Abstract
Purpose: The risk factors of pneumothorax after lung radiofrequency (RF) ablation are long known. The objective was to demonstrate that the visualisation of an aeric RF path after the needle withdrawal was predictive of pneumothorax occurrence and chest tube placement.
Materials and methods: A total of 70 patients were retrospectively included in this study. For each patient, we determined the pneumothorax risk factors (age, gender, previous surgery, emphysema, lesion size, distance between pleura and lesion), visualisation of a RF track, length and thickness, presence of pneumothorax, volume, chest tube placement, duration of drainage and hospital stay.
Results: Among 70 patients included retrospectively, 26 needed a chest tube placement (37%). Considering the group with path visualisation (37 patients, group A) and the patients without path visualisation (group B), the 2 groups were comparable for pneumothorax risk factors. Considering the patients who needed a chest drain, the visualisation of the path was significatively more important (23 cases, 88.4%) (p< 10−3) than in the group without (8 patients, 31.8%). Multivariate analyses were significant in the three analyses after adjustments on the risk factors for the occurrence of pneumothorax. Incidence of drains was significantly more (p < 10−3) important in group A (23 drainages 62%) than in group B (4 drainages or 12%). The length and thickness of the tracks were not predictable of drain placement.
Conclusions: Besides the well-known risk factors of severe pneumothorax after lung RFA, the simple visualisation of an aeric path just after the RF needle withdrawal is significantly associated with chest tube placement and can be considered as a risk factor as itself.
Introduction
Pneumothorax is the most common complication following a percutaneous radiofrequency (RF) lung intervention. According to recent studies [Citation1] on large population, there is an estimated incidence of pneumothorax of 25%, 77.5% requiring chest tube placement.
Putting in a thoracic tube is the most common reason for an extended hospitalisation [Citation2].
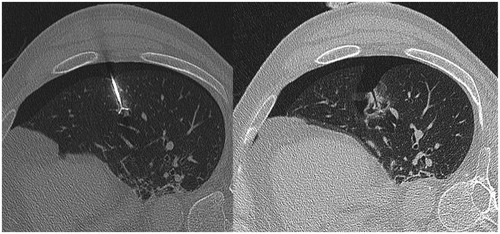
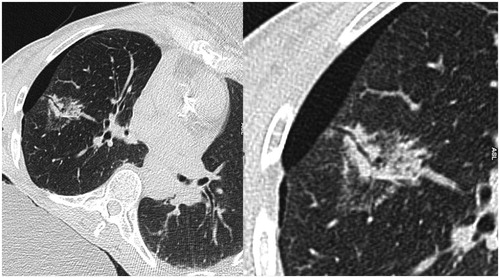
Even if the post RF pulmonary mortality is very low (s< 1% [Citation3]), an estimated one in two deaths is due to a pneumothorax or morbidity induced by its occurrence [Citation3,Citation4]. The risk factors of pneumothorax secondary to pulmonary RF ablation recognised and demonstrated in many reviews are increased age; male gender, no history of lung surgery, emphysema, number of tumours ablated and increased length of the aerated lung traversed by the electrode [Citation5]. Besides these risk factors, according to our experience, the simple visualisation of a persistant aeric path after the RF needle withdrawal () seemed to increase the incidence of severe “pneumothorax” needing chest tube placement.
The main objective was to show that visualisation of a visible “open” pathway pursuant to lung ablations RF track after the needle withdrawal was a risk factor of drain installation.
Materials and methods
The main objective was to demonstrate that the visualisation of the RF path after the needle withdrawal was predictive of chest tube placement. Considering this primary outcome measure, we retrospectively included all patients treated with pulmonary RF for primary or secondary lesions in our centre between January 2014 and June 2016. A total of 70 patients were included. Patients treated for multiple lesions in the same lung were excluded since the possibility of a pneumothorax requiring drainage on the first injury might distort any results observed when treating other injuries.
Population characteristics
For each patient we identified:
✓ the potential pneumothorax risk factors (advanced age, gender, history of ipsilateral thoracic surgery and distance between the pleura and the lesion along the RF needle pathway),
✓ the presence of emphysema on a computerised tomography (CT) scan,
✓ the visualisation of a well-defined air path in place of the RF coaxial needle’s intra-parenchyma pathway between the visceral pleura and the tumour, its length and thickness (),
✓ the number and volume of immediate pneumothorax,
✓ the pneumothorax management (no treatment, exsufflation and drainage),
✓ the duration of drainage,
✓ a pneumothorax at 48 h and 1-month examination so as to avoid missing any delayed occurrence,
✓ The length of hospital stay.
