Abstract
Purpose: To assess the value of bowel preparation plus targeted antibiotics for preventing intrahepatic infections after MWA of liver tumours in BEA patients.
Materials and methods: This retrospective study included 21 patients (divided into two groups) with a history of BEA undergoing ultrasound-guided MWA of liver tumours from November 2008 to June 2014. Group A (n = 10) received single-antibiotic therapy (cefazedone 2 g bid 4, amoxicillin and flucloxacillin sodium 2 g bid 3, levofloxacin 0.5 g qd 3) after ablation, and group B (n = 11) received bowel preparation before ablation plus combined antibiotic therapy (imipenem and cilastatin sodium 1 g 1/12 h, linezolid 0.6 g 1/12 h). Patients were followed for 3 months. Incidences of fever, bacteraemia, and intrahepatic infections were compared, including the duration of fever and length of hospital stay.
Results: Following ablation, in group A, 90% of the patients (9/10) had fever, 60% (6/10) had bacteraemia, 60% (6/10) had liver abscess, and 10% (1/10) had biliary tract infection. In group B, no cases of bacteraemia or intrahepatic infection were reported, and the incidences of fever, bacteraemia, and liver abscess were substantially lower than group A (p = 0.002, 0.004, 0.004). Duration of fever and length of hospital stay were markedly shorter than group A (p = 0.002 0.003).
Conclusions: Bowel preparation plus targeted antibiotic therapy can significantly reduce the incidences of fever, bacteraemia, and intrahepatic infections in BEA patients undergoing MWA of liver tumours. These preliminary results need to be further validated in randomised trials.
Introduction
Liver metastases often occur in patients undergoing tumour resection and biliary-enteric anastomosis (BEA) for the treatment of hilar hepatocellular carcinoma, hilar hepatocholangiocarcinoma, extrahepatic cholangioma, pancreatic carcinoma and ampullary carcinoma. The efficacy of radiochemotherapy is limited for the above metastatic liver cancers, and only a minority of patients are candidates for surgical resection [Citation1–4].
MWA is a minimally invasive procedure that has excellent efficacy and good tolerance, as well as low complication rates and repeatability [Citation5–8]. In recent years, MWA has been widely used for the treatment of primary and metastatic liver cancers [Citation9–15]. However, a high incidence of secondary intrahepatic infections has been reported following MWA of liver tumours in patients who have undergone BEA [Citation16,Citation17].
Patients who undergo BEA often have reflux cholangitis due to an incompetent ampullary sphincter and a continuous connection of the bile duct and bowel [Citation18]. These patients have a relatively high incidence of intrahepatic infections after liver tumour ablation (both radiofrequency and MWA) [Citation16], mainly due to the secondary formation of liver abscesses in the ablation zone. The incidence of liver abscess is 44%-100% in BEA patients undergoing thermal ablation (MWA or radiofrequency) of liver tumours [Citation17,Citation19,Citation20]. Therefore, prevention and control of secondary intrahepatic infections is very important for a good prognosis in these patients.
In this study, clinical observation and a comparative study were performed on patients with prior BEA undergoing MWA for metastatic liver cancer. The aim of the study was to evaluate the clinical efficacy of bowel preparation plus targeted antibiotic use during the periablation period for preventing and reducing intrahepatic infections following MWA.
Materials and methods
Clinical data and methods
This retrospective study was approved by the hospital’s ethics committee. Informed consent was obtained from all the patients prior to the treatment. We analysed the clinical, pathological, and follow-up data of 21 patients with prior BEA who underwent ultrasound-guided percutaneous MWA for metastatic liver cancer in our department from 1 January 2010 to 30 April 2014. All the patients had been diagnosed with metastatic liver cancer by MRI/CT and contrast-enhanced ultrasound (CEUS) of the liver before the treatment.
The 21 patients enrolled in the study were divided into two groups: group A (n = 10) received conventional single-antibiotic therapy after ablation, while group B (n = 11) received bowel preparation before ablation in addition to targeted antibiotic therapy in the periablation period.
Ablation therapy was performed using a KY-2000 MWA system (Kangyou Medical Instruments, Nanjing, China) equipped with a 15 G MWA electrode. The microwave emission frequency was set to 2450 MHz, and the power was 50 W. The duration of ablation for each lesion was 5–20 min, depending on the size of the lesion. During treatment, we monitored the hyperechoic area of the lesion and the hyperechoic area of ablation using real-time US to determine the end point of treatment. When the hyperechoic region on greyscale US covered the entire target region, the treatment session was stopped.
