Abstract
Aim: The aim of this study was to assess the outcomes of patients operated on for peritoneal metastases from unusual cancer sites of origin, meaning apart from peritoneal metastases (PM) from colorectal, gastric and epithelial ovarian carcinomas, pseudomyxoma peritonei and mesothelioma.
Patients and methods: A questionnaire concerning patients treated with cytoreductive surgery (CRS) plus hyperthermic intraperitoneal chemotherapy (HIPEC) for PM arising from unusual cancer sites of origin was sent to all centres, which routinely performed HIPEC, through the Peritoneal Surface Oncology Group International and the RENAPE network.
Results: Between September 1990 and June 2016, 850 procedures for unusual cases were performed in 781 patients, in 53 centres worldwide. Nearly two-thirds of the procedures were performed for three indications: rare ovarian carcinoma (n = 224), sarcoma (n = 189) and neuroendocrine tumours (n = 127). The median PCI was 12 [0–39]. Grade III–IV postoperative complications occurred in 272 patients (41%). Nineteen patients (2.9%) died postoperatively. After a median follow-up of 46 months, median overall survival (OS) was 39 months [33.18–44.05]. Five-year OS rate was 38.7%. For the three main indications, 5-year OS was significantly greater in patients with PM from rare ovarian carcinoma (57.7%), than that of patients with PM from neuroendocrine tumours (39.9%), and from sarcoma (29.3%) (p < 0.0001).
Conclusions: CRS and HIPEC appear to be safe and effective in patients with peritoneal metastases from unusual cancer sites of origin, especially from rare ovarian carcinomas, PM from neuroendocrine tumours. The respective roles of CRS and HIPEC remain unclear and should be evaluated.
Introduction
Complete cytoreductive surgery (CRS) followed by hyperthermic intraperitoneal chemotherapy (HIPEC) is the gold standard for curative treatment of isolated peritoneal metastases (PM) of colorectal origin and rare primary peritoneal malignancies, peritoneal pseudomyxoma peritonei and malignant peritoneal mesotheliomas [Citation1–4]. For PM arising from epithelial ovarian or gastric carcinomas, the potential benefits of CRS plus HIPEC remain controversial [Citation5–12], and are currently being evaluated in clinical trials.
Rarely, this combined treatment has been used for PM arising from other cancer sites of origin. In those cases, indications for CRS and HIPEC are exceptional because most of the time PM are associated with extra-peritoneal dissemination. However, there are also abdominal malignancies with a known major peritoneal tropism that may benefit from CRS and HIPEC but whose rarity is such that their description in the literature does not exceed sporadic clinical case reports [Citation13]. Consequently, the efficacy of CRS followed by HIPEC for patients with non-colorectal PM and non-primary peritoneal malignancies remains unclear because of the heterogeneity of primary tumour types, and the limited number of patients reported.
The aim of this study was to assess the outcomes of patients operated on for peritoneal metastases from unusual cancer sites of origin, meaning apart from PM from colorectal, gastric and epithelial ovarian carcinomas, pseudomyxoma peritonei and mesothelioma.
Methods
A retrospective analysis of patients treated with CRS plus HIPEC for PM arising from unusual cancer sites of origin other than gastrointestinal carcinomas, pseudomyxoma peritonei and mesothelioma were collected from member centres of the PSOGI. A questionnaire (supplementary Table 1) was sent to each centre through PSOGI for centres outside France and through BIG-RENAPE network for French centres. Rare indications, for which fewer than 10 patients were operated on, were excluded from the analysis. Only the data concerning the first procedure were analysed for patients who underwent more than one procedure.
The extent of peritoneal carcinomatosis (PC) was routinely measured using the peritoneal index (formerly called the Sugarbaker score) which ranges from 0 to 39 [Citation14]. The quality of cytoreductive surgery was defined according to the “Sugarbaker completeness of cytoreduction score” (CC): CC-0: no macroscopic residual tumour; CC-1: residual tumour less than 2.5 mm; CC-2: residual tumour between 2.5 mm and 25 mm; CC-3: residual tumour >25 mm [Citation8]. Cytoreductive surgery was considered to be complete when a CC-0 or CC-1 score was attained. Intraperitoneal treatment consisted of HIPEC either done with the open abdomen or the closed abdomen technique, as previously described [Citation15,Citation16]. Surgical complications were retrospectively graded according to the NCI CTCAE V4 [Citation17].
Long-term follow-up was carried out according to the underlying pathology of the PC. Recurrences were diagnosed based on clinical, radiological or histological findings and were consistently confirmed in multidisciplinary team meetings.
Statistical analysis
Descriptive data were expressed as means (± standard deviation) and medians (range) for quantitative variables, and as numbers (percentage) for qualitative data.