Pneumothorax
The volume of pneumothorax was calculated using the Collins profile inter-pleural distance measurements [Citation6]. RF procedures were performed via CT or CT angiography on all patients in a standardised manner using a coaxial needle system 15 Gauge Boston Scientific. For all patients, the RF needle and the coaxial were completely removed, the patients underwent chest X-ray after RFA and a CT scan was performed at the end of the percutaneous procedure to evaluate pneumothorax. The visualisation of a path was determined on this acquisition (120 kVp, 20 mAs collimation 4 × 1 mm, normalised pitch 1.75, slice thickness 1.25 mm, reconstruction increment 0.8 mm; Somatom, Siemens). The needle tracks were not ablated, at the end of the ablation, the needles were simply removed.
The international recommendations for pneumothorax management were followed [Citation7]. All patients with a low-abundance pneumothorax who were stable at 5 min received no treatment. For those with a mean abundance <543 cc pneumothorax, we performed exsufflation by using a short venous catheter (18 gauge). Finally patients with an immediate and/or persistent abundant pneumothorax despite exsufflation received an eight French chest tube (Prodimed, Plastimed®).
All patients had a chest radiograph before they returned to hospitalisation room and received a scan at 48 h and at 1 month.
Statistical analysis
Continuous characteristics, such as age, the distance between the pleura and the lesion, the lesion size, the path length and thickness and the quantity of pneumothorax, were expressed as a mean, SD, 95% confidence intervals, median, interquartile, minimum and maximum. The categorical characteristics, such as gender, surgical history, emphysema, pneumothorax occurrence and pneumothorax treatments were expressed as numbers and frequency. The bivariate comparisons of the continuous variables were made by the Wilcoxon–Mann–Whitney test and those for qualitative variables by the Chi-squared test or, when their conditions of application were not met, and by the Fisher’s exact test. A multivariate logistic regression was carried out in order to take into account the prevalence of pneumothorax risk factors in the evaluation of the association between the visualisation of the needle path and the occurrences of pneumothorax and chest tube placement.
All statistical analyses were two-tailed and considered statistically significant when p values was <0.05. They were performed using SPSS PAWS Statistics version 20.0 (IBM Inc., New York, NY) and R version 3.3.1 (Stanford University, CA).
Results
Description of the patient population
The two groups (group A “with path visualization” and group B “without path visualization”) were comparable for different pneumothorax risk factors ().
Table 1 Comparability of groups “without” and “with” RF pulmonary path visualisation.
Age: (average 64.4 years [17.2] in Group B vs. 68.5 years in the Group A [12.7], p = 0.49).
Gender: (14 women and 23 men in the Group A compared to 11 women and 22 men in Group A; p = 0.8).
Previous surgery: (22 patients with history in the Group A compared with 13 in Group B; p = 0.15).
Presence of emphysema (22 patients in Group B as compared to 21 in the Group A; p = 0.46).
The only differences between the two groups was the lesion size (17.2 [7.8] in Group B vs. 13.6 [7.1] in the Group A p = 0.02) and the distance from lesion to pleura (24.8 in group B [18.6] vs. 30.2 in group A [Citation14] p = 0.04). Thus, a persistent pathway was more often seen for small and deep lesions.
Results regarding pneumothorax
The incidence of pneumothorax was larger in the group A “with path visualization” (32 patients, 86.4%) than in group B “without path visualization” (18 patients, 54.5%) (p < 0.05). The volume of pneumothorax was significantly more important in group A (28%) than in group B (11%) (p < 0.05). Among the 70 patients included, 26 needed a chest tube placement (37%). The incidence of drains was significantly more (p < 0.05) important in group A (23 drainages 62%) than in the group B (4 drainages or 12%).
For patients needing a chest drain, the path visualisation was larger (23 cases, 88%) (p < 0.05) than in the group without drain (14 patients, 31.8%).
The multivariate analyses were focussed on three aspects:
✓ the risk of pneumothorax on the whole population,
✓ the risk of drainage on whole population, and
✓ the risk of drainage in patients with pneumothorax.
Multivariate analysis demonstrated that pathway visualisation had a marked association with pneumothorax and chest drainages in the three analyses after adjustments of the risk factors ().
Table 2. Multivariate analysis in the three analyses (the risk of pneumothorax on whole population, the risk of drainage on whole population, the risk of drainage in patients with pneumothorax) after adjustments on the risk factors.
The drains were removed 28.3 h [7.8] after the RFA for group A and 24 h after RFA for group B (p = 0.3). No patients presented a pneumothorax on the CT scanner examination at 48 h and at one month. The hospital stay was the same for all patients (48 h) except for one patient of each group for the occurrence of hemothorax.
Discussion
A simple pathway visualisation after the RFA needle withdrawal is associated with an increased risk of pneumothorax and an increased chest tube placement.
