Abstract
Introduction: Hyperthermic intraperitoneal chemotherapy (HIPEC) is an effective treatment for peritoneal carcinomatosis (PC). Laparoscopic surgery is performed in the treatment of colorectal and appendiceal cancer, and PC from diverse origin in selected patients. HIPEC management by laparoscopic approach after cytoreductive surgery (CRS) completed locoregional treatment of PC, and may be feasible and safe after appropriate patient selection.
Objective: Development of an experimental model of HIPEC by laparoscopic approach, with CO2 recirculation. Clinical translation in two patients with PC and low peritoneal cancer index.
Material and methods: We performed CRS in a porcine model of 5 pigs (35–38 kg) by laparoscopic approach. Laparoscopic HIPEC by CO2 recirculation system was performed; laparoscopic access was used for catheter input and output placement (Paclitaxel 175 mg/m2 for 60 min at 42 °C). The experimental variables were: blood gases, haemodynamic and intra-abdominal and central temperature. Clinical model application was performed in three cases with PC from colorectal origin.
Results: No statistically significant differences was found in blood gases, haemodynamic or temperature in the experimental study. In clinical study, there were no technical complications during laparoscopic-HIPEC approach, and we observed no changes in haemodynamic variables during the procedure.
Conclusions: CRS and HIPEC laparoscopic model by CO2 recirculation system is safe and feasible technique in selected patients, that include low PC index, local and accessible tumour recurrences or high-risk of PC tumours.
Introduction
Hyperthermic intraperitoneal chemotherapy (HIPEC) is an effective treatment for patients with peritoneal metastases (PM). Laparoscopic surgery is well established for colorectal and appendiceal cancer treatment, and for peritoneal carcinomatosis (PC) of diverse origins [Citation1]. HIPEC management by laparoscopic approach after cytoreductive surgery (CRS) completed locoregional treatment of PM, and may be feasible and safe after appropriate patient selection. This treatment (CRS + HIPEC laparoscopic) has been administered by other groups in animal models and patients [Citation2,Citation3], with no higher morbidity and mortality than other HIPEC approaches, in selected cases.
Based on our model of closed-HIPEC and CO2 recirculation system (experimental and clinical study) [Citation4,Citation5], we have developed closed-HIPEC by a laparoscopic approach. Safety and efficacy were checked in a pig model; we analysed haemodynamic and temperature variables, and the development of technology.
The main objective is to develop an experimental laparoscopic HIPEC via a CO2 recirculation system and clinical translation in patients with peritoneal metastases of colorectal origin.
Material and methods
Animals
The pig model included 5 female mini-pigs weighing 35–38 kg. A CRS (pelvic lymphadenectomy and interaortocaval) plus HIPEC with a laparoscopic approach was performed (). Azaperone (0.4 ml/kg IM), ketamine (0.2 ml/kg IM) and morphine (0.4 mg/kg SC) were used for the initial pre-anaesthetic phase. The pigs were then anaesthetised using midazolam (0.4 ml/10 kg IV) and atracurium (0.2 ml/10 kg IV). Anaesthesia was maintained using inhaled isoflurane (2.5%) and oxygen (100%). Heart rate, blood oxygen saturation, electrocardiogram and blood pressure were monitored non-invasively. Central temperature was monitored using an oesophageal thermometer.
After surgery, laparoscopic ports were used for catheter placement to input and output the perfusion solution and also to perform laparoscopic HIPEC CO2 recirculation system. We put inflow, outflow and CO2 catheters through the laparoscopic ports (). No leak fluid was observed during the laparoscopic HIPEC procedure. A gas exchanger was placed in the middle and upper abdominal wall, to check the turbulent flow and that the abdominal cavity had filled. A complete laparoscopic HIPEC was done. The drug used was Paclitaxel 175 mg/m2 for 60 min at 42 °C. Blood gases and haemodynamic and temperature variables were analysed at different times of surgery: (1) at the start of surgery (T1); (2) during surgery (T2); (3) after surgery (T3); (4) during HIPEC (T4); and (5) after HIPEC (T5).
Our study was in accordance with the Ethical Standards of the Regional Committee on animal experimentation, according to the laws of animal protection for experimentation and other specific regulations.