Treatment procedure
All the patients underwent relevant tests and examinations after admission to our hospital. Ablation was performed under intravenous anaesthesia by two surgeons from our department who each had more than 5 years of experience in ablation therapy. Patients in group A received antibiotic therapy for 3 days following MWA; the duration of antibiotic use was extended for patients who presented with fever or an elevated white blood cell (WBC) count following ablation. Patients in group B underwent bowel preparation before ablation and received a targeted combination of antibiotics with a spectrum of activity covering Enterobacter and Enterococcus faecalis/faecium. Antibiotic therapy began the day before ablation and continued for 5 days following ablation.
Bowel preparation
For group B patients, a non-residue general diet was provided for 3–4 days prior to ablation. A total of seven treatments of oral metronidazole (3*0.4 g × 2.5 days) plus gentamicin sulphate (3*8 WU × 2.5 days) were given until noon of the day before ablation. Polyethylene glycol electrolyte powder (246.6 g) was administered at 7 pm on the night before ablation to empty the bowels.
Antibiotic use
From the afternoon of the day before ablation until 5 days after the procedure, group B patients were administered a continuous intravenous infusion of imipenem and cilastatin sodium (MSD Pharmaceutical Co., Ltd., Hangzhou, China; 1 g every 12 h) plus linezolid (Norway; 0.6 g every 12 h).
Follow-up
CEUS or enhanced MRI/CT was performed 3–4 days after ablation in all the patients to confirm complete ablation of the metastatic lesions. All the patients were re-examined with routine blood tests, liver and kidney function tests, tumour marker analysis, CEUS, and enhanced MRI or CT of the liver at 1 and 3 months after ablation.
Outcome measures
Following ablation, the two groups of patients were compared with respect to the incidences of fever, bacteraemia, and intrahepatic infections (including secondary formation of liver abscess in the ablation zone and biliary tract infection); in addition, the duration of fever (in days) and the length of hospital stay (in days) were compared between the groups.
Statistical methods
The statistical analysis was performed using IBM SPSS 19.0 Statistics (IBM SPSS, Somers, NY). Quantitative data were compared between the two groups using Student’s t-test. Incidence rates were compared using the χ2 test. Differences with a value of p < 0.05 were considered statistically significant.
Results
A total of 48 liver metastatic lesions in 21 patients were treated with 28 MWA procedures in this study. The characteristics of the two groups of patients are summarised in . The median interval from resection of primary tumours to ablation of liver metastatic lesions was 7 months (range, 2–25 months) in group A vs. 10 months (range, 2–91 months) in group B; the difference was not statistically significant (p = 0.69). No significant differences were found between the two groups in terms of age, gender, maximum diameter of metastatic lesions, prevalence of diabetes mellitus, WBC count before ablation, or ablation duration ().
Table 1. Clinicopathological features of the 21 patients included in this study.
In group A, 90% (9/10) of patients developed fever after ablation. Fever was abated in two cases using anti-infective therapy. The other seven patients who presented with fever had secondary intrahepatic infections after ablation, and US and CT scans identified liver abscess formation in six of these patients 7–10 days following ablation (). These six patients had bacteraemia, and their blood cultures were positive for Escherichia coli (one patient), Enterobacter cloacae (one patient), Enterococcus faecalis (three patients), and Enterococcus faecium (one patient). Four of the six patients had concomitant right chest infection (); these included three patients with empyema (two with E. faecalis infection and one with E. coli infection). The six patients with liver abscesses had continuous fever for 18–50 days with varying degrees of chills and with the maximum temperature reaching 41 °C. One patient with recurrent fever had no evidence of abscess formation, and antibiotic therapy failed to alleviate the fever. Two months after ablation, US examination of this patient revealed intrahepatic bile duct dilatation with poor sound transmission. US-guided percutaneous catheter drainage was performed, and culture of the drained bile grew E. coli.
Figure 1. Abdominal CT of a 56-year-old male patient. Over 1 year after surgery for hilar hepatocellular carcinoma, this patient underwent ablation of a recurrent lesion in the left lobe of the liver. Following ablation, he developed intermittent fever and chills. An abdominal CT scan at 6 days after ablation shows the abscess in the ablation area.