The visualisation of this pathway was more frequently seen for deep and small lesions. The genesis of post RF pneumothorax is the result of complex and specific phenomena not found during biopsies. The literature reports the origins of post RF pneumothorax were studied on swine models [Citation8]. In this study, the authors compared the changes induced only by biopsy and those induced by RF’s needle’s pathway. In the first case, with biopsy only, traces of a haemorrhage with a pulmonary parenchymal protected environment is found while for RF there is an accompaniment of serum effusion fluid, an emphysema-type inflation in the alveolar spaces and the presence of intravascular coagulation phenomena [Citation9,Citation10].
All of these elements are responsible for the formation of a dense crown of parenchyma reformed around the RF pathway. This pathway seems specific to lung tissue (“oven effect”), which is probably due to a heat loss along the RF’s coaxial needle.
This phenomenon has been described in several cases reports [Citation11], described with the term of bronchopleural fistulae, which was already associated with malignant pneumothorax. The incidence of intractable pneumothorax due to bronchopleural fistula is approximately 0.6% [Citation12].
These cases reports described several solutions to treat fistulas associated with severe pneumothorax [Citation13–16] without mentioning a potential preventive treatment.
Our study makes a distinction between the simple visualisation of an aeric track and the presence of a real bronchopleural fistula since the path visualisation was seen in a few patients without a thoracic drain and could not be considered as real persistent fistulas.
While our study is limited by a modest number of patients, the results are nonetheless meaningful. Another limiting factor is that previous surgeries and the distance between the pleura and the lesion are the only significant risk factors in our population.
Conclusion
Besides the classical and well-known risk factors of malignant pneumothorax after lung RFA, the simple visualisation of a persistent aeric track after RF needle withdrawal is significantly associated with chest tube placement and can be considered as a risk factor as itself.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Kashima M. (2010). Complications of lung radiofrequency ablation in 892 treatments. J Vasc Interv Radiol 21:69.
- Rose SC. (2006). Lung cancer and radiofrequency ablation. J Vasc Interv Radiol 17:927–51.
- De Baère T, Auperin A, Deschamps F, et al. (2015). Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol Adv Access 26:987–91.
- Palussiere J, Lagarde P, Aupérin A, et al. (2015). Percutaneous lung thermal ablation of non-surgical clinical N0 non-small cell lung cancer: results of eight years’ experience in 87 patients from two centers. Cardiovasc Interv Radiol 38:160–6.
- Kennedy SA, Milovanovic L, Dao D, et al. (2014). Risk factors for pneumothorax complicating radiofrequency ablation for lung malignancy: a systematic review and meta-analysis. J Vasc Interv Radiol 25:1671–81.e1.
- Collins CD, Lopez A, Mathie A, et al. (1995). Quantification of pneumothorax size on chest radiographs using interpleural distances: regression analysis based on volume measurements from helical CT. AJR Am J Roentgenol 165:1127–30.
- Nour-Eldin NE. (2011). Outcomes of an algorithmic approach to management of pneumothorax complicating thermal ablation of pulmonary neoplasms. J Vasc Interv Radiol 22:1279–86.
- Cohen F, Souteyrand P, Varoquaux A, et al. (2009). Origine des pneumothorax compliquant les ablations pulmonaires par radiofréquence. Etude sur un modèle porcin Faculté de Médecine Marseille, France. Congrés Des Journées Françaises De Radiologie.
- Izaaryene J. (2008). Pathological effects of lung radiofrequency ablation that contribute to pneumothorax, using a porcine model. Int J Hyperthermia.
- Pawel M, Dewhirst MW, Halpern E, et al. (2008). Radiofrequency ablation: the effect of distance and baseline temperature on thermal dose required for coagulation. Int J Hyperthermia 24:550–9.
- York JA. (2013). Treating bronchopleural fistulae percutaneously with N-butyl cyanoacrylate glue. J Vasc Interv Radiol 24:1581–3.
- Sakurai J. (2007). Intractable pneumothorax due to bronchopleural fistula after radiofrequency ablation of lung tumors. J Vasc Interv Radiol 18:141–5.
- Cahalane AM. (2012). Bronchopleural cutaneous fistula after pulmonary radiofrequency ablation: treatment with low-adherent paraffin gauze dressing. J Vasc Interv Radiol 23:283–5.
- Petsas T, Siamblis D, Giannakenas C, et al. (1995). Fibrin glue for sealing the needle track in fine-needle percutaneous lung biopsy using a coaxial system: part II–clinical study. Cardiovasc Intervent Radiol 8:373–7.
- Alexander ES. (2012). Use of endobronchial valves for the treatment of bronchopleural fistulas after thermal ablation of lung neoplasms. J Vasc Interv Radiol 23:1236–40.
- Kim KH. (2006). Bronchopleural fistula treatment with use of a bronchial stent-graft occluder. J Vasc Interv Radiol 17:1539–43.