Patients
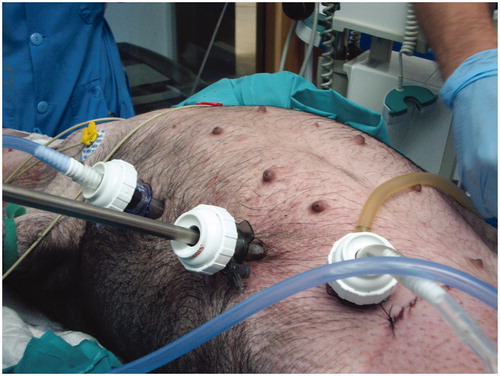
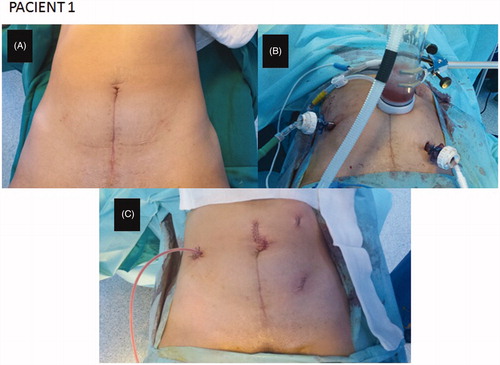
Once the technical safety had been verified in the experimental phase, the HIPEC laparoscopic model was used in two patients. Functional Eastern Cooperative Oncology Group (ECOG) performance status was 1 in both. The first patient, a 39-year-old woman, was diagnosed with an appendiceal mucinous tumour and an ovarian metastases. A left oophorectomy and infraumbilical laparotomy appendectomy were performed. After first surgery, CRS was completed by the laparoscopic approach with 4 ports (12 mm: optical port in umbilical region, left lower, left upper and right upper abdomen). A right hemicolectomy and omentectomy were performed. A 5-cm laparotomy was done to take the specimen off, and make an intestinal section and ileocolic anastomosis before HIPEC. Laparoscopic ports were used to place the input and output catheter through them (). The input catheter was placed in the left upper abdomen, the output catheter was placed in the left lower abdomen, and the input CO2 catheter was placed in the right lateral abdomen. A hand-assist through the small laparotomy was used for the placement of all catheters into the abdominal cavity. The 5-cm laparotomy was closed around the gas exchange device of our model. Mitomycin C 35 mg/m2 was administered for 60 min at 42.5 °C, by the protocol approved in our Hospital.
Figure 3. Closed-abdomen laparoscopic HIPEC by CO2 recirculation. Patient 1. A: before surgery; B: during laparoscopic HIPEC; C: after surgery.
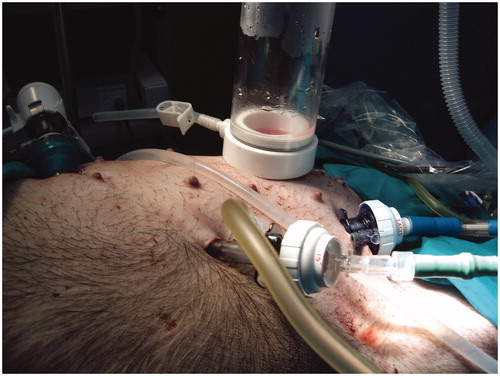
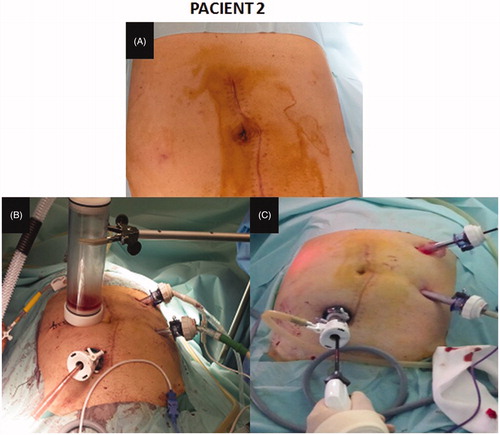
The second case was a 67-year-old male with appendiceal mucinous adenocarcinoma and local peritoneal metastases (pT4aN1bM1b). A right hemicolectomy was performed by laparotomy, because it was a large tumour. CRS (omentectomy and right parietal peritonectomy) plus HIPEC was completed 2 weeks after the first surgery. A laparoscopic approach was used: we performed a pneumoperitoneum by an open technique using the Hasson port in the right upper abdomen; two ports of 12 mm were placed in the left and right lateral abdomen. A right abdomen 5-cm laparotomy was performed to remove the specimen and place the gas exchange device (). HIPEC was performed with Mitomycin C 35 mg/m2 for 60 min at 42.5 °C. The last patient was a 55-year-old man with colon cancer with local peritoneal implants. A CRS (left hemicolectomy + omentectomy + left parietal peritonectomy) plus HIPEC was performed by laparoscopic approach by the same way.