Overall, survival time was defined as the time from the first HIPEC procedure to the date of death, the date of the last follow-up, or the cut-off date, whichever came first. Perioperative deaths were not excluded from the survival analysis. Recurrence-free survival was defined as the time from the first HIPEC procedure until recurrence or the last follow-up. Deceased patients were censored at the date of death. Patients with recurrent disease at the time of the HIPEC procedure or patients who had undergone a CC-2 resection were considered as having an immediate relapse and were not included in the recurrence-free survival analysis. Median survival times and survival rates were computed using the Kaplan–Meier method. Hazard ratios and p values were obtained with the Cox proportional hazards model. Multivariate overall survival was also adjusted for age. Factors with a significance degree ≤0.20 and with less than 25% of missing data were introduced into multivariate models. A p values of less than 0.15 was retained in the final model. SAS statistical software (V9.3) (SAS Institute Inc., Cary, NC) was used for all analyses.
Results
Patients and indications
Between September 1990 and June 2016, 850 procedures consisting of CRS plus HIPEC for PM from unusual cancer sites of origin were performed in 53 centres worldwide. Thirty procedures for rare indications were performed in fewer than 10 patients and were excluded (adrenal cancer, n = 8; renal carcinoma, n = 3; bladder cancer, n = 1; lymphoma, n = 1). In total, 820 procedures performed in 781 patients were included in the analysis. More than 70% of the procedures were performed during the last 10 years (n = 565 (72.3%) since 2006). Most of the patients underwent one procedure (n = 744), 35 patients had two procedures and two patients had three procedure. There were 548 women (70.2%) and 233 men (29.8%), with a median age of 52.8 years (range: 12.4–84.4).
Twelve unusual cancer sites of origin of PM were included. Nearly two-thirds of the procedures were performed for three indications: less common ovarian carcinoma histologic subtypes (n = 224), sarcoma (n = 189) and neuroendocrine tumours (n = 127). Other indications were PM from hepato-pancreato-biliary carcinomas in 99 patients (including cholangiocarcinoma in 44 patients, pancreatic adenocarcinoma in 32 and hepatocellular carcinoma in 23), from gynaecologic cancers in 37, from desmoplastic small round cell tumours in 37, from the urachus in 39 and from breast carcinoma in 18 patients.
Preoperative systemic chemotherapy was administered to 395 patients (53.2%), and 199 (34.3%) received postoperative systemic chemotherapy. The median interval between the diagnosis of PM and CRS plus HIPEC was 5.98 months (−15.01 to 270.96).
Procedures and postoperative courses
During the first procedure, the median PCI, reported in 564 patients, was 12/39 (range 0–39). It was equal to or more than 10 in 61.7% of the patients. CRS was complete in most of the patients (CC-0: 71.7%, CC-1: 17.8%).
Regarding the HIPEC procedure, 417 patients (55.6%) and 333 patients (44.4%) were submitted to an open and a closed abdomen technique, respectively. The median duration of HIPEC was 90 min [25–180] and the mean temperature of the bath was 41.9 (±1.33). The chemotherapeutic agents used for HIPEC were cisplatin at a median dose of 60 [20–100] mg/m2, mitomycin C at a median dose of 30 [3.3–60] mg/m2, oxaliplatin at a median dose of 360 [200–480] mg/m2, doxorubicin at a median dose of 15 [10–60] mg/m2, and irinotecan at a median dose of 200 mg/m2. A combination of two chemotherapeutic agents was used in 315 patients (40.5%).
Regarding the main three indications, the median duration of HIPEC were 90 [30–135], 60 [25–123], and 90 [30–123] for neuroendocrine tumours, common ovarian carcinoma histologic subtypes and sarcoma, respectively. The most frequently chemotherapy agents used were mitomycin C (56%) and oxaliplatin (40%) for neuroendocrine tumours, cisplatin (50%) and mitomycin (33%) for common ovarian carcinoma and doxorubicin (42%) and association doxorubicin/cisplatin (36%) for sarcoma.
Grade III–IV (NCI CTCAE V4.0) postoperative complications occurred in 272 patients (41%). Nineteen patients (2.9%) died postoperatively. The cause of death in two patients was not reported. In the remaining patients, death was related to septic complications and multi-organ failure in eight, multi-organ failure in three, intraperitoneal bleeding and shock in two, respiratory failure in two, pulmonary embolism in one and pancreatic leak and renal failure in one patient. For the three main indications, occurrence of major complications and mortality rate were 38.5% (n = 30) and 5% (n = 4), 42% (n = 86) and 3% (n = 6), and 35% (n = 56) and 2.5% (n = 4), for neuroendocrine tumours, common ovarian carcinoma histologic subtypes and sarcoma, respectively.
Survival and prognostic factors
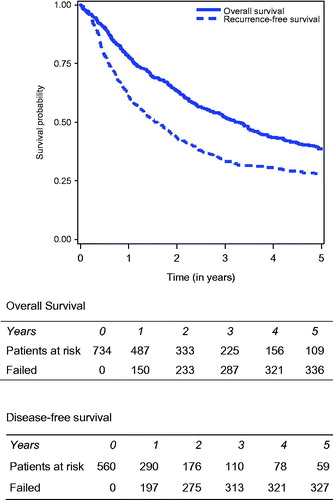
After a median follow-up of 46.48 months and a mean follow-up of 59.08 months, 364 patients (49.2%) were alive, 376 (50.8%) had died mostly due to disease progression (n = 247), and for 41 patients, the status was not known. An analysis of overall survival (OS) was performed on 734 patients (status unknown for 41 patients, plus 6 unknown dates of death, among the 781), and among them 371 had died.