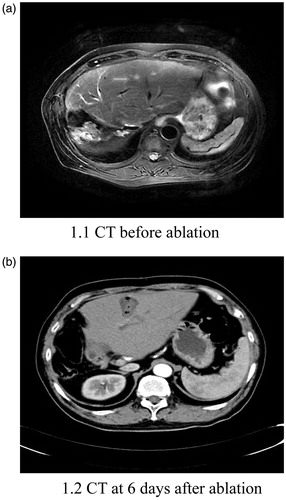
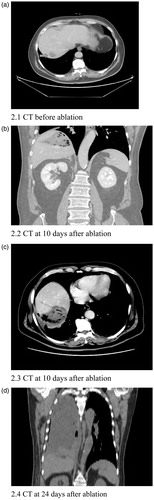
Figure 2. A 59-year-old male patient underwent ablation of a metastatic lesion in the right lobe of the liver 2 years and 1 month after surgery for ampullary carcinoma. Following ablation, the patient developed intermittent fever and chills. An abdominal CT scan at 10 days after ablation shows an abscess in the ablation area; the lung CT scan at 24 days after ablation shows a right empyema.
Antibiotic susceptibility testing was performed in the seven patients with secondary intrahepatic infections. Escherichia coli and E. cloacae were found to be sensitive to imipenem and cilastatin sodium, and E. faecalis/E. faecium was sensitive to linezolid. Among the six patients with liver abscess, one was changed to combination antibiotic therapy with imipenem and cilastatin sodium plus linezolid injection according to the susceptibility testing results and then discharged after being cleared of infection. Three others underwent catheter drainage of the liver abscesses and were subsequently changed to combination antibiotic therapy with imipenem and cilastatin sodium plus linezolid injection and then discharged after being cured. Two patients died.
In group B, the incidence of fever was 18.2% (2/11). These two patients did not experience bacteraemia or intrahepatic infection after ablation; their body temperatures were never higher than 38 °C and returned to normal within 1 week after ablation. Group B patients presented with no bacteraemia, intrahepatic infections, or liver abscess formation after ablation during the 3-month follow-up period. Significant differences were observed between the two groups in the incidence of fever, bacteraemia, and liver abscesses, as well as the duration of fever and length of hospital stay. No significant difference was observed in the incidence of intrahepatic biliary tract infection ().
Table 2. Postoperative complications in patients undergoing microwave ablation.
Discussion
Liver abscess is a serious complication that occurs after ablation of liver tumours, with an incidence rate of 0.3–1% [Citation21,Citation22]. The primary reasons for liver abscess formation after the thermal ablation of liver tumours are decreased WBC counts, diabetes mellitus, bile duct inflammation, and the reflux of bowel bacteria after sphincter of Oddi dysfunction caused by a biliary stent or the absence of the sphincter of Oddi caused by BEA [Citation22,Citation23]. The incidence of liver abscess is markedly higher in patients with prior BEA undergoing thermal (radiofrequency or microwave) ablation of liver tumours than in patients without prior BEA.
An increased risk of liver abscess formation in BEA patients undergoing ablation of liver tumours occurs for two primary reasons. (1) Biliary tract infection may be the result of the prior BEA [Citation18]. Studies have suggested that biliary tract infection following Roux-en-Y choledochojejunostomy is associated with increased pressure of the bile outflow tract, inhibited bile discharge, and food stasis and reflux in the bowels [Citation24–26]. (2) Following MWA of liver tumours, necrosis of the lesion can cause local damage to small bile ducts, leading to the release of pathogens that have refluxed into the biliary tract. This results in secondary infection in the treatment zone and induces liver abscess formation [Citation27,Citation28].
Geschwind et al. [Citation29] reported that patients treated with piperacillin/tazobactam and bowel preparation before radiofrequency ablation did not experience liver abscess; however, only four total cases were examined. Bowel cleansing may be useful for reducing the incidence of liver abscess in patients with BEA; no evidence has suggested that bowel preparation alone can prevent the occurrence of liver abscess after MWA. Some studies have reported that the use of antibiotics can reduce the incidence of liver abscess after liver tumour ablation in patients who have undergone BEA, but other studies have reported contradictory results [Citation22]. Liver abscess formation can prolong hospital stays, which can affect patients’ quality of life and increase medical expenses for patients.
We analysed the causes of secondary infection after MWA of liver tumours in BEA patients and assessed culture and susceptibility tests in group A. We found that the pathogenic bacteria were negative bacilli or positive cocci. The patients had no pulmonary infection or infections in other organs of the body prior to the procedure. Considering that the pathogenic bacteria all originated from intestinal sources, we formulated a preventative intrahepatic infection regimen for patients in group B consisting of bowel preparation plus targeted antibiotics.
