence of distant spreading [Citation42]. Based on these in-depth understandings of GC metastasis, more attention should be paid to GC PM in terms of both basic research and clinical practice.
Second, CRS + HIPEC offer promising benefits for treating GC PM. As an integrated treatment strategy using the combination of surgical resection, cytotoxic chemotherapy, hyperthermic tumour ablation and hydrodynamic washing [Citation43,Citation44], CRS + HIPEC is the only possible treatment strategy that offers concrete clinical benefits for patients with GC PM, and it has attracted increasing and expanding attention from the oncology community across the world, particularly over the past decade. Although the published trials have not yielded unequivocal conclusions, evidence has been obtained to support the idea that CRS + HIPEC is promising for GC PM. After 30 years of exploration and development, adequate evidence is available showing that CRS + HIPEC could improve the median OS up to approximately 13 months, double the median OS associated with conventional therapy. Moreover, CRS + HIPEC could offer a survival benefit for selected GC PM patients with acceptable safety profiles.
Third, to maximise the efficacy and minimise the adverse events of CRS + HIPEC, careful patient selection is critical. Well-planned, structured pre-operative examinations are essential, including a performance status evaluation, serum levels of tumour makers, and medical imaging assessments, as reported in a recent expert consensus statement [Citation6]. PCI and CCR, which were first described by Sugarbaker et al. [Citation45,Citation46], are two key prognostic factors for a better OS, and they are considered as inclusion criteria written into expert consensus of different countries [Citation6] and large specialised PM institutions [Citation22,Citation47,Citation48]. Therefore, a whole set of screening approaches is important, including routine physical examination, performance status evaluation, blood tests of the nutrition status, major organ function parameters, tumour markers, and specialised medical imaging studies [Citation6]. Although efforts have been made to better define resectability before implementing surgical exploration, surgical exploration, preferably laparoscopic surgery, provides accurate evaluation of the resectability [Citation22].
Fourth, the optimal technical approaches to HIPEC remains an unanswered question. In terms of the technical aspect of CRS + HIPEC, there is no evidence supporting the choice of open vs. closed HIPEC. Both techniques are acceptable in the current clinical practice. Similarly, in terms of drugs used in HIPEC, both the traditional mitomycin C- and cisplatin-based regimens as well as platinum- or docetaxel-based drugs are acceptable [Citation49]. The selection of cytotoxic drugs used for HIPEC is mainly based on their depth of infiltration and their cytotoxic effects, but it is not strictly related to the pathology of the primary tumour [Citation7,Citation48,Citation50]. There is still no standard protocol for the HIPEC duration, flow rate, temperature, drug selection or technique. Additionally, there is no control study evaluating these HIPEC-related parameters. In the present review, we found that an open HIPEC using mitomycin C- and cisplatin-based drug regimens for 60–90 min at 40–43 °C with a flow rate of 500 ml/min was the most frequently used approach.
Fifth, important issues remain unsettled in the field of CRS + HIPEC for GC PM in terms of clinical trials. The most important issue is in additional multi-centre trials and broader international collaborations. Multi-centre collaborations for RCTs are required to generate higher levels of evidence. Currently, most trials are retrospective or prospective single-centre trials. Furthermore, at present, there is only a single-centre RCT conducted by Yang et al. [Citation13] that was conducted in China, and two registered multi-centre RCTs (GASTRIPEC and GASTRICHIP) are ongoing in Europe. Many countries have developed specialised peritoneal cancer centres and have accumulated sufficient surgical experience. It is now time for international collaborations in multi-centre RCTs, and the field is well prepared for developing guidelines to use CRS + HIPEC as part of GC PM treatment. In addition, standardised protocols are required for widespread application of the technique.
In conclusion, the combination of CRS and HIPEC is a promising integrated treatment strategy for GC PM that has encouraging initial results and calls for urgent further evaluation of this strategy in RCTs.
Acknowledgements
This work was supported by the Key Discipline Development Fund of Beijing Shijitan Hospital, Capital Medical University (2016fmzlwk), and the Special Fund for the Capital Characteristic Clinical Medicine Development Project (Z161100000516077) (both to Yan Li).
Disclosure statement
The authors declare that there are no conflicts of interest to report for this work.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, et al. (2015). Global cancer statistics, 2012. CA Cancer J Clin 65:87–108.