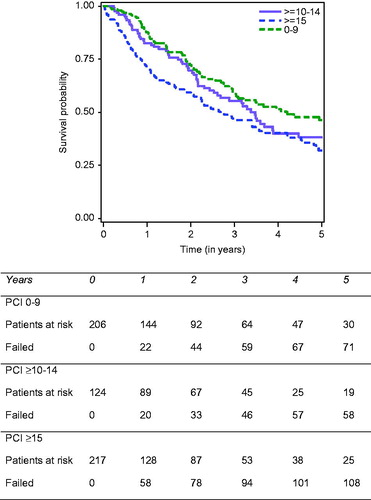
Regarding the entire cohort, median OS was 39.45 months [33.18–44.05]. One year, 3-year and 5-year OS were, respectively, 77.8%, 52.2% and 38.7%. OS decreased significantly for higher PCI scores, with a 5-year OS equal to 46.1% when the PCI was strictly below 10, 38.2% when the PCI was comprised between 10 and 14, and 31.9% when the PCI was equal to or more than 15 ().
Figure 1. Overall survival according to the PCI in patients who underwent cytoreductive surgery plus HIPEC for peritoneal metastases from rare cancer sites of origin.
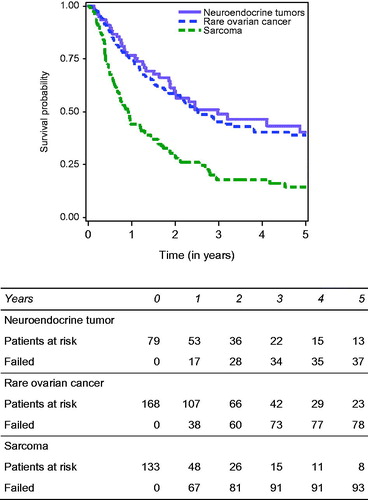
Regarding survival for the three indications with more than 50 patients – rare ovarian carcinoma, neuroendocrine tumours and sarcoma – 5-year OS was significantly greater in patients with PM from rare ovarian carcinoma (57.7%), than that of patients with PM from neuroendocrine tumours (39.9%), and from sarcoma (29.3%) (p < 0.0001) ().
Figure 2. Overall survival according to the origin of PM in patients who underwent cytoreductive surgery plus HIPEC for peritoneal metastases from ovarian carcinoma, neuroendocrine tumours and sarcoma.
In the univariate analysis (), negative prognostic factors for OS were male gender (p = 0.002), aetiology of PM (p < 0.0001), a PCI score strictly greater than 10 (p = 0.005), CC-1,2 or 3 (p < 0.0001), the closed abdomen technique (p = 0.0004), HIPEC exceeding 60 min (p < 0.0001), poorly differentiated tumours (p = 0.0004), lymph node involvement (p < 0.0001), pre- and postoperative systemic chemotherapy (p = 0.001). The number of chemotherapeutic agents used for HIPEC did not significantly influence OS. The multivariate analysis in revealed five independent negative prognostic factors: preoperative chemotherapy (HR 1.72, 95%CI: 1.26–2.35, p = 0.0006), lymph node involvement (HR 1.99, 95%CI:1.39–2.85, p = 0.0002), postoperative chemotherapy (HR 1.54, 95%CI:1.11–2.14, p = 0.001), residual tumour CC 2 (HR 2.57, 95%CI:1.65–4.02, p < 0.0001), and aetiology (cholangiocarcinoma, pancreas, GIST, sarcoma, endometrium vs. rare ovarian cancer). The extent of the disease evaluated with the PCI was not identified as an independent prognostic factor. The type of chemotherapeutic agent used for HIPEC was not identified as an independent predictive factor for survival.
Table 1. Univariate analysis for overall survival.
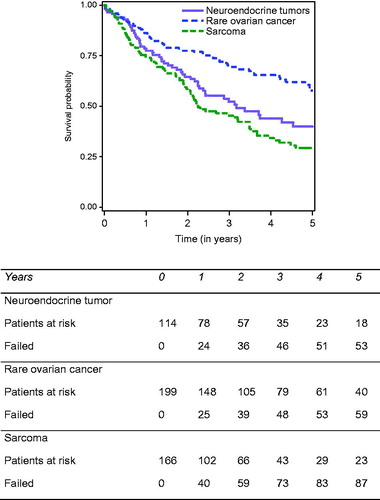
Patients with incomplete cytoreduction (CC2) were excluded from the disease-free survival (DFS) analysis. In total, 665 patients were analysed for disease-free survival, and information concerning the recurrence was available for 591 among them. Thirty-one patients were excluded because the date of relapse was not valid. At the end of follow-up, 372/560 patients (62.9%) had developed recurrent disease. Thus, the median DFS of the entire cohort was 18.9 months [15.7–22.4]. At 1, 3 and 5 years, DFS rates were, respectively, 61.2%, 33.2% and 28% (). Concerning the three indications with more than 50 patients – rare ovarian carcinoma, neuroendocrine tumours and sarcoma – 5-year DFS was significantly greater in patients with PM from ovarian carcinoma (38.9%), and from neuroendocrine tumours (40.2%), compared to that of patients with PM from sarcoma (14.3%) (p < 0.0001) (). The univariate and multivariate analysis identified independent predictors for poor DFS, identical to those identified for OS, except for the type of CC.