Figure 4. Closed-abdomen laparoscopic HIPEC by CO2 recirculation. Patient 2. A: before surgery; B: during laparoscopic HIPEC; C: during cytoreductive laparoscopic surgery.
Informed consent was obtained from both patients. The procedures were performed in accordance with Ethics Committee of our Institution and with the Declaration of Helsinki of 1975, revised in 1983.
Results
Experimental phase
Five animals were included in the experimental study. The mean time of surgery (CRS + HIPEC) was 120 min. Intra-abdominal temperature was 42.5 °C. Haemodynamic variables analysed (Cardiac Index (CI), Global End-Diastolic Volume (GEDV), Stroke Volume Variation (SVV), Extravascular Lung Water Index (ELWI), Systemic Vascular Resistance (SVR), Central Venous Pressure (CVP), median blood pressure (MBP)) and blood gas variables (pCO2, pH and HCO3) are described in and . There were no technical complications during the procedure. All animals were sacrificed at the end of the procedure with an IV dose of sodium pentobarbital (100 mg/Kg).
Table 1. Haemodynamic values in pigs HIPEC laparoscopic surgery.
Table 2. Blood gases values in pigs HIPEC laparoscopic surgery.
Clinical phase
In all patients, HIPEC was performed by a laparoscopic approach and CO2 recirculation technique. There were no haemodynamic complications during the procedure. The mean time of surgery (CRS + HIPEC) was 240 min. The patients started an oral diet on the second postoperative day. Intensive Care Unit stay was 72 h, and hospital stay was 6 days. No complications in the first 30 days after surgery were recorded.
Discussion
CRS plus HIPEC administration by laparoscopic approach has been developed and applied in selected patients with peritoneal metastases of diverse origins. This approach can provide HIPEC pharmacokinetic advantages related to intraperitoneal administration of the drug plus the advantages of minimally invasive surgery.
Intra-abdominal pressure may improve drug penetration into the tumoural node. Some experimental studies have shown higher drug penetration in tumoural nodes with higher and controlled intra-abdominal pressure. Solass et al. [Citation6] showed higher drug penetration in the intra-abdominal pressure group called therapeutic pneumoperitoneum. Facy et al. [Citation7] showed a different Oxaliplatin penetration under different thermal conditions and intra-abdominal pressure in a porcine model. This author described the highest drug penetration with hyperthermic conditions (42 °C) and high intra-abdominal pressure (25 cm H2O or 18.3 mmHg). Gesson-Paute et al. [Citation8] explained increased drug penetration by higher intra-abdominal pressure: hand-assisted laparoscopy-HIPEC was performed in the first group, while open-abdomen HIPEC was performed in the second. Oxaliplatin penetration was higher in the laparoscopy-HIPEC group than in the open-abdomen HIPEC group. No peritonectomy procedures were performed in this study, and there are no intra-abdominal pressure data, but they concluded increased drug tissue penetration under higher intra-abdominal pressure (laparoscopy-HIPEC).
In other pig model study by Ferron et al. [Citation2], a CRS (parietal and pelvic peritonectomy) plus HIPEC was performed by a laparoscopic approach, using a hand-assisted device. In our model, that of closed-abdomen laparoscopic HIPEC by CO2 recirculation system, a turbulent flow is created into the abdominal cavity which is observed through the device placed in the middle of the abdominal cavity [Citation4]. This closed-abdomen HIPEC showed optimal fluid and intra-abdominal temperature distribution (methylene blue stain), with haemodynamic stability.