- Chen W, Zheng R, Baade PD, et al. (2016). Cancer statistics in China, 2015. CA Cancer J Clin 66:115–32.
- Bijelic L, Sugarbaker PH. (2012). The role of intraperitoneal chemotherapy in the treatment of patients with advanced gastric cancer. Ann Ital Chir 83:224–31.
- Yonemura Y, Canbay E, Li Y, et al. (2016). A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur J Surg Oncol 42:1123–31.
- Thomassen I, Van Gestel YR, Van Ramshorst B, et al. (2014). Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer 134:622–8.
- Li Y, Zhou YF, Liang H, et al. (2016). Chinese expert consensus on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal malignancies. World J Gastroenterol 22:6906–16.
- Roviello F, Marrelli D, De Manzoni G, et al. (2003). Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg 90:1113–19.
- Sakuramoto S, Sasako M, Yamaguchi T, et al. (2007). Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–20.
- Yu JI, Lim Do H, Ahn YC, et al. (2015). Effects of adjuvant radiotherapy on completely resected gastric cancer: a radiation oncologist’s view of the ARTIST randomized phase III trial. Radiother Oncol 117:171–7.
- Cao L, Selby LV, Hu X, et al. (2016). Risk factors for recurrence in T1-2N0 gastric cancer in the United States and China. J Surg Oncol 113:745–9.
- Lee JH, Lee CM, Son SY, et al. (2014). Laparoscopic versus open gastrectomy for gastric cancer: long-term oncologic results. Surgery 155:154–64.
- Sadeghi B, Arvieux C, Glehen O, et al. (2000). Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 88:358–63.
- Yang XJ, Huang CQ, Suo T, et al. (2011). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 18:1575–81.
- Glehen O, Gilly FN, Arvieux C, et al. (2010). Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol 17:2370–7.
- Rudloff U, Langan RC, Mullinax JE, et al. (2014). Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol 110:275–84.
- Yang XJ, Li Y, Yonemura Y. (2010). Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat gastric cancer with ascites and/or peritoneal carcinomatosis: Results from a Chinese center. J Surg Oncol 101:457–64.
- Glehen O, Schreiber V, Cotte E, et al. (2004). Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch Surg 139:20–6.
- Beaujard AC, Glehen O, Caillot JL, et al. (2000). Intraperitoneal chemohyperthermia with mitomycin C for digestive tract cancer patients with peritoneal carcinomatosis. Cancer 88:2512–9.
- Wu HT, Peng KW, Ji ZH, et al. (2016). Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with lobaplatin and docetaxel to treat synchronous peritoneal carcinomatosis from gastric cancer: results from a Chinese center. Eur J Surg Oncol 42:1024–34.
- Tu Y, Tian Y, Fang Z, et al. (2016). Cytoreductive surgery combined with hyperthermic intraperitoneal chemoperfusion for the treatment of gastric cancer: a single-centre retrospective study. Int J Hyperthermia 32:587–94.
- Boerner T, Graichen A, Jeiter T, et al. CRS-HIPEC prolongs survival but is not curative for patients with peritoneal carcinomatosis of gastric cancer. Ann Surg Oncol 23:3972–7.
- Passot G, Vaudoyer D, Villeneuve L, et al. (2016). What made hyperthermic intraperitoneal chemotherapy an effective curative treatment for peritoneal surface malignancy: a 25-year experience with 1,125 procedures. J Surg Oncol 113:796–803.
- Desantis M, Bernard JL, Casanova V, et al. (2015). Morbidity, mortality, and oncological outcomes of 401 consecutive cytoreductive procedures with hyperthermic intraperitoneal chemotherapy (HIPEC). Langenbecks Arch Surg 400:37–48.
- Yarema RR, Ohorchak MA, Zubarev GP, et al. (2014). Hyperthermic intraperitoneal chemoperfusion in combined treatment of locally advanced and disseminated gastric cancer: results of a single-centre retrospective study. Int J Hyperthermia 30:159–65.
- Magge D, Zenati M, Mavanur A, et al. (2014). Aggressive locoregional surgical therapy for gastric peritoneal carcinomatosis. Ann Surg Oncol 21:1448–55.
- Muller H, Hotopp T, Tofeili A, Wutke K. (2014). Systemic chemotherapy using FLOT – regimen combined with cytoreductive surgery plus HIPEC for treatment of peritoneal metastasized gastric cancer. Hepatogastroenterology 61:703–6.