Discussion
Since the introduction of CRS followed by HIPEC in 1980 by Spratt [Citation18], the indications and criteria for selecting the best candidates for HIPEC have been identified and refined, to obtain the best survival results with acceptable morbidity [Citation19–21]. Thus, CRS and HIPEC are now routinely performed for colorectal peritoneal metastases, pseudomyxoma peritonei and mesothelioma, when the disease is completely resectable and without extra-peritoneal extension [Citation1,Citation3,Citation4]. CRS and HIPEC have also been performed for other PM arising from frequent diseases such as gastric and epithelial ovarian carcinomas, but with debated results, and are still under evaluation. That is the reason why this study was focussed on rare indications excluding patients with PM from colorectal, gastric and epithelial ovarian carcinomas, and those with pseudomyxoma and peritoneal mesothelioma.
Our study assessed the outcomes for 781 patients treated in 53 centres worldwide. The majority of the patients (72%) were operated on during the last 10 years, confirming the recent development and expansion of this combined treatment. This analysis determined that OS following CRS and HIPEC was 38.5% at 5 years, which suggested that the combined treatment in those rare indications was safe and virtually as effective as CRS plus HIPEC for colorectal PM, with reported 5-year survival rates of around 40% [Citation22–26]. These favourable outcomes are certainly related to the application of stringent selection criteria when choosing patients for CRS and HIPEC, as in the case of colorectal cancer. First, most of the patients (89.6%) underwent complete cytoreductive surgery (CC0-1), which is one of the major prognostic factors. Second, the mean PCI was relatively low (PCI = 12 [0–39]) and we know that the extent of the disease evaluated by the PCI is the other main prognostic factor. Thus, for patients with PM from colorectal cancer, it has been demonstrated that a PCI exceeding 17–20 jeopardised the prognosis [Citation27–30], and in fact, such patients are not considered for CRS and HIPEC. Third, a majority of the patients received pre- and/or postoperative chemotherapy, which can help select patients before surgery, by excluding those with disease progression under chemotherapy, and may also decrease the risk of recurrence after surgery.
The long-term survival observed in this series and wider knowledge of CRS and HIPEC in patients with PM from colorectal cancer could encourage us to extend our indications. However, we must proceed cautiously and analyse the results according to the aetiology. Three main indications were identified, with more than a hundred patients treated, for whom we can try to draw conclusions for decision making. Patients with PM from rare ovarian carcinoma enjoyed the best prolonged survival, with 5-year OS and DFS rates of 57.7% and 38.9%, respectively. Among patients operated on for rare ovarian carcinomas, the most frequent aetiologies were mucinous, granulosa cell and endometroid ovarian cancer. The prolonged survival rates obtained for mucinous ovarian carcinoma with PM, confirmed that this histologic type can be likened to pseudomyxoma peritonei, with extended mucinous peritoneal implants, and thus should be treated similarly, with complete radical surgery and HIPEC. Concerning granulosa cell ovarian tumours, a recent systematic review from the Cochrane database [Citation30] comprising five retrospective cohort studies [Citation31–34] with data on 535 women, reported overall 5- and 10-year disease-specific survival rates ranging between 93 and 97%, and between 87 and 95%, respectively [Citation32–34]. The type of surgery (incomplete versus radical) was associated with an increased risk of recurrence in two of those five studies [Citation33,Citation34]. However, the potential benefit of combined HIPEC was not evaluated in any of those studies, and its exact role continues to fuel debate [Citation35,Citation36].
Concerning PM from neuroendocrine tumour, the potential benefit of CRS and HIPEC is also highly debated. In fact, few studies have focussed on these patients as most of them also have extra-peritoneal involvement, and the progression of TNE is generally slow. However, it has been shown that the presence of PM from TNE negatively influences the prognosis [Citation37,Citation38], and that debulking surgery could increase survival [Citation39]. Thus, in the recent ENETS Consensus Guidelines [Citation40,Citation41], experts proposed that PM from TNE should be treated aggressively, in patients with favourable benefit/risk ratios, and in high-volume centres, if and only if complete resection is feasible, and this after a full assessment of the disease. Again, the exact role of HIPEC in this indication is not clear, as suggested by the studies reported by Elias et al. [Citation42,Citation43]. In the present study, the 5-year survival rate was comparable to that reported in studies of patients operated on without HIPEC [Citation44], and lower than that of patients who had received HIPEC. This is confusing, but it can be explained by the completeness or not of the resection, as well as by the presence of extra-peritoneal metastases. It is therefore impossible to draw a clear conclusion and a randomised study to evaluate the impact of HIPEC should be initiated.