Minimally invasive surgery is implemented in normal surgical practice [Citation9–13]. In colon cancer surgery, several prospective randomised studies have found no difference in the overall survival between the laparoscopic or laparotomy approaches. CRS + HIPEC by laparoscopic approach has shown efficacy and safety in some studies, always with correct preoperative patient selection. Peritoneal cancer index (PCI) can help to select patients who will benefit from laparoscopic-CRS and HIPEC. A PCI <10 in CT scan before surgery or during laparoscopic surgery may indicate that laparoscopic CRS and HIPEC surgery may be feasible and safe by this approach [Citation14–16]. In case of local peritoneal metastases recurrence, minimally invasive surgery + HIPEC laparoscopy has shown to be a safe procedure in ovarian cancer (less surgical time, less blood losses and shorter hospital stay than open surgery). The laparoscopic approach has shown no statistically significant differences in morbidity and mortality in comparison with the open approach; HIPEC is not a contraindication for a minimally invasive approach in a localised recurrence. Due to HIPEC effect in microscopic residual effect, there are many studies about peritoneal metastases prevention in high risk of peritoneal metastases tumours [Citation17]. They are anticipating the occurrences of macroscopic peritoneal disease, and it could by perform by laparoscopic approach. Adjuvant HIPEC in T4 colon cancer or perforated tumour can reduce the peritoneal metastases development from 40% to 10% [Citation18].
The laparoscopic HIPEC approach in these cases offers minimally invasive surgery benefits and should not be a contraindication for the administration of HIPEC in selected cases.
One first laparoscopic HIPEC model was a system called Continuous Hyperthermic Peritoneal Perfusion (CHPP) [Citation19]. This method was developed and used by laparotomy, and appears similar to the classic closed HIPEC technique. In laparoscopic HIPEC, the authors have included patients with certain characteristics: refractory ascites, CRS + HIPEC or as a neoadjuvant before second CRS. HIPEC is performed by closed abdominal cavity and manual fluid agitation during the procedure. The study presented optimal peritoneal pharmacokinetics (Cisplatin and mitomycin-C). In our model, CO2 recirculation produces a turbulent flow inside the abdominal cavity, and no manual agitation is performed.
There is another HIPEC treatment, called PIPAC (pressurised intraperitoneal aerosol chemotherapy), that has been used in humans [Citation20]. PIPAC method applied aerosol chemotherapy into the abdomen for 30 min at a pressure of 12 mmHg and a temperature of 37 °C. We use a CO2 and pressure of 12 mmHg too, but at 42° and for 60 min. We have applied a controlled high pressure during the laparoscopic HIPEC (12 mmHg, but at 42° and for 60 min), similar to the pressures used during conventional laparoscopic abdominal pressure. Both methods may improve the tissue chemotherapy, due to high pressure. Although we have not analysed the depth of penetration of the drug into the peritoneum, this could be a line of future research that would provide important data to the developed model.
As demonstrated in other pharmacokinetic studies, laparoscopic HIPEC may improve drug penetration into tumour cells, and our study was safe in clinical and technical terms in both phases (experimental and clinical). Correct patient selection can benefit this approach, and it is key to optimise cancer treatment with no morbidity. In our case, the peritoneal cancer index was the most important factor for the laparoscopic approach. Therefore, laparoscopic HIPEC with CO2 recirculation system after debulking surgery is safe and reproducible in local peritoneal metastases, with minimally invasive surgery benefits and no high morbidity. It is necessary to use a larger cohort of patients to confirm these observations and to perform prospective randomised trials to compare laparoscopic HIPEC and CO2 recirculation system versus closed-abdomen HIPEC and CO2 recirculation system.
Conclusions
Laparoscopic CRS and HIPEC by CO2 recirculation system is safe and feasible technique in selected patients, such as patients with low peritoneal cancer index before surgery or a tumour technically accessible recurrence.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Fagotti A, Petrillo M, Costantini B, et al. (2014). Minimally invasive secondary cytoreduction plus HIPEC for recurrent ovarian cancer: a case series. Gynecol Oncol 132:303–6.