- Konigsrainer I, Horvath P, Struller F, et al. (2014). Initial clinical experience with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in signet-ring cell gastric cancer with peritoneal metastases. J Gastric Cancer 14:117–22.
- Canbay E, Mizumoto A, Ichinose M, et al. (2014). Outcome data of patients with peritoneal carcinomatosis from gastric origin treated by a strategy of bidirectional chemotherapy prior to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a single specialized center in Japan. Ann Surg Oncol 21:1147–52.
- Schildberg CW, Weidinger T, Hohenberger W, et al. (2014). Metastatic adenocarcinomas of the stomach or esophagogastric junction (UICC stage IV) are not always a palliative situation: a retrospective analysis. World J Surg 38:419–25.
- Kang LY, Mok KT, Liu SI, et al. (2013). Intraoperative hyperthermic intraperitoneal chemotherapy as adjuvant chemotherapy for advanced gastric cancer patients with serosal invasion. J Chin Med Assoc 76:425–31.
- Hultman B, Lind P, Glimelius B, et al. (2013). Phase II study of patients with peritoneal carcinomatosis from gastric cancer treated with preoperative systemic chemotherapy followed by peritonectomy and intraperitoneal chemotherapy. Acta Oncol 52:824–30.
- Shen P, Stewart JHT, Levine EA. (2009). Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: non-colorectal indications. Curr Probl Cancer 33:168–93.
- Scaringi S, Kianmanesh R, Sabate JM, et al. (2008). Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: a single western center experience. Eur J Surg Oncol 34:1246–52.
- Zhu ZG, Tang R, Yan M, et al. (2006). Efficacy and safety of intraoperative peritoneal hyperthermic chemotherapy for advanced gastric cancer patients with serosal invasion. A long-term follow-up study. Dig Surg 23:93–102.
- Yonemura Y, Kawamura T, Bandou E, et al. (2005).Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg 92:370–5.
- Hall JJ, Loggie BW, Shen P, Beamer S, Douglas Case L, Mcquellon R, et al. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J Gastrointest Surg 2004; 8:454–63.
- Fujimura T, Yonemura Y, Nakagawara H, et al. (2000). Subtotal peritonectomy with chemohyperthermic peritoneal perfusion for peritonitis carcinomatosa in gastrointestinal cancer. Oncol Rep 7:809–14.
- Hirose K, Katayama K, Iida A, et al. (1999). Efficacy of continuous hyperthermic peritoneal perfusion for the prophylaxis and treatment of peritoneal metastasis of advanced gastric cancer: evaluation by multivariate regression analysis. Oncology 57:106–14.
- Yonemura Y, Fujimura T, Nishimura G, et al. (1996). Effects of intraoperative chemohyperthermia in patients with gastric cancer with peritoneal dissemination. Surgery 119:437–44.
- Yonemura Y, Fujimura T, Fushida S, et al. (1991). Hyperthermo-chemotherapy combined with cytoreductive surgery for the treatment of gastric cancer with peritoneal dissemination. World J Surg 15:530–5.
- Fujimoto S, Takahashi M, Mutou T, et al. (1997). Improved mortality rate of gastric carcinoma patients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined with surgery. Cancer 79:884–91.
- Averbach AM, Jacquet P. (1996). Strategies to decrease the incidence of intra-abdominal recurrence in resectable gastric cancer. Br J Surg 83:726–33.
- Sugarbaker PH. (2016). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: progress toward a new standard of care. Cancer Treat Rev 48:42–9.
- Seshadri RA, Glehen O. (2016). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in gastric cancer. World J Gastroenterol 22:1114–30.
- Sugarbaker PH. (2001). Cytoreductive surgery and peri-operative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome. Eur J Surg Oncol 27:239–43.
- Sugarbaker PH. (1999). Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol 43:S15–S25.
- Glehen O, Gilly FN, Boutitie F, et al. (2010). Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer 116:5608–18.
- Levine EA, Stewart JHT, Shen P, et al. (2014).Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg 218:573–85.
- Glehen O, Mohamed F, Gilly FN. (2004). Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol 5:219–28.
- Coccolini F, Campanati L, Catena F, et al. (2015). Hyperthermic intraperitoneal chemotherapy with cisplatin and paclitaxel in advanced ovarian cancer: a multicenter prospective observational study. J Gynecol Oncol 26:54–61.