Regarding the third most frequent indication, PM from soft-tissue sarcomas, 5-year OS reached nearly 30%, with a 5-year DFS rate of 14%. These results may seem disappointing; however, survival rates with chemotherapy alone are worse. Indeed, it has been demonstrated that complete resection of recurrent disease, when feasible, improves survival, up to 48 months [Citation45]. Therefore, CRS should improve survival, in certain subgroups of patients with PM from sarcoma, as suggesting in the study reported by Baratti et al. about 37 patients [Citation46]. In this study, the 5-year OS was 24.3%, with a greater proportion of long survivors in the subgroup of uterine leiomyosarcoma. Patients with PM from GIST had the worst survival rates and all of those with retroperitoneal liposarcoma experiences peritoneal relapse. Again, the respective roles of CRS and HIPEC in prolonging survival are difficult to determine, and this has been underlined in other studies [Citation47–49].
This large analysis of more than 800 procedures shows that different methods (either an open or closed abdomen), various types of chemotherapeutic agents, alone or combined, were used. These variations represent the inter-centre variability in achieving HIPEC, and this study cannot afford to show the superiority of a technique or a chemotherapy agent over others. Indeed, none of these techniques have demonstrated superiority and it is now accepted that the open or closed abdomen technique can be used indifferently, but clinicians are advised to always use the same technique in the same centre [Citation50,Citation51]. Regarding intraperitoneal chemotherapy, the agents most frequently used worldwide are mitomycin C and oxaliplatin (cisplatin was mainly used in ovarian carcinomas). Again, both of these agents can be indifferently used, with a preference for oxaliplatin for PM from colorectal cancer and for mitomycin for mesothelioma and pseudomyxoma peritonei.
There are certainly limitations to this retrospective analysis conducted on patients treated over a long-time interval. Despite the large number of patients included in the study, this was a highly selected cohort, representing only a fraction of the total number of patients with PM from uncommon cancer sites. However, the first question that our study addressed was whether CRS plus HIPEC was effective in patients with PM from rare aetiologies. The results on safety and on overall survival support the use of surgical resection plus HIPEC for such rare indications, in selected patients, provided complete resection can be achieved. Selecting patients for such treatment could be based on their general status and the evaluated risk of postoperative complications, the aggressiveness of the disease according to the disease-free interval, response to systemic chemotherapy and the extent of the peritoneal disease. Surprisingly, the PCI was not identified as an independent prognostic factor. This could be due to the fact that PM arose from greatly diverse aetiologies, with very different prognoses. If these aetiologies were considered individually, the PCI would more likely than not retain its prognostic value as we know it.
However, the exact role of HIPEC has to be elucidated. It is very difficult even impossible to answer the question by a randomised trial for each aetiology [Citation52]. The attitude which we could propose regarding the efficiency of HIPEC for these rare indications for PM arising from unusual cancer sites, in the absence of a strong level of evidence, would be to adapt it to the patient, to the cancer site of origin, the histologic type and to the presence or not of extra-peritoneal metastases. Thus, HIPEC should be preferentially offered to young patients, in good general condition, without extra-peritoneal disease, with PM from an intra-abdominal primary, or from mucinous histology.
In conclusion, CRS and HIPEC appear to be safe and effective in patients with peritoneal metastases from rare cancer sites of origin. The best candidates for such procedures seem to be patients with rare ovarian carcinomas and PM from neuroendocrine tumours. The respective roles of CRS and HIPEC remain unclear and warrant evaluation for the most frequent causes of PM.
Supplemental File
Download PDF (198.9 KB)Acknowledgements
The authors thank all the contributors of the PSOGI Group and the BIG-RENAPE Group for their collaboration, Peggy Jourdan-Enfer and Anaïs Poulet for the quality of data collected. The authors acknowledge Lorna Saint Ange for editing, Christelle Maurice and Evelyne Decullier for her contribution to the data analysis.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
The collaborators of the BIG-RENAPE Working Group include: J. Abba (Department of Surgical Oncology, CHU Grenoble University, Grenoble, France); K. Abboud (Department of Surgical Oncology, CHU St Etienne, St Etienne, France); Alyami, M. (Department of Surgical Oncology, Centre Hospitalier Lyon Sud - EMR 3738, Lyon 1 University, Lyon, France); C. Arvieux (Department of Surgical Oncology, CHU Grenoble University, Grenoble, France); N. Bakrin (Department of Surgical Oncology, Centre Hospitalier Lyon Sud - EMR 3738, Lyon 1 University, Lyon, France); J-M. Bereder (Department of Surgical Oncology, CHU L'Archet 2, Nice, France); D. Bouzard (Department of Surgical Oncology, CHU Louis Mourier, Colombes, France); C. Brigand (Department of Surgical Oncology, CHRU Hautepierre, Strasbourg, France); S. Carrère (Department of Surgical Oncology, Institut du Cancer de Montpellier, Montpellier, France); D. Delroeux (Department of Surgical Oncology, CHU Jean Minjoz, Besançon, France); F. Dumont (Department of Surgical Oncology, ICO - René