In our study, group B patients had substantially lower incidences of bacteraemia and liver abscess than group A patients. Group B patients benefitted from (1) the cleaning of the bowel and reduction of bowel pressure before ablation (non-residue general diet, metronidazole, gentamicin sulphate, and polyethylene glycol electrolyte powder), which helped minimise the reflux of bowel contents into the biliary tract after ablation and (2) provision of targeted antibiotic therapy to eradicate likely infecting organisms.
Patients in group A received single-antibiotic therapy after ablation. Blood culture of the six patients with liver abscess and bile culture of the lone patient with biliary tract infection in group A showed that BEA patients who presented with ablation-related fever were mostly infected with Enterobacter and E. faecalis/E. faecium. Enterobacter infections were sensitive to carbapenems, and E. faecalis/E. faecium infections were sensitive to linezolid.
Group B patients underwent anti-infective therapy during the periablation period (1 day before ablation to 5 days after ablation) with a targeted combination of antibiotics comprised of a spectrum covering Enterobacter and E. faecalis/E. faecium. None of the group B patients developed bacteraemia or liver abscess, suggesting that bowel preparation in addition to targeted antibiotic use in the periablation period can significantly reduce the incidence of secondary intrahepatic infections in BEA patients undergoing MWA for liver tumours.
The incidence of intrahepatic biliary tract infection was not significantly different between the two groups of patients. The possible reasons for this include (1) the number of patients was small in the present study; (2) following ablation of liver tumours, both transaminase elevation and intravenous anaesthesia could affect bowel motility and reduce bowel movements, leading to bloating and further inducing reflux cholangitis; and (3) there is a possibility that the biliary tract infections were the result of prior BEA and not related to the ablation procedure per se.
In summary, cleaning of the bowel and the use of antibiotics effective against Enterobacter and E. faecalis/E. faecium in the periablation period can greatly reduce the incidence of secondary intrahepatic infections in BEA patients undergoing MWA for liver tumours. Intrahepatic infections following MWA of liver tumours in BEA patients are mainly caused by pathogens derived from the bowel. This was confirmed by the results of blood and bile culture performed for group A patients who had intrahepatic infections following MWA. Presently, no effective or standard therapeutic regimens exist for the prevention and control of intrahepatic infections in BEA patients undergoing MWA of liver tumours. In the current study, the antibiotics used in group B were determined by the results of culture and susceptibility tests for patients with intrahepatic infections in group A. The patients in group B had a significantly lower incidence of fever after ablation than those in group A. Moreover, bacteraemia and intrahepatic infection were entirely absent in group B. BEA patients have a high incidence of liver abscess after MWA. Fewer BEA patients with liver metastases were treated with MWA. Obtaining a sufficiently large patient population to achieve adequate statistical power in this study would be very time-consuming and difficult. Our study includes the largest number of patients in this category to date. Although the groups are small, the preliminary results suggest that bowel preparation in addition to targeted antibiotic therapy can reduce the incidence of intrahepatic infections in BEA patients after MWA. The results are preliminary but have a guiding value for clinical treatment and suggest important implications for bowel cleansing with antibiotic treatment in preventing liver abscess in patients with prior BEA undergoing MWA of liver tumours.
The two primary limitations in this study were (1) the number of patients was small, and the results only represent the treatment experience at one hospital and (2) the therapeutic regimen for the patients in group B was chosen mainly based on the results of antibiotic susceptibility testing in patients with intrahepatic infections in group A. The grade of antibiotics used was relatively high, and the effectiveness needs to be further validated in randomised trials using larger samples.
Conclusion
In conclusion, this study shows that bowel preparation plus targeted antibiotic therapy during the periablation period can effectively reduce the incidence of intrahepatic infections following MWA of liver tumours in patients who have undergone prior BEA.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. (1997). Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg 21:195–200.
- Sperti C, Moletta L, Merigliano S. (2015). Multimodality treatment of recurrent pancreatic cancer: myth or reality? World J Gastrointest Oncol 7:375–82.
- Takada T, Amano H, Yasuda H, et al. (2002). Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer 95:1685–95.
- Sikora SS, Balachandran P, Dimri K, et al. (2005). Adjuvant chemo-radiotherapy in ampullary cancers. Eur J Surg Oncol 31:158–63.
- Liang P, Yu J, Lu MD, et al. (2013). Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J Gastroenterol 19:5430–8.
- Robert CG, Martin MD, Charles R, et al. (2010). Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol 17:171–8.