- Ferron G, Gesson-Paute A, Classe JM, et al. (2005). Feasibility of paroscopic peritonectomy followed by intra-peritoneal chemohyperthermia: an experimental study. Gynecol Oncol 99:358–61.
- Fagotti A, Costantini B, Gallotta V, et al. (2015). Minimally invasive secondary cytoreduction plus HIPEC versus open surgery plus HIPEC in isolated relapse from ovarian cancer: a retrospective cohort study on perioperative outcomes. J Minim Invasive Gynecol 22:428–32.
- Sánchez-García S, Padilla Valverde D, Villarejo Campos P, et al. (2014). Experimental development of an intra-abdominal chemohyperthermia model using a closed abdomen technique and a PRS-1.0 Combat® CO2 Recirculation System. Surgery 155:719–25.
- Sánchez-García S, Villarejo Campos P, Padilla Valverde D, et al. (2016). Intraperitoneal chemotherapy hyperthermia (HIPEC) for peritoneal carcinomatosis of ovarian cancer origin by fluid and CO2 recirculation using the closed-abdomen technique (Combat PRS-1.0): a clinical pilot study. Int J Hyperth 32:488–98.
- Solass W, Hetzel A, Nadiradze G, et al. (2012). Description of a novel approach for intraperitoneal drug delivery and the related device. Surg Endosc 26:1849–55.
- Facy O, Al Samman S, Magnin G, et al. (2012). High pressure enhances the effect of hyperthermia in intraperitoneal chemotherapy with oxaliplatin: an experimental study. Ann Surg 256:1084–8.
- Gesson-Paute A, Ferron G, Thomas F, et al. (2008). Pharmacokinetics of oxaliplatin during open versus laparoscopically assisted heated intraoperative intraperitoneal chemotherapy (HIPEC): an experimental study. Ann Surg Oncol 15:339–44.
- Park DA, Yun JE, Kim SW, Lee SH. (2016). Surgical and clinical safety and effectiveness of robot-assisted laparoscopic hysterectomy compared to conventional laparoscopy and laparotomy for cervical cancer: a systematic review and meta-analysis. Eur J Surg Oncol 5:30687–4.
- Ocuin LM, Tsung A. (2016). Minimally invasive hepatic surgery. Surg Clin North Am 96:299–313.
- Son T, Hyung WJ. (2016). Laparoscopic gastric cancer surgery: current evidence and future perspectives. World J Gastroenterol 22:727–35.
- Pascual M, Salvans S, Pera M. (2016). Laparoscopic colorectal surgery: current status and implementation of the latest technological innovations. World J Gastroenterol 22:704–17.
- Ramos RF, Scalon FM, Scalon MM, Dias DI. (2016). Staging laparoscopy in gastric cancer to detect peritoneal metastases: a systematic review and meta-analysis. Eur J Surg Oncol 42:1315–21.
- Esquivel J, Averbach A. (2012). Laparoscopic cytoreductive surgery and HIPEC in patients with limited pseudomyxoma peritonei of appendiceal origin. Gastroenterol Res Pract 2012:981245.
- Esquivel J, Averbach A, Chua TC. (2011). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with limited peritoneal surfaces malignancies: initial feasibility and safety results. Ann Surg 253:764–8.
- Passot G, Bakrin N, Isaac S, et al. (2014). Postoperative outcomes of laparoscopic vs open cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for treatment of peritoneal surface malignancies. Eur J Surg Oncol 40:957–62.
- Tentes AA, Stamou K, Pallas N, et al. (2016). The effect of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) as an adjuvant in patients with resectable pancreatic cancer. Int J Hyperthermia 32:895–9.
- Klaver CE, Musters GD, Bemelman WA, et al. (2015). Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with colon cancer at high risk of peritoneal carcinomatosis: the COLOPEC randomized multicenter trial. BMC Cancer 15:428.
- Chang E, Alexander R, Libutti SK, et al. (2001). Laparoscopic continuous hyperthermic peritoneal perfusion. J Am Coll Surg 193:225–9.
- Solass W, Kerb R, Mürdter T, et al. (2014). Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol 21:553–9.