Gauducheau, St Herblain, France); C. Eveno (Department of Surgical Oncology, CHU Lariboisière, Paris, France); O. Facy (Department of Surgical Oncology, CHU Dijon, Dijon, France); F. Guyon (Department of Surgical Oncology, Institut Bergonié, Bordeaux, France); G. Ferron (Department of Surgical Oncology, IUCT Oncopole, Toulouse, France); R. Kianmanesh (Department of Surgical Oncology, CHU Robert Debré, Reims, France); R. Lo Dico (Department of Surgical Oncology, CHU Lariboisière, Paris, France); G. Lorimier (Department of Surgical Oncology, CHU Angers, Angers, France); F. Marchal (Department of Surgical Oncology, Institut de Cancérologie de Lorraine, Vandoeuvre-lès-Nancy, France); P. Mariani (Department of Surgical Oncology, Institut Curie, Paris, France); P. Meeus (Department of Surgical Oncology, Centre Léon Bérard, Lyon, France); S. Msika (Department of Surgical Oncology, CHU Louis Mourier, Colombes, France); P. Ortega-Deballon (Department of Surgical Oncology, CHU Dijon, Dijon, France); B. Paquette (Department of Surgical Oncology, CHU Jean Minjoz, Besançon, France); P. Peyrat (Department of Surgical Oncology, Centre Léon Bérard, Lyon, France); N. Pirro (Department of Surgical Oncology, CHU La Timône, Marseille, France); M. Pocard (Department of Surgical Oncology, CHU Lariboisière, Paris, France); J. Porcheron (Department of Surgical Oncology, CHU St Etienne, St Etienne, France); F. Quenet (Department of Surgical Oncology, Institut du Cancer de Montpellier, Montpellier, France); P. Rat (Department of Surgical Oncology, CHU Dijon, Dijon, France); O. Sgarbura (Department of Surgical Oncology, Institut du Cancer de Montpellier, Montpellier, France); E. Thibaudeau (Department of Surgical Oncology, ICO - René Gauducheau, St Herblain, France); J-J. Tuech (Department of Surgical Oncology, CHU Charles Nicolle, Rouen, France); F. Zinzindohoue (Department of Surgical Oncology, Hôpital Européen Georges Pompidou, Paris, France).
The collaborators of the PSOGI Working Group include: S. A. Ahrendt (Department of Surgery, University of Pittsburgh Medical Center Shaydyside Hospital, Pittsburgh, USA); E. Akaishi (Department of Surgical Oncology, Centro de Oncologia Hospital Sirio Libanes, Sao Paolo, Brazil); S. H. Baik (Department of Surgery, Gangnam Severance Hospital - Yonsei University College of Medicine, Seoul, Korea); D. Baratti (Department of Gastrointestinal Surgery, San Raffaele Scientific Institute, Milan, Italy); A. Bhatt (Department of Surgical Oncology, Fortis Hospitals Limited, Bangalore, India); P. Cachin (Department of Surgery, Akademiska sjukhuset, Uppsala University Hospital, Uppsala, Sweden); W. Ceelen (Department of Gastrointestinal Surgery, Gent University Hospital, Ghent, Belgium); I. De Hingh (Department of Surgery, Catharina Ziekenhuis, Eindhoven, Netherlands); M. De Simone (Department of Surgical Oncology, Candiolo Cancer Institute - FPO, IRCCS, Turin, Italy); R. P. Edwards (Department of Surgery, University of Pittsburgh Medical Center Shaydyside Hospital, Pittsburgh, USA); J. Franko (Department of Surgical Oncology, Mercy Medical Center, Baltimore, USA); L. Gonzalez-Bayon (Department of Surgical Oncology, Hospital Gregorio Marañón, Madrid, Spain); V. Gushchin (Department of Surgical Oncology, Mercy Medical Center, Baltimore, USA); M. P. Holtzman (Department of Surgery, University of Pittsburgh Medical Center Shaydyside Hospital, Pittsburgh, USA); M-C. Hsieh (Department of General Surgery, Wan-Fang Hospital, Taipei, Taiwan); D. Kecmanovic (Department of Surgery, First Surgical Clinic, Clinical Center of Serbia, Belgrade, Serbia); K. W. Lee (Department of Surgery, University of Pittsburgh Medical Center Shaydyside Hospital, Pittsburgh, USA); K. Lehmann (Department of Surgery and Transplantation, University Hospital of Zurich, Zurich, Switerland); Y. Liu (NPO to Support Peritoneal Surface Malignancy Treatment, Kyoto, Japan); S. Mehta (Division of Peritoneal Surface Oncology, Saifee Hospital, Mumbai, India); D. L. Morris (Department of Surgery, University of New South Wales, Sydney, Australia); S. O'Dwyer (Department of Colorectal Surgery, Christie Cancer Center, Manchester, United Kingdom); E. Orsenigo (Department of Gastrointestinal Surgery, San Raffaele Scientific Institute, Milan, Italy); P. K. Pande (Department of Surgical Oncology, BLK Superspeciality Hospital, New Delhi, India); E. J. Park (Department of Surgery, Gangnam Severance Hospital - Yonsei University College of Medicine, Seoul, Korea); J. F. Pingpank (Department of Surgery, University of Pittsburgh Medical Center Shaydyside Hospital, Pittsburgh, USA); P. Piso (Department of Surgery, University of Regensburg, Regensburg, Germany); F. Rajan (Department of Surgical Oncology, Kovai Medical Centre, Coimbatore, India); B. Rau (Department of Surgical Oncology, Charite Campus Mitte University of Berlin, Berlin, Germany); A. Sardi (Department of Surgical Oncology, Mercy Medical Center Baltimore, USA); A. Sommariva (Melanoma and Sarcoma Unit, Istituto Oncologico Veneto, Padua, Italy); J. Spiliotis (First Department of Surgical Oncology, Metaxa Cancer Memorial Hospital, Piraeus, Greece); A. A. K. Tentes (Department of Surgery, Metropolitan Hospital, Athens, Greece); M. Teo (Department of Surgical Oncology, National Cancer Centre Singapore, Singapore, Singapore); R. Yarema (Department of Oncology and Medical RadiologyDanylo Halytsky Lviv National Medical University, Lviv, Ukraine); Y. Yonemura (NPO to Support Peritoneal Surface Malignancy Treatment, Kyoto, Japan); S. S. Zaveri (Department of Surgical Oncology, Manipal Hospital, Bangalore, India); H. J. Zeh (Department of Surgery, University of Pittsburgh Medical Center Shaydyside Hospital, Pittsburgh, USA).