- Ikai I, Itai Y, Okita K, et al. (2004). Report of the 15th follow-up survey of primary liver cancer. Hepatol Res 28:21–9.
- Yu J, Liang P. (2016). Status and advancement of microwave ablation in China. Int J Hyperthermia 20:1–10.
- Ong SL, Gravante G, Metcalfe MS, et al. (2009). Efficacy and safety of microwave ablation for primary and secondary liver malignancies: a systematic review. Eur J Gastroenterol Hepatol 21:599–605.
- Lloyd DM, Lau KN, Welsh F, et al. (2011). International multicentre prospective study on microwave ablation of liver tumours: preliminary results. HPB (Oxford) 13:579–85.
- Bhardwaj N, Strickland AD, Ahmad F, et al. (2010). Microwave ablation for unresectable hepatic tumours: clinical results using a novel microwave probe and generator. Eur J Surg Oncol 36:264–8.
- Jiao D, Qian L, Zhang Y, et al. (2010). Microwave ablation treatment of liver cancer with 2,450-MHz cooled-shaft antenna: an experimental and clinical study. J Cancer Res Clin Oncol 136:1507–16.
- Boutros C, Somasundar P, Garrean S, et al. (2010). Microwave coagulation therapy for hepatic tumors: review of the literature and critical analysis. Surg Oncol 19:e22–32.
- Inokuchi R, Seki T, Ikeda K, et al. (2010). Percutaneous microwave coagulation therapy for hepatocellular carcinoma: increased coagulation diameter using a new electrode and microwave generator. Oncol Rep 24:621–7.
- Meloni MF, Chiang J, Laeseke PF, et al. (2017). Microwave ablation in primary and secondary liver tumours: technical and clinical approaches. Int J Hyperthermia 33:15–24.
- Shibata T, Yamamoto Y, Yamamoto N, et al. (2003). Cholangitis and liver abscess after percutaneous ablation therapy for liver tumors: incidence and risk factors. J Vasc Interv Radiol 14:1535–42.
- Yu MA, Liang P, Yu XL, et al. (2011). Liver abscess as a complication of microwave ablation for liver metastatic cholangiocarcinoma after bilioenteric anastomosis. Int J Hyperthermia 27:503–9.
- Chuang JH, Chen WJ, Lee SY, Chang NK. (1998). Prompt colonization of the hepaticojejunostomy and translocation of bacteria to liver after bile duct reconstruction. J Pediatr Surg 33:1215–8.
- Elias D, Di Pietroantonio D, Gachot B, et al. (2006). Liver abscess after radiofrequency ablation of tumors in patients with a biliary tract procedure. Gastroenterol Clin Biol 30:823–7.
- de Baère T, Risse O, Kuoch V, et al. (2003). Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol 181:695–700.
- Liang P, Wang Y, Yu XL, Dong B. (2009). Malignant liver tumors: treatment with percutaneous microwave ablation — complications among cohort of 1136 patients. Radiology 251:933–40.
- Livraghi T, Solbiati L, Meloni MF, et al. (2003). Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 226:441–51.
- Decadt B, Siriwardena AK. (2004). Radiofrequency ablation of liver tumours: systematic review. Lancet Oncol 5:550–60.
- Laukkarinen J, Chow P, Sand J, et al. (2007). Long-term changes in hepatobiliary physiology after Roux-en-Y hepaticojejunostomy. J Surg Res 143:270–5.
- Summers GE, Jr, Hocking MP. (1992). Preoperative and postoperative motility disorders of the stomach. Surg Clin North Am 72:467–86.
- Johnson CP, Sarna SK, Cowles VE, et al. Motor activity and transit in the autonomically denervated jejunum. Am J Surg 1994; 167:80–8.
- Choi D, Lim HK, Kim MJ, et al. (2005). Liver abscess after percutaneous radiofrequency ablation for hepatocellular carcinomas: frequency and risk factors. AJR Am J Roentgenol 184:1860–7.
- Hoffmann R, Rempp H, Schmidt D, et al. (2012). Prolonged antibiotic prophylaxis in patients with bilioenteric anastomosis undergoing percutaneous radiofrequency ablation. J Vasc Interv Radiol 23:545–51.
- Geschwind JF, Kaushik S, Ramsey DE, et al. (2002). Influence of a new prophylactic antibiotic therapy on the incidence of liver abscesses after chemoembolization treatment of liver tumors. J Vasc Interv Radiol 13:1163–6.