References
- Chua TC, Moran BJ, Sugarbaker PH, et al. (2012). Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 30:2449–56.
- Yan TD, Black D, Savady R, Sugarbaker PH. (2007). A systematic review on efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol 14:484–92.
- Yan TD, Deraco M, Baratti D, et al. (2009). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 27:6237–42.
- Esquivel J, Elias D, Baratti D, et al. (2008). Consensus statement on the loco regional treatment of colorectal cancer with peritoneal dissemination. J Surg Oncol 98:263–7.
- Morgan RJ, Jr, Armstrong DK, Alvarez RD, et al. (2016). Ovarian Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 14:1134–63.
- Le Brun JF, Campion L, Berton-Rigaud D, et al. (2014). Survival benefit of intraperitoneal chemotherapy for recurrent ovarian cancer: a multi-institutional case control study. Ann Surg Oncol 21:3621–7.
- Bakrin N, Bereder JM, Decullier E, et al. (2013). Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol 39:1435–43.
- Smyth EC, Verheij M, Allum W, et al. (2016). ESMO Guidelines Committee. Gastric Cancer: ESMO Clinical Practice Guidelines. Ann Oncol 27:v38–49.
- Yang XJ, Huang CQ, Suo T, et al. (2011). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 18:1575–81.
- Glehen O, Gilly FN, Arvieux C, et al. (2010). Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol 17:2370–7.
- Chia CS, You B, Decullier E, et al. (2016). Patients with peritoneal carcinomatosis from gastric cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is cure a possibility? Ann Surg Oncol 23:1971–9.
- Yonemura Y, Canbay E, Li Y, et al. (2016). A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur J Surg Oncol 42:1123–31.
- Honoré C, Goéré D, Macovei R, et al. (2016). Peritoneal carcinomatosis from unusual cancer origins: is there a role for hyperthermic intraperitoneal chemotherapy? J Visc Surg 153:101–7.
- Jacquet P, Sugarbaker PH. (1996). Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J Exp Clin Cancer Res 15:49–58.
- Sugarbaker PH. (1996). Peritoneal carcinomatosis: natural history and rational therapeutic interventions using intraperitoneal chemotherapy. Cancer Treat Res 81:149–68.
- Elias D, Antoun S, Goharin A, et al. (2000). Research on the best chemohyperthermia technique for treatment of peritoneal carcinomatosis after complete resection. Int J Surg Investig 1:431–9.
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2010. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14.
- Spratt JS, Adcock RA, Sherrill W, Travathen S. (1980). Hyperthermic peritoneal perfusion system in canines. Cancer Res 40:253–5.
- Chua TC, Saxena A, Schellekens JF, et al. (2010). Morbidity and mortality outcomes of cytoreductive surgery and perioperative intraperitoneal chemotherapy at a single tertiary institution: towards a new perspective of this treatment. Ann Surg 251:101–6.
- Glehen O, Osinsky D, Cotte E, et al. (2003). Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol 10:863–9.
- Kwakman R, Schrama AM, van Olmen JP, et al. (2016). Clinicopathological parameters in patient selection for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer metastases a meta-analysis. Ann Surg 263:1102–11.
- Verwaal VJ, van Ruth S, de Bree E, et al. (2003). Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis from colorectal cancer. J Clin Oncol 21:3737–43.
- Elias D, Lefevre JH, Chevalier J, et al. (2009). Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 27:681–5.
- Elias D, Gilly F, Boutitie F, et al. (2010). Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 28:63–8.
- Goéré D, Malka D, Tzanis D, et al. (2013). Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg 257:1065–71.
- de Cuba EM, Kwakman R, Knol DL, et al. (2013). Cytoreductive surgery and HIPEC for peritoneal metastases combined with curative treatment of colorectal liver metastases: systematic review of all literature and meta-analysis of observational studies. Cancer Treat Rev 39:321–7.
- Gomes da Silva RG, Sugarbaker PH. (2006). Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg 203:878–86.
- Cotte E, Passot G, Gilly FN, Glehen O. (2010). Selection of patients and staging of peritoneal surface malignancies. World J Gastrointest Oncol 2:31–5.
- Cashin PH, Graf W, Nygren P, Mahteme H. (2012). Patient selection for cytoreductive surgery in colorectal peritoneal carcinomatosis using serum tumor markers: an observational cohort study. Ann Surg 256:1078–83.
- Goéré D, Souadka A, Faron M, et al. (2015). Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol 22:2958–64.
- Gurumurthy M, Bryant A, Shanbhag S. (2014). Effectiveness of different treatment modalities for the management of adult-onset granulosa cell tumours of the ovary (primary and recurrent). Cochrane Database Syst Rev 21:CD006912.
- Hauspy J, Beiner ME, Harley I, et al. (2011). Role of adjuvant radiotherapy in granulosa cell tumors of the ovary. Int J Radiation Oncol, Biol, Phys 79:770–4.
- Mangili G, Ottolina J, Gadducci A, et al. (2013). Long-term follow-up is crucial after treatment for granulosa cell tumours of the ovary. Br J Cancer 109:29–34.
- Sun H-D, Lin H, Jao MS, et al. (2012). A long-term follow-up study of 176 cases with adult-type ovarian granulosa cell tumors. Gynecol Oncol 124:244–9.
- Gouy S, Uzan C, Pautier P, et al. (2013). Results of oxaliplatin-based hyperthermic intraperitoneal chemotherapy in recurrent ovarian granulosa cell tumors. Eur J Obstet Gynecol Reprod Biol 170:464–7.
- Al-Badawi IA, Abu-Zaid A, Azzam A, et al. (2014). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for management of recurrent/relapsed ovarian granulosa cell tumor: a single-center experience. J Obstet Gynaecol Res 40:2066–75.
- Norlén O, Stålberg P, Öberg K, et al. (2012). Long-term results of surgery for small intestinal neuroendocrine tumors at a tertiary referral center. World J Surg 36:1419–31.
- Norlén O, Edfeldt K, Akerstrom G, et al. (2014). Peritoneal carcinomatosis from small intestinal neuroendocrine tumors: clinical course and genetic profiling. Surgery 156:1512–21.
- Boudreaux JP, Wang YZ, Diebold AE, et al. (2014). A single institution's experience with surgical cytoreduction of stage IV, well-differentiated, small bowel neuroendocrine tumors. J Am Coll Surg 218:837–4.
- Pavel M, O'Toole D, Costa F, et al. (2016). ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 103:172–85.
- Kianmanesh R, Ruszniewski P, Rindi G, et al. (2010). ENETS consensus guidelines for the management of peritoneal carcinomatosis from neuroendocrine tumors. Neuroendocrinology 91:333–40.
- Elias D, Sideris L, Liberale G, et al. (2005). Surgical treatment of peritoneal carcinomatosis from well-differentiated digestive endocrine carcinomas. Surgery 137:411–6.
- Elias D, David A, Sourrouille I, et al. (2014). Neuroendocrine carcinomas: optimal surgery of peritoneal metastases (and associated intra-abdominal metastases). Surgery 155:5–12.
- de Mestier L, Lardière-Deguelte S, Brixi H, et al. (2015). Updating the surgical management of peritoneal carcinomatosis in patients with neuroendocrine tumors. Neuroendocrinology 101:105–11.
- Jaques DP, Coit DG, Hajdu SI, Brennan MF. (1990). Management of primary and recurrent soft-tissue sarcoma of the retroperitoneum. Ann Surg 212:51–9.
- Rossi CR, Deraco M, De Simone M, et al. (2004). Hyperthermic intraperitoneal intraoperative chemotherapy after cytoreductive surgery for the treatment of abdominal sarcomatosis: clinical outcome and prognostic factors in 60 consecutive patients. Cancer 100:1943–50.
- Bonvalot S, Cavalcanti A, Le Péchoux C, et al. (2005). Randomized trial of cytoreduction followed by intraperitoneal chemotherapy versus cytoreduction alone in patients with peritoneal sarcomatosis. Eur J Surg Oncol 31:917–23.
- Barratti D, Pennacchioli E, Kusamura S, et al. (2010). Peritoneal sar-comatosis: is there a subset of patients who may benefitfrom cytoreductive surgery and hyperthermic intraperitonealchemotherapy? Ann Surg Oncol 17:3220–8.
- Baumgartner JM, Ahrendt SA, Pingpank JF, et al. (2013). Aggressive locoregional management of recurrent peritoneal sarcomatosis. J Surg Oncol 107:329–34.
- Sarnaik AA, Sussman JJ, Ahmad SA, et al. (2007). Technology for the delivery of hyperthermic intraoperative intraperitoneal chemotherapy: a survey of techniques. Recent Results Cancer Res 169:75–82.
- Ortega-Deballon P, Facy O, Jambet S, et al. (2010). Which method to deliver hyperthermic intraperitoneal chemotherapy with oxaliplatin? An experimental comparison of open and closed techniques. Ann Surg Oncol 17:1957–63.
- Systemic chemotherapy with or without intraperitoneal chemohyperthermia in treating patients undergoing surgery for peritoneal carcinomatosis from colorectal cancer. Available from: https://clinicaltrials.gov/show/NCT00